Abstract
This work addresses the scarcity of energy resources and environmental issues by concentrating on the synthesis of biodiesel by the transesterification of waste cooking oil with methanol. Marble sludge (MS), a novel heterogeneous catalyst, was used to speed up the rate of reaction. The catalyst’s physical and chemical characteristics were thoroughly examined using a variety of methods, including X-ray diffraction, X-ray Fluorescence, SEM, particle size distribution, and BET analysis. Using the MS catalyst, the study investigated the impact of important parameters on the yield of biodiesel from waste cooking oil with the aid of response surface methodology using Design-Expert version 13 software. These parameters included temperature (50–70℃), reaction time (1–4 h), catalyst concentration (1–5 wt%), and methanol-to-oil molar ratio (5–20 mol/mol). Optimization of the parameters was performed for economic targets to lower the production cost of biodiesel. The results showed that a methanol-to-oil molar ratio of 20:1, a catalyst of 5 wt%, and a reaction time of 1 h at 57℃ were the ideal parameters for obtaining a biodiesel yield of 93.5%. The resultant biodiesel revealed promising characteristics, such as a flash point of 160℃, a kinematic viscosity of 4 mm2/s, and a density of 0.871 g/cm3. The study demonstrates the significant consequences and real-world advantages of using rational engineering methods to use MS as a very effective, stable, and easily recoverable catalyst for the long-term, sustainable generation of biodiesel from waste cooking oil.
Similar content being viewed by others
Introduction
One important outcome of global social and economic growth is the availability of energy. Activity and productivity may be reduced by manufacturers worldwide who are dealing with a sharp rise in the price of oil, which is required to generate electricity1. Furthermore, there is an immediate need to address climate change and global warming, which are brought on by the unchecked emissions of greenhouse gases from burning fossil fuels. One promising way to lessen the aforementioned issues is to use renewable energy2. Biodiesel is one of several alternative energy sources that is known for being an energy-efficient fuel that can be used in internal combustion engines with little to no modification. It is also non-toxic, biodegradable, and produces less hazardous gasses when it burns3,4. Within this framework, biodiesel, as a biofuel, represents a renewable energy source with the potential to displace fossil fuels. However, one major challenge that still has to be addressed is the whole cost of producing biodiesel, which includes the cost of feedstocks, catalysts, and the production process5.
Usually, transesterification which involves interactions between triglycerides and methanol or ethanol in the presence of homogeneous basic catalysts like KOH and NaOH is used to synthesize biodiesel6. The process of transesterification produces long-chain fatty acid methyl esters (FAMEs), which are the main ingredients of biodiesel. Various feedstocks, including vegetable oils, animal fats, leftover cooking oils, and microalgae, can produce FAMEs. This process accelerates the reaction between the feedstock and alcohol (often methanol or ethanol) by using a catalyst. Because the resulting biodiesel esters don’t include sulfur, nitrogen, or aromatic compounds, they are perfect for low-emission engines. The properties of biodiesel can vary greatly depending on the feedstock. Compared to diesel fuel, it typically has a greater cetane number, flash point, and viscosity7,8. High catalytic activity, brief reaction times, and gentle reaction conditions are only a few advantages of homogeneous catalysts9. However, issues including inadequate recyclability and expensive separation have been linked to their use. These issues might be resolved by using less expensive recoverable heterogeneous catalysts, less corrosive, and less susceptible to free fatty acids and moistness10,11,12,13.
Alkaline-earth metal oxides, alkali-loaded calcium and magnesium, basic zeolites, and clays are only a few of the many catalyst supports that have been documented to date. However, few were utilized extensively due to the high expense of their synthesis. However, less corrosive, less expensive alternatives to ty acids and moisture may be able to solve these issues14,15,16. In order to address the issue, highly efficient and reasonably priced heterogeneous catalysts made of waste materials were used, including dolomites, leftover egg, oyster, and scallop shells as well as bivalve clam shells and bones17,18,19,20,21,22.
200 million tons of marble sludge (MS) are generated worldwide from the marble industry23. Egypt produces 6.4 million tons of MS annually as a result of processing marble products24. There are several problems with waste management, ecology, and landfill scarcity when such large volumes of MS wastes are dumped in landfills25,26,27. The growing hotel and restaurant industries, as well as the packaged food business, are major sources of waste cooking oil (WCO). It is not appropriate to dispose of these oils inappropriately28. In addition to offering a less expensive substitute for the increasing need for fossil fuels, WCO as a feedstock has also added benefits of severing the food supply chain, which is the cause of many deadly diseases. Moreover, the price of biodiesel is lowered by using WCO as a feedstock. Saravanan et al. examined the potential of waste bull bone-based heterogeneous catalyst for biodegradation and reusability in the generation of alternative fuel from WCO, as well as its potential for use as a fuel substitute29. Cement kiln dust (CKD) was proposed by Eslam et al. as a catalyst to produce biodiesel from WCO30. Ahmed et al. also utilized ostrich bone wastes as a reusable catalyst31. ZnO/BiFeO3 was described by Salimi and Hosseini as a unique magnetic catalyst in the transesterification of canola oil to yield biodiesel32. Joorasty et al. studied the production of biodiesel by transesterifying amygdalus scoparia oil with a magnetic catalyst consisting of Fe3O4 and NaOH/Clinoptilolite33. Mohebolkhames et al. examined the viability of using leftover calcined salmon fish bones as low-cost, reusable catalyst support for sunflower oil transesterification to produce biodiesel34. Shobana et al. used calcium oxide, a heterogeneous catalyst generated from oyster shells, to generate biodiesel from Capparis spinosa L seed oil35. Chinedu et al. used heterogeneous catalysts from catfish born to produce biodiesel from waste catfish oil36. Maafa37 developed a heterogeneous carbon-based sulfonated tire polymer char (TPC-SO3H) solid acid catalyst from scrap tires via two-step process. They first pyrolyzed the scrap tire at 500 °C, then performed a thermal acid treatment with concentrated H2SO4 at 180 °C. This catalyst was then successfully employed to produce biodiesel from waste chicken fat. More recently, Alhomaidi et al. produced biodiesel from plant seed oil employing eco-friendly iron-modified clay nano-catalyst38. They also conducted a detailed chromatographic analysis of the produced biodiesel and concluded that this method could be scaled up for large-scale biodiesel production. Additionally, Maafa et al. utilized low-cost soybean deodorized distillate oil (SDDO) to economically produce biodiesel employing a solid-base heterogeneous catalyst known as CPW-CaO39. They found that this catalyst, derived from chitosan production wastes (CPW), is highly efficient and environmentally friendly.
