Abstract
The prevalence of adolescent myopia is remarkably increasing. Previous studies have indicated that an unhealthy diet is a risk factor for myopia. However, the link between diet-related inflammation and myopia is unclear. To explore their correlation, we used dietary inflammation index (DII) that is a parameter to quantify the inflammatory potential of diet, to reveal the relationship between DII and myopia in adolescents. We extracted sociodemographic data, information of diets and eye refractive status of adolescents from National Health and Nutrition Examination Survey (NHANES) for period 1999–2008. Dietary intake data was used to calculate DII scores, which were then categorized into quartiles. Multivariable regression models and subgroup analyses were conducted to investigate the association between DII and myopia. Subsequently, smoothed curve analyses were conducted to discern the trend of correlation between DII and myopia across diverse population. A total of 7191 juveniles aged at 12 to 18 years with complete information were included in our study, consisting 3367 participants with diagnosis of myopia. Among these participants, a trend towards an increasing prevalence of myopia was observed with a higher DII. After adjusting for all covariates, stratified logistic regression analyses showed that among the population aged in 16 to 18 years old or with 9-11th grade educational level, the prevalence of myopia was significantly increased with higher DII score (OR = 1.06, 95% CI = 1.01, 1.11, P = 0.006; OR = 1.06, 95% CI = 1.01, 1.11, P = 0.010). In the two subgroups, participants in the highest quartile of DII had a 31.00% higher risk of myopia and a higher 27.00% risk of myopia respectively, compared to those in the lowest quartile of DII. Our results revealed an increasing trend in the prevalence of myopia with increased DII score in adolescents. Particularly, DII was positively associated with the risk of myopia among the population aged in 16 to 18 years old and with 9-11th grade educational level.
Similar content being viewed by others
Introduction
Myopia is a global health problem that bothers hundreds of thousands of people. The onset age of myopia tends to be younger in recent years, which is thought to be caused by some modifiable risk factors such as increased intensive education, increased near-work activities, excessive use of electronic products, and frequent consumption of sweets1. Currently, accumulating evidence supports that systemic inflammation plays an essential role in the pathogenesis and progression of myopia2,3. Elevated circulation levels of inflammatory biomarkers (e.g., interleukin (IL)-1β, tumor necrosis factor (TNF)-α) caused by abnormal immune activation contribute to myopia progression either directly or indirectly by inducing scleral remodeling3. Besides, some medications with anti-inflammatory potential can prevent myopia effectively. In Syrian hamsters with monocular form deprivation (MFD) eyes, myopia progression as well as expression levels of c-Fos, nuclear factor κB (NF-κB), IL-6, and TNF-α were slowed by immunosuppressive agent cyclosporine A (CSA) application4. However, inflammatory stimulators lipopolysaccharide (LPS) or peptidoglycan (PGN) administration led to opposite result4. These findings support that chronic low-grade inflammation is a key feature of myopia.
In addition, considerable attentions have been paid to the player of diet in systemic inflammation, and most non-communicable diseases5,6. Unhealthy food patterns such as a higher intake of high-sugar products can aggravate chronic inflammation7 and contribute to the development of myopia8. Adherence to a healthy diet has been confirmed to be favorable for lowering systemic inflammation9 and offers a valuable intervention to compete against myopia progression10. To quantify the pro-inflammatory or anti-inflammatory capacities of more comprehensive diet accurately rather than a single-nutrient, dietary inflammatory index (DII) was proposed based on the summary of published literatures by Shivappa et al. in 201411. It has been reported to be associated with increasing circulating inflammatory biomarkers, including IL-1β, IL-4, IL-6, IL-10, TNF-α, and C-reactive protein (CRP)12. Many researchers have widely applied DII to evaluate the roles of diet-induced inflammation in the development of various systemic diseases, which contain cardiovascular disease, diabetes, obesity13. Nevertheless, the association between DII and myopia remains unclear.
In this case, we intended to explore whether the inflammatory degree of diet, measured via DII, was linked to the risk of myopia from the National Health and Nutrition Examination Surveys (NHANES), a cross-sectional study launched by National Center for Disease Control and Prevention (CDC) in the United States at two-year intervals14. The present study was performed on participants from 12 to 18 years old, because non-cycloplegic refraction examination data of individuals older than 12 years old was collected in NHANES during 1999 to 2008, and myopia was prevalent during childhood and adolescent. It was hoped to provide evidence for developing appropriate dietary guidance to prevent myopia in adolescents.
Materials and methods
Study population
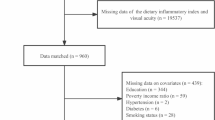
The NHANES is an ongoing cross-population-based survey to collect a plethora of information on the nutrition and health of the US population. Five cycles of data from 1999 to 2008 in the NHANES database were combined in this study, based on the availability of both refraction exanimation and dietary data. Participants who met the following exclusion criteria were included: (1) without complete data on refraction examination; (2) missing or without dietary data; (3) received refractive or cataract survey; (4) hyperopia that was defined as refractive error > 0.5 diopter (D) in any eye; (5) aged < 12 years or > 18 years; (6) missing demographic data, BMI data, education data and PIR data. Finally, the research comprised 7191 participants. The selection process is depicted in Fig. 1.
Assessment of myopia
All subjects aged ≥ 12 years were eligible to undergo objectively refractive measurements using an autorefractor/keratometry (Nidek ARK-760 A, Nidek Co. Ltd., Gamagori, Japan) in the non-cycloplegic conditions15,16. Average of three consecutive measurements were recorded for each eye. The spherical equivalent (SE) was calculated as sum of spherical error and half of cylindrical error. Because the two eyes of individuals were correlated, only the right eye of each participant was used for analyses15,16. Then, myopia was defined as SE value ≤ -0.5 diopters (D)17,18. Emmetropia was defined as the SE value between -0.5 D and 0.5 D, while hyperopia was defined as a refractive error > 0.5 D17,18.
Assessment of dietary inflammation index
Dietary intake data of NHANES participants were captured from the 24-hour dietary recall interview. To assess overall dietary inflammation, the dietary inflammation index (DII) was calculated. A total of 45 components were considered, and each parameter was assigned a specific DII score depending on their effects on six inflammatory biomarkers (IL-1β, IL-4, IL-6, IL-10, TNF-α, CRP). In present study, 26 nutrients out of 45 food parameters were used, including energy, protein, carbohydrate, dietary fiber, total fat, saturated fat, monounsaturated fatty acids, polyunsaturated fatty acids (PUFAs), cholesterol, vitamin A, β-carotene, vitamin B1, vitamin B2, niacin, vitamin B6, total folate, vitamin B12, vitamin C, vitamin D, vitamin E, iron, magnesium, zinc, selenium, caffeine, and alcohol. To evaluate the inflammatory potentials of each participant, firstly, we subtracted global standard mean from estimated intake of the individual, and then divided by the standard deviation. Secondly, Z-score value of each food parameter was measured and converted to centered proportions. Thirdly, each centered percentile was multiplied by the corresponding inflammatory effect index. Finally, the final score was summed for each participant. A positive DII score represents pro-inflammatory potential, while a negative DII score indicates an anti-inflammatory effect.
Assessment of covariates
To assess the influence of potential confounding factors, age, race, gender, education attainment, body mass index