The usefulness and viability of using MS for the synthesis of biodiesel are the subject of a literature gap that needs to be filled. Exploring a locally accessible trash resource for environmentally friendly catalyst assistance is made possible by it. Examining the catalytic characteristics of MS and refining its application in the production of biodiesel could lead to new opportunities for the Middle East’s sustainable energy generation. Furthermore, by comprehending the distinct features and capabilities of MS catalysts, one can expand the array of reasonably priced materials available for biodiesel production and diversify catalyst possibilities.
Response surface methodology (RSM) is a statistical and mathematical method used to design a set of experiments. It is used to optimize the parameters that affecting on the response. RSM is used as a linear or second-order polynomial to decrease the cost and the number of experiments performed. The value of lack of fit is determined to check whether the model is adequate or not. It is used to identify the divergence between the experimental data and the fitted model. When the pure error is less than the residual error, the lack of fit is significant indicating the model has several issues. RSM is classified into full factorial design (FFD), Box-Behnken design (BBD), and central composite design (CCD). CCD is a widely used response surface design. It is used to build factorial experiments in the presence of axial and center points. It can be used to determine first and second-order terms and also to evaluate the response of variables using curvature in the presence of axial and center points to a factorial design. CCD is preferred over BBD due to its insensitivity to missing data, its use of five levels for each factor (compared to BBD’s three levels), and its excellent prediction capability near the center of the design space. In BBD, any missed runs can significantly affect the accuracy of the remaining runs, making the model less dependable40.
In this sense, the present research utilized MS as a catalyst to produce biodiesel from WCO. Although numerous investigations on producing biodiesel from spent cooking oil have been recognized, none of the studies systematically optimize the factor influencing the reaction of transesterification for the produced biodiesel using MS as a catalyst. The research’s proposed biodiesel meets the requirements for commercial use. Also, the physiochemical properties of the raw MS and WCO were carried out to investigate the possible impacts of transesterification on the oil properties. The goal of this study was to reduce fuel costs by employing waste materials such as WCO and MS-based heterogeneous catalysts, which could be reused again. The sources that have been found are internationally accessible and sustainable. The study also aims to optimize the variables influencing the transesterification process to produce biodiesel which is the merit and the novelty of this study, namely the reaction temperature, catalyst concentration, reaction time, and methanol-to-oil molar ratio, to produce a good yield of biodiesel using the suggested waste MS catalyst. It also studies the reusability of the MS catalyst that helps in saving production costs.
Materials and methodology
Raw materials
The sunflower waste cooking oil (WCO) was supplied from the Sweet Food factory, in Al Asher Men Ramadan, Egypt. The properties of the waste cooking oil are listed in Table 1. Marble sludge (MS) was supplied from Shaq Al-Thu’ban Cluster, Egypt. Methanol (99.7%) was purchased from Alsharq company for the chemical industry, in Egypt.
Assessment of marble sludge
MS elemental analysis uses X-ray fluorescence (XRF) to measure the concentration of various elements. The phases that are present in the material are displayed using X-ray diffraction (XRD). To determine the particle size distribution of calcinated marble sludge, a conventional sieving process is employed as described by ASTM D42241. The sieve used complies with ASTM E11 standards42. SEM analysis is performed on the calcinated marble sludge to discover the active sites present in the catalyst with the aid of the JEOL5410 apparatus. The pore size distribution and surface area abundance in the calcined MS have been identified by Brunauer-Emmett-Teller (BET) analysis43. BET was carried out using a 3 Flex 3500 microscopic model analyzer at 77 K in the presence of nitrogen adsorption-desorption isotherm analysis.
Catalyst preparation from marble sludge
The biodiesel was produced using processed marble sludge. Process variables in the biodiesel synthesis, such as reaction temperature, catalyst concentration, reaction time, and the molar ratio of alcohol to oil, were optimized in light of the marble sludge catalyst’s performance based on the biodiesel yield. In an oven, the marble sludge was dried at 105℃ and further calcined at 900℃ for 2 h subsequently, to disassociate into their oxide forms such as CaO and MgO44.
Biodiesel synthesis
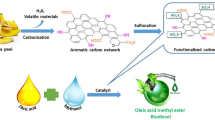
Using a reflux condenser and a circular glass batch reactor, the trans-esterification reaction was conducted. MS was blended with sunflower waste cooking oil and methanol and heated with a magnetic heat stirrer until the target temperature was reached. To condense the evaporated methanol during the reaction, a reflux condenser was placed at one of the reactor necks. A temperature probe was used to measure the temperature at the other reactor neck. The glycerol was extracted from the biodiesel using a separating funnel after two hours of settling to ensure a better separation. To remove the catalyst from the final product, the mixture was further washed with deionized water. The final product was heated for two hours at 105℃ to remove any remaining moisture and unreacted methanol45. The synthesis of biodiesel is illustrated in Fig. 1. The biodiesel yield was calculated using Eq. (2).
Experimental design of biodiesel synthesis
The experimental design and response surface methodology (RSM) were used to examine how the process variables namely; A: reaction time (h), B: reaction temperature (℃), C: methanol-to-oil molar ratio (M/O), and D: catalyst concentration (wt%) affect the biodiesel yield (%). Table 2 illustrates these parameters with their limits.
At the maximum limit, Central Composite Design (CCD) experiments yielded a biodiesel conversion that was equal to 99.7%. The experimental study conducted at the range’s low end revealed that the biodiesel conversion was equal to 70.2%. An undesirable conversion of biodiesel will come from lowering the values below them46.
The review articles done by El-Sheltawy and Al-Sakkari47, Sulaiman48, and Ling et al.49 were used as a basis for selecting the reaction parameters and ranges. In all experiments, the rate of stirring was 750 rpm. Using the central composite design technique (CCD), 30 trials were conducted as a means of reducing the number of experiments, as indicated in Table 3. The identical conditions, known as the center point of the design, are present in runs 25 through 30 of the experiment. Economic and environmental aspects have been taken into consideration when selecting the suggested optimization targets. These include minimizing reaction conditions, especially reaction time and temperature, to maximize biodiesel production while lowering energy costs. Design-Expert version 13 (https://www.statease.com/software/design-expert/) software was used to examine the trial runs, their order, regression analysis, graphical analysis, and numerical optimization. In order to produce eco-friendly biodiesel, solid waste was used as a heterogeneous catalyst. B100 biodiesel was tested for total, free, and triglyceride content using the technique specified in EN 14,105 (2011).
Characteristics of the optimum biodiesel synthesis
The physicochemical characteristics of the biodiesel produced at the optimum conditions were analyzed and compared with ASTM D675150 of the International Standard of Biodiesel and EN 14,21451 of Biodiesel specifications. The total fatty acids methyl esters (FAMEs) were determined with the aid of EN 14,103 procedure52. Mono, di, triglycerides, total glycerol, and free glycerides were also determined using the EN 14,105 53 procedure in biodiesel. Fourier Transform Infrared Spectroscopy (FTIR) was performed to determine the functional group of the produced biodiesel (FAME) using IEC/EN 60825-1/A2:2001 class 1, Avatar Series placed in Egyptian Academy for Engineering and Advanced Technology.
Reusability of calcined marble sludge
After obtaining the optimal biodiesel sample, the biodiesel was filtrated to remove solid particles; following a methanol wash to eliminate glycerol, the solid particles were lastly dried to eliminate any remaining methanol corpuscle. Without the need for a fresh solid catalyst and under the same ideal reaction conditions, this MS was reused to catalyze the cross-esterification reaction of sunflower oil. After performing the cross-esterification process reactions and conversions were calculated for each experiment.
Results and discussion
Characterization of marble sludge waste
Chemical analysis of marble sludge waste
The chemical analysis of the marble sludge waste used in the current study and compared with previous studies are illustrated in Table 4. The results indicated that the waste of marble sludge has about 30% loss on ignition (LOI) and about 53% calcium oxide. The total percentage of sodium, aluminum, and potassium oxides is 0.86%. Marble sludge waste has a low iron oxide percentage compared with Prošek et al.24.
Mineralogical analysis of marble sludge waste
Marble sludge was calcined at a temperature of 900℃ for 2 h and is used for oxidation of calcium carbonate to produce calcium oxide after the elimination of carbon dioxide in gas form. The characterization of marble sludge waste and its calcination was analyzed using XRD analysis. The results of the analysis of the XRD of both samples are illustrated in Fig. 2. It can be observed that the calcinated MS has a sharper peak than the MS due to the presence of calcium oxide which has a crystalline nature35.
Sieve analysis of calcium oxide catalyst
The cumulative sieve analysis of the calcinated marble sludge waste (CaO catalyst) is shown in Fig. 3, it is found that the mean particle size of the catalyst is 0.09 mm which indicates that the catalyst is very fine.
SEM analysis of calcium oxide catalyst
Figure 4 shows the SEM analysis of the calcinated marble sludge catalyst (CaO). The catalyst has an irregular shape and also a porous structure with different shapes and sizes of particles. It is indicated that the catalyst consists of a large surface area suitable for the biodiesel reaction.
Analysis of specific surface area (BET) of calcined marble sludge
BET was used to examine the specific surface area of MS calcined at 900℃; the results are shown in Table 5. It was observed that the MS has a 16.16 m2/g surface area, pore diameter of 3.34 nm, pore volume of 0.0287 cm3/g, and 0.0327 cm3/g total pore volume. The calcined MS has a large specific surface area, which makes it a viable catalyst option. The BET analysis of the calcined MS and several catalysts from previous studies is displayed in Table 5. According to IUPAC regulations55, the pores of the catalyst are generally categorized as micropores, mesopores, and macropores with diameters less than 2 nm, ranging from 2 to 50 nm and more than 50 nm, respectively. The results obtained indicate that the mesopore type of adsorbent catalyst generated is the most accessible form of pores for usage as a catalyst.
Figure 5 shows the nitrogen adsorption-desorption isotherms of the MS catalyst calcined at 900℃. The isotherm displayed the characteristic curve of type IV, which is typical of mesoporous materials. Most typical pore diameters are in the range of 3.34 nm, which allows triglyceride molecules to easily enter the catalyst and for the transesterification reaction to occur when most of the catalyst particles’ active sites come into touch with one another57.
ANOVA of biodiesel synthesis
Table 6 shows the actual and predicted biodiesel yield for each experiment. Design Expert program version 13 was used to generate the regression model Eq. 3 which relates the reaction parameters with the reaction response (biodiesel yield). When the P-value is less than 0.1, the model is considered as significant. The ANOVA model was used to determine the confidence level of the model at 95%. The program was used to determine the critical F-value of each model according to the number of parameters, significant level, and number of samples. To determine the the model significance and also the critical values of F which are compared with the value of F for each model. The F-test and P-values were found to be 3.3 and less than 0.0001 respectively as shown in Table 7. The ANOVA analysis shows that the model was quadratic which can be simplified after neglecting the terms that have a P-value more than 0.1.
Where A: reaction time (h), B: reaction temperature (℃), C: Methanol-to-oil molar ratio (M/O), and D: catalyst concentration (wt%). It can be noted that all the variables have a positive impact on the yield of biodiesel.
The coefficient of determination (R2) serves as an indicator of a model’s predictive efficiency. It quantifies the proportion of the total variation in the predicted or model values that can be attributed to the mean. For a model to exhibit high predictive efficiency, the R2 value should be close to 1.0. As shown in Table 8, the proposed model demonstrates a relatively high R2 value of 0.9728, suggesting that the regression model accounts for most of the data variation. The model leaves only 2.72% of the variations unexplained. Additionally, the small difference between the high adjusted R2 value (0.9474) and the predicted R2 value (0.8588) further confirms the model’s reliability and underscores its high significance. The significance of all the linear factors (A, B, C, and D) and their interactions is determined by the p-values, with p-values below 0.1 indicating significance at a 95% confidence interval. According to the results in Table 8, the p-values for the linear factors B, C, and D, as well as their interactions with other factors, are below 0.1, signifying their significance in the biodiesel yield. The result of the p-value assures that the interaction between time and catalyst and that between temperature and catalyst are significant. While the interaction between the molar ratio and the catalyst is not significant.
Figure 6 shows the plot of the predicted values according to the regression model Eq. 3 versus the actual experimental biodiesel yield which shows an excellent agreement between the actual data obtained from the experiments and the output from the model.
Effect of reaction conditions on the biodiesel yield
The effect of the reaction conditions on the yield of biodiesel is illustrated in Fig. 7. Increasing time causes an increase in yield till it reaches 2 h, then the yield almost becomes constant due to the reversible behavior of the transesterification reaction as illustrated in Fig. 7a60,61. Increasing the temperature causes an increase in the biodiesel. It can be observed that the yield of biodiesel at 50℃ and 70℃ increased from 85 to 95% as shown in Fig. 7b. Increasing the reaction temperature causes increasing the rate of the reaction that enhances the yield of biodiesel. Increasing the reaction temperature above the boiling point of methanol causes the appearance of bubbles of the methanol at the surface of the catalyst due to the evaporation of methanol and hinders the direct contact between the oil and the catalyst which lowers the rate of the reaction and decrease the yield of the biodiesel.
On the other hand, increasing the methanol-to-oil molar ratio causes increasing in the yield of the biodiesel as a result of shifting the equilibrium to produce the fatty acid methyl ester till reaches a certain value causes decreasing in the yield of biodiesel due to the dilution of the waste cooking oil and leads to decreasing the rate of the reaction as shown in Fig. 7c62.
Figure 7d shows the effect of the catalyst on the yield of the biodiesel, as the catalyst concentration increases from 1 wt% to 5 wt%, the yield of biodiesel increases from 91.6–93.12% at a temperature of 60℃, the reaction time of 2.5 h (center point conditions) and the methanol-to-oil molar ratio 12.5. Increasing the addition of catalysts causes the formation of soap and makes the separation between the glycerol and the biodiesel more difficult63.
Figure 8 illustrates the percentage contributions of individual factors to biodiesel yield. The percentage of contribution was determined by dividing the sum of squares for each factor by the total sum of squares multiplied by 100. The factors’ influence follows the order: B > C > A > D. This indicates that reaction temperature (B), methanol-to-oil molar ratio (C), and reaction time (A) are the primary factors affecting biodiesel yield. In contrast, catalyst concentration (D) has the least impact, contributing almost 1%.
Effect of the interaction parameters on the biodiesel yield
Figure 9 displays the response surface graphs based on the quadratic regression Eq. 3. These graphs, shown in Fig. 9a–r, illustrate the interactions between different variables. Figure 9a–c represent the interaction of reaction time and reaction temperature at minimum, center point, and maximum values of catalyst concentration and methanol-to-oil molar ratio (M/O). It demonstrates that increasing the (M/O) and catalyst concentration from 5:1 and 1% to 20:1 and 5% significantly increases the biodiesel yield from 80% to about 100% at a temperature of 70℃ for 1 h.
Figure 9d–f illustrate the interaction of reaction time and (M/O) molar ratio at minimum, center point, and maximum values of catalyst concentration and reaction temperature. It demonstrates that increasing temperature causes a sharp increase in the biodiesel yield.
Figure 9g–i illustrate the interaction of reaction time and catalyst concentration at minimum, center point, and maximum values of (M/O) molar ratio and reaction temperature. It shows that increasing both the (M/O) molar ratio and temperature causes a significant increase in biodiesel yield. In contrast, the combination of temperature and reaction time also increases biodiesel yield, but the effect of the reaction temperature is more dominant than the effect of reaction time and (M/O) molar ratio, as shown in Fig. 9c and f.
Figure 9j–l illustrate the interaction of reaction temperature and (M/O) molar ratio at minimum, center point, and maximum values of catalyst concentration and reaction time. It can be observed that there is no change in the yield when increasing catalyst concentration and reaction time from 3 to 5% and 2.5 h to 4 h respectively as illustrated in Fig. 9k, l.
A similar trend is observed on changing (M/O) molar ratio and reaction time, where biodiesel yield is more influenced by the (M/O) molar ratio than by reaction time, as depicted in Fig. 9m and n, and 9o. Notably, the increasing reaction time and reaction temperature as illustrated in Fig. 9p and q, and r cause a little improvement in the yield of biodiesel compared to the effect of reaction temperature and (M/O) molar ratio as shown in Fig. 9i.
Response surface plots of the interactive parameters: a–c effect of reaction time and temperature: d–f effect of reaction time and (M/O) molar ratio: g–i effect of reaction time and catalyst concentration: j–l effect of reaction temperature and (M/O) molar ratio: m–o effect of catalyst concentration and reaction temperature: p–r effect of catalyst concentration and (M/O) molar ratio.
The percentage contributions of these interaction factors are presented in Fig. 10. Among these, the interactions between AD and AC are the most significant, contributing 35.53% and 25.47% respectively. In contrast, the interaction between CD has a negligible effect on biodiesel yield, as indicated by its p value of 0.7614.
Contributions of the interaction of reaction time (A)-reaction temperature (B), reaction time (A)-methanol to oil molar ratio (C), reaction time (A)-catalyst concentration (D), reaction temperature (B)-methanol to oil molar ratio (C), reaction temperature (B)-catalyst concentration (D) and methanol to oil molar ratio (C)-catalyst concentration (D).
Optimization of biodiesel synthesis
Table 9 presents the optimization constraints for biodiesel synthesis, while Table 10 illustrates the first 12 solutions from the 100 solutions generated by the program, each with varying levels of desirability based on the optimization constraints. The optimal sample was selected according to the highest desirability score. The optimal conditions were determined to be a temperature of 57℃, a methanol-to-oil molar ratio of 20:1, a reaction time of 1 h, and a catalyst concentration of 5 wt%. Under these conditions, a biodiesel yield of approximately 93.5% was achieved. Five replicant experiments were performed at the optimum conditions to determine the standard deviation and error, and it was found to be 1.43% and 1.176%, respectively.
FTIR of biodiesel
FTIR spectroscopy was performed on the produced biodiesel to determine the functional groups related to various stretching and bending vibrations. FTIR was conducted in the range of 4000 to 500 cm-1, as shown in Fig. 11. Several studies have confirmed the presence of C = O in the ester group ranging from 1700 to 1800 cm-1 64,65. This existed in the absorption peak of 1741 cm-1. It is also present in the absorption peak of 1167 cm-1. The absorption peaks of 2926 cm-1 and 2856 cm-1 denote the presence of the aliphatic group -CH2 while the peak of 1452 cm-1 is related to the -CH3 alkane group, which is similar to the results obtained by Mohebolkhames et al.34 and Abati et al.66. Finally, the absorption band at 3437 cm-1 is attributed to the presence of the -OH group in the sample due to the air contact67.
Characteristics of biodiesel at optimum conditions
The optimum sample of the produced biodiesel from waste cooking oil using the waste of marble sludge as a heterogenous catalyst was characterized to obtain the physiochemical properties according to the standard limits. Table 11 shows that these properties are in agreement with EN 14,21451 and ASTM D6751 50. Table 12 illustrates the gas chromatography of the produced biodiesel at the optimum conditions according to Standard European limits EN 14,10352 and EN 14,10553.
Table 13 illustrates a comparison between the current study with other previous work. It can be indicated that the current study has several advantages such as:
-
1.
The marble sludge waste needs a simple preparation before usage in biodiesel synthesis.
-
2.
The utilization of waste cooking oil in the synthesis of biodiesel.
-
3.
The highest yield of biodiesel can be obtained at minimum reaction time and temperature saving operating cost and energy compared with the previous studies.
-
4.
The prepared catalyst can be utilized several times before disposal.
-
5.
Marble sludge waste and waste cooking oil were used in the synthesis of a valuable product with minimum energy consumption instead of disposal.
Catalyst reusability
Figure 12 shows the reusability of calcined marble sludge catalyst in the synthesis of biodiesel. It can be observed that the biodiesel synthesis decreases from 93% in the first use to 87% after the second usage followed by 80% in the third usage. The decreased yield of the biodiesel synthesis is due to glycerol contamination at the active sites causing loss of active positions. It is also due to the leaching of the catalyst by converting from the solid state to the liquid phase. The results indicated that the catalyst can be replaced after two times usage.
Conclusion
The cost of producing biodiesel is significantly influenced by the choice of feedstock, catalyst, and reaction conditions. In this study, waste cooking oil was selected as the feedstock due to its availability and cost-effectiveness, addressing both the environmental concerns associated with its disposal and the overall cost of biodiesel production. This choice not only helps in managing waste but also provides an economical raw material for biodiesel synthesis.
The catalyst used demonstrated excellent catalytic activity, proving to be a crucial component in the efficiency of the process. Waste marble sludge was simply prepared for use in biodiesel synthesis, offering a sustainable and low-cost alternative to conventional catalysts. This innovative use of waste materials aligns with the principles of green chemistry and circular economy, further enhancing the environmental benefits of the biodiesel production process.
The highest yield of biodiesel achieved was 93.5%, obtained under optimal conditions: a reaction time of 1 h, a temperature of 57 °C, a molar ratio of methanol to oil (M/O) of 20:1, and a catalyst loading of 5%. These conditions not only maximize the biodiesel yield but also reduce operational costs and energy consumption compared to previous studies, making the process more economically viable. The ability to reuse the catalyst twice before disposal further improves its cost-effectiveness and reduces waste, contributing to a more sustainable production cycle.
This study aims to promote the sustainable production of fine materials by evaluating the catalyst’s feasibility and practical application through rational engineering approaches. This includes assessing the efficiency, reusability, and overall impact of the catalyst on the biodiesel production process. Additionally, this research presents a promising method to expedite the adoption of a new catalyst for biodiesel production via catalytic transesterification. The findings suggest that this approach not only supports environmental sustainability but also offers a cost-effective and efficient pathway for large-scale biodiesel manufacturing.
By examining the practical applications and long-term viability of the catalyst, this study contributes valuable insights into the development of more sustainable industrial processes. The results highlight the potential for significant advancements in biodiesel production technology, paving the way for broader implementation and commercialization. This approach underscores the importance of integrating innovative materials and methods to achieve more sustainable and efficient production systems, ultimately contributing to a greener and more sustainable future.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abd El-Malek, F. et al. Microorganism-mediated algal biomass processing for clean products manufacturing: current status, challenges and future outlook. Fuel. 311, 122612 (2022).
Basumatary, S., Nath, B., Das, B., Kalita, P. & Basumatary, B. Utilization of renewable and sustainable basic heterogeneous catalyst from Heteropanax fragrans (Kesseru) for effective synthesis of biodiesel from Jatropha curcas oil. Fuel. 286, 119357 (2021).
Osagiede, C. A. & Aisien, F. A. Biochar-based bi-functional catalyst derived from rubber seed shell and eggshell for biodiesel production from waste cooking oil. Fuel. 358, 130076 (2024).
Amenaghawon, A. N., Obahiagbon, K., Isesele, V. & Usman, F. Optimized biodiesel production from waste cooking oil using a functionalized bio-based heterogeneous catalyst. Clean. Eng. Technol. 8, 100501 (2022).
Mansoorsamaei, Z., Mowla, D., Esmaeilzadeh, F. & Dashtian, K. Sustainable biodiesel production from waste cooking oil using banana peel biochar-Fe2O3/Fe2K6O5 magnetic catalyst. Fuel 357, 129821 (2024).
Al-Sakkari, E. G., Elozeiri, A. A., Abdeldayem, O. M., Likozar, B. & Boffito, D. C. Fish and animal waste as catalysts for biodiesel synthesis. in Waste and Biodiesel: Feedstocks and Precursors for Catalysts (2022). https://doi.org/10.1016/B978-0-12-823958-2.00003-3.
Bousba, D. et al. Efficient biodiesel production from recycled cooking oil using a NaOH/CoFe2O4 magnetic nano-catalyst: synthesis, characterization, and process enhancement for sustainability. Energy Convers. Manag. 300, 118021 (2024).
Yaşar, F. Comparision of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel. 264, 116817 (2020).
Abdul, H. et al. A review on non-edible oil as a potential feedstock for biodiesel: physicochemical properties and production technologies. RSC Advances vol. 11 at (2021). https://doi.org/10.1039/d1ra04311k.
Khan, I. W. et al. Biodiesel production by valorizing waste non-edible wild olive oil using heterogeneous base catalyst: Process optimization and cost estimation. Fuel 320, 123828 (2022).
Esmaeilnejad Ahranjani, P., Kazemeini, M. & Arpanaei, A. Green biodiesel production from various plant oils using nanobiocatalysts under different conditions. Bioenergy Res. 13, 552–562 (2020).
Zahid, A. et al. Efficient production of biodiesel by the transesterification of neem oil using Ni-doped ZnO nanoparticles as heterogeneous catalysts. Arab. J. Sci. Eng. 49, 1–10 (2024).
Jumah, M. N. B. et al. Enhancing the catalytic performance of NiO during the transesterification of waste cooking oil using a diatomite carrier and an integrated Ni0Metal: Response surface studies. ACS Omega 6, 12318–12330 (2021).
Mahloujifar, M. & Mansournia, M. A comparative study on the catalytic performances of alkali metals-loaded KAlSiO4 for biodiesel production from sesame oil. Fuel 291, 120145 (2021).
Attari, A., Abbaszadeh-Mayvan, A. & Taghizadeh-Alisaraie, A. Process optimization of ultrasonic-assisted biodiesel production from waste cooking oil using waste chicken eggshell-derived CaO as a green heterogeneous catalyst. Biomass Bioenergy 158, 106357 (2022).
Farooq, M. & Ramli, A. Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renew. Energy 76, 362–368 (2015).
Parida, S., Singh, M. & Pradhan, S. Biomass wastes: A potential catalyst source for biodiesel production. Bioresour. Technol. Rep. 18, 101081 (2022). https://doi.org/10.1016/j.biteb.2022.101081.
Chen, G., Shan, R., Shi, J., Liu, C. & Yan, B. Biodiesel production from palm oil using active and stable K doped hydroxyapatite catalysts. Energy Convers. Manag. 98, 463–469 (2015).
Obadiah, A., Swaroopa, G. A., Kumar, S. V., Jeganathan, K. R. & Ramasubbu, A. Biodiesel production from Palm oil using calcined waste animal bone as catalyst. Bioresour. Technol. 116, 512–516 (2012).
Aghel, B., Mohadesi, M., Razmehgir, M. H. & Gouran, A. Biodiesel production from waste cooking oil in a micro-sized reactor in the presence of cow bone-based KOH catalyst. Biomass Convers. Biorefin. 13, 13921–13935 (2023).
Rahman, M. A. Valorization of harmful algae E. compressa for biodiesel production in presence of chicken waste derived catalyst. Renew. Energy 129, 132–140 (2018).
Tan, Y. H. et al. Biodiesel production from used cooking oil using green solid catalyst derived from calcined fusion waste chicken and fish bones. Renew. Energy 139, 696–706 (2019).
Munir, M. J., Kazmi, S. M. S., Gencel, O., Ahmad, M. R. & Chen, B. Synergistic effect of rice husk, glass and marble sludges on the engineering characteristics of eco-friendly bricks. J. Build. Eng. 42, 102484 (2021).
Prošek, Z., Nežerka, V. & Tesárek, P. Enhancing cementitious pastes with waste marble sludge. Constr. Build. Mater. 255, 119372 (2020).
Munir, M. J., Abbas, S., Nehdi, M. L., Kazmi, S. M. S. & Khitab, A. Development of eco-friendly fired clay bricks incorporating recycled marble powder. J. Mater. Civ. Eng. 30, 04018069 (2018).
Munir, M. J., Kazmi, S. M. S., Wu, Y. F., Hanif, A. & Khan, M. U. A. Thermally efficient fired clay bricks incorporating waste marble sludge: An industrial-scale study. J. Clean. Prod. 174, 1122–1135 (2018).
Kazmi, S. M. S., Munir, M. J., Wu, Y. F., Hanif, A. & Patnaikuni, I. Thermal performance evaluation of eco-friendly bricks incorporating waste glass sludge. J. Clean. Prod. 172, 1867–1880 (2018).
Khodadadi, M. R., Malpartida, I., Tsang, C. W., Lin, C. S. K. & Len, C. Recent advances on the catalytic conversion of waste cooking oil. Mol. Catal. 494, 111128 (2020).
Saravanan, R. et al. Waste bull bone based reusable and biodegradable heterogeneous catalyst for alternate fuel production from WCO, and investigation of its usability as fuel substitute. Fuel. 355, 129436 (2024).
Al-Sakkari, E. G. et al. Esterification of high FFA content waste cooking oil through different techniques including the utilization of cement kiln dust as a heterogeneous catalyst: A comparative study. Fuel. 279, 118519 (2020).
Ahmed, Y. M. Z., El-Sheikh, S. M. & Zaki, Z. I. Changes in hydroxyapatite powder properties via heat treatment. Bull. Mater. Sci. 38, 1807–1819 (2015).
Salimi, Z. & Hosseini, S. A. Study and optimization of conditions of biodiesel production from edible oils using ZnO/BiFeO3 nano magnetic catalyst. Fuel 239, 1204–1212 (2019).
Joorasty, M., Hemmati, A. & Rahbar-Kelishami, A. NaOH/clinoptilolite-Fe3O4 as a novel magnetic catalyst for producing biodiesel from Amygdalus scoparia oil: Optimization and kinetic study. Fuel 303, 121305 (2021).
Mohebolkhames, E., Kazemeini, M. & Sadjadi, S. Utilization of Salmon fish bone wastes as a novel bio-based heterogeneous catalyst-support toward the production of biodiesel: Process optimizations and kinetics studies. Mater. Chem. Phys. 311, 128522 (2024).
Shobana, R., Vijayalakshmi, S., Deepanraj, B. & Ranjitha, J. Biodiesel production from Capparis spinosa L seed oil using calcium oxide as a heterogeneous catalyst derived from oyster shell. Mater. Today Proc. 80, (2023).
Agu, C. M. et al. Biodiesel production from waste cat fish oil using heterogeneous catalyst from cat fish born: A viable waste management approach, and ANN modeling of biodiesel yield. Waste Manag. Bull. 1, 172–181 (2024).
Maafa, I. M. Biodiesel synthesis from high free-fatty-acid chicken fat using a scrap-tire derived solid acid catalyst and KOH. Polymer (Basel) 14, 643 (2022).
Alhomaidi, E., Aljabri, M., Alsharari, S. S. & Alsam, A. Chromatographic assessment of biodiesel production from Peganum harmala seed oil using environmentally benign nano-catalysts. Biomed. Chromatogr. 38, e5794 (2024).
Maafa, I. M., Sayed Alahl, A., Abd El-Magied, A., Cui, M. O., Dhmees, A. S. & X. & Eco-friendly self-terminated process for preparation of CaO catalyst based on chitosan production wastes for biodiesel production. J. Mater. Res. Technol. 30, 1217–1227 (2024).
Veza, I., Spraggon, M., Fattah, I. M. R. & Idris, M. Response surface methodology (RSM) for optimizing engine performance and emissions fueled with biofuel: Review of RSM for sustainability energy transition. Results Eng. vol. 18 at (2023). https://doi.org/10.1016/j.rineng.2023.101213.
ASTM-D422-63-. e2. Standard Test Method for Particle-Size Analysis of Soils (Withdrawn 2016). ASTM Int. (2007). (2007).
ASTM. ASTM E11-17, Standard Specification for Woven Wire Test Sieve Cloth and Test Sieves. ASTM Stand. (2017).
Arokiasamy, P. et al. Metakaolin/sludge based geopolymer adsorbent on high removal efficiency of Cu2+. Case Stud. Constr. Mater. 17, e01428 (2022).
Khosa, A. A., Xu, T., Xia, B. Q., Yan, J. & Zhao, C. Y. Technological challenges and industrial applications of CaCO3/CaO based thermal energy storage system: A review. Solar Energy 193 (2019). https://doi.org/10.1016/j.solener.2019.10.003.
Kumar, R., Ghosh, A. K. & Pal, P. Sustainable Production of Biofuels through Membrane-Integrated Systems. Sep. Purif. Rev. 49 (2020). https://doi.org/10.1080/15422119.2018.1562942.
Khodary, K. E., Naeem, M. M. & Roushdy, M. H. Utilization of electric arc furnace dust as a solid catalyst in biodiesel production. Clean. Technol. Environ. Policy 25, 1–11 (2023).
El-Sheltawy, S. T. & Al-Sakkari, E. G. Recent trends in solid waste utilization for biodiesel production. J. Solid Waste Technol. Manage. 42, 1 (2016).
Talha, N. S. & Sulaiman, S. Overview of catalysts in biodiesel production. ARPN J. Eng. Appl. Sci. 11, 439–442 (2016).
Ling, J. S. J. et al. A review of heterogeneous calcium oxide based catalyst from waste for biodiesel synthesis. SN Appli. Sci. 1 (2019). https://doi.org/10.1007/s42452-019-0843-3.
ASTM D6751–20a (2020) Standard specification for biodiesel fuel blend stock (B100) for Middle Distillate Fuels. (ASTM International, West Conshohocken, PA).
BS EN 14214:2019, Fatty acid methyl esters (FAME) for use in diesel engines and heating applications. (Requirements and test methods, the British adoption of European standards).
BS EN 14103:2020 Fat and oil derivatives. Fatty Acid Methyl Esters (FAME). (Determination of ester and linolenic acid methyl ester contents).
CSN EN 14105 - Fat and oil derivatives - Fatty Acid Methyl Esters (FAME). (Determination of free and total glycerol and mono-, di-, triglyceride contents).
El-Sayed, H. A., Farag, A. B., Kandeel, A. M., Younes, A. A. & Yousef, M. M. Characteristics of the marble processing powder waste at Shaq El-Thoaban industrial area, Egypt, and its suitability for cement manufacture. HBRC J. 14, 171–179 (2018).
Abdel Hamid, E. M., Aly, H. M. & El Naggar, K. A. M. Synthesis of nanogeopolymer adsorbent and its application and reusability in the removal of methylene blue from wastewater using response surface methodology (RSM). Sci. Rep. 2024. 141 14, 1–24 (2024).
Zhu, Z. et al. Soybean biodiesel production using synergistic CaO/Ag nano catalyst: Process optimization, kinetic study, and economic evaluation. Ind. Crops Prod. 166, 113479 (2021).
Krishnamurthy, K. N., Sridhara, S. N. & Ananda Kumar, C. S. Optimization and kinetic study of biodiesel production from Hydnocarpus wightiana oil and dairy waste scum using snail shell CaO nano catalyst. Renew. Energy 146, 280–296 (2020).
Ismail, S., Ahmed, A. S., Anr, R. & Hamdan, S. Biodiesel Production from Castor Oil by Using Calcium Oxide Derived from Mud Clam Shell. J. Renew. Energy (2016).
Akens, T. F. A. & Ekeinde, E. B. H. A. Synthesis of biodiesel from blend of seeds oil-animal fat employing agricultural wastes as base catalyst. Case Stud. Chem. Environ. Eng. 5, 100202 (2022).
Ogunkunle, O., Oniya, O. O. & Adebayo, A. O. Yield response of biodiesel production from heterogeneous and homogeneous catalysis of milk bush seed (Thevetia peruviana) oil. Energy Policy Res. 4, (2017).
Abdel Hamid, E. M. et al. Box-Behnken design (BBD) for optimization and simulation of biolubricant production from biomass using aspen plus with techno-economic analysis. Sci. Rep. 141 (14), 1–20 (2024).
Zhang, C. Y. et al. Biodiesel production by esterification reaction on k + modified mgal-hydrotalcites catalysts. Catalysts 9, 742 (2019).
Gupta, V. & Pal Singh, K. The impact of heterogeneous catalyst on biodiesel production; a review. Mater. Today Proc. 78, 364–371 (2023).
Kamaronzaman, M. F. F., Kahar, H., Hassan, N., Hanafi, M. F. & Sapawe, N. Analysis of biodiesel product derived from waste cooking oil using fourier transform infrared spectroscopy. in Mater. Today Proc. 31 (2020).
Chowdhury, S. et al. Fabrication and performance analysis of keratin based-graphene oxide nanocomposite to remove dye from tannery wastewater. Heliyon 10, 1 (2024).
Modupe Abati, S. et al. Biodiesel production from spent vegetable oil with Al2O3 and Fe2O3-biobased heterogenous nanocatalysts: Comparative and optimization studies. Fuel. 364, 130847 (2024).
Mirghani, M. E. S., Kabbashi, N. A., Alam, M. Z., Qudsieh, I. Y. & Alkatib, M. F. R. Rapid method for the determination of moisture content in biodiesel using FTIR spectroscopy. JAOCS J. Am. Oil Chem. Soc. 88, 1897–1904 (2011).
ASTM D5865/D5865M-19 (2019), Standard Test Method for Gross Calorific Value of Coal and Coke. (ASTM International, West Conshohocken, PA).
ASTM D93-20 (2020), Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. (ASTM International, West Conshohocken, PA).
ASTM D97-17b(2022), Standard Test Method for Pour Point of Petroleum Products. (ASTM International, West Conshohocken, PA).
ASTM D445-21e2 (2022), Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). (ASTM International, West Conshohocken, PA).
ASTM D4052. -22 (2022), Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. (ASTM International, West Conshohocken, PA).
Singh, A., Choudhary, A. K., Sinha, S., Panchal, H. & Sadasivuni, K. K. Analysis of vibrations in a diesel engine produced by Jatropha biodiesel using heterogeneous catalyst. Energy Environ. 34, 407–428 (2023).
Al-Hamamre, Z., Sandouqa, A., Al-Saida, B., Shawabkeh, R. A. & Alnaief, M. Biodiesel production from waste cooking oil using heterogeneous KNO3/Oil shale ash catalyst. Renew. Energy. 211, 470–483 (2023).
Saad, M., Siyo, B. & Alrakkad, H. Preparation and characterization of biodiesel from waste cooking oils using heterogeneous Catalyst(Cat.TS-7) based on natural zeolite. Heliyon 9, 6 (2023).
Saetiao, P. et al. Catalytic conversion of palm oil into sustainable biodiesel using rice straw ash supported-calcium oxide as a heterogeneous catalyst: Process simulation and techno-economic analysis. Case Stud. Chem. Environ. Eng. 8, 100432 (2023).
Brahma, S. et al. Biodiesel production from quinary oil mixture using highly efficient Musa chinensis based heterogeneous catalyst. Fuel. 336, 127150 (2023).
Acknowledgements
The authors gratefully acknowledge the funding of the Deanship of Graduate Studies and Scientific Research, Jazan University, Saudi Arabia, through project number: RG24-M037.
Author information
Authors and Affiliations
Contributions
Conceptualization, Kamilia A. El-Naggar, Eman S. Mansor, Ibrahim M. Maafa, Ahmed Abutaleb, Ayman Yousef, Saleh M. Matar, Eman M. Abdel Hamid; Data curation, Ahmed Abutaleb, Ayman Yousef, Eman M. Abdel Hamid; Investigation, Kamilia A. El-Naggar, Eman S. Mansor, Saleh M. Matar, Eman M. Abdel Hamid; Methodology, Kamilia A. El-Naggar, Eman S. Mansor, Eman M. Abdel Hamid; Validation, Kamilia A. El-Naggar, Eman S. Mansor, Ibrahim M. Maafa, Ahmed Abutaleb, Ayman Yousef, Saleh M. Matar, Eman M. Abdel Hamid Hameed; Writing – original draft, Ibrahim M. Maafa, Ahmed Abutaleb, Ayman Yousef, Eman M. Abdel Hamid; Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
El-Naggar, K.A., Mansor, E.S., Maafa, I.M. et al. Valorization of marble sludge waste in biodiesel production using a central composite design. Sci Rep 14, 28136 (2024). https://doi.org/10.1038/s41598-024-77819-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-77819-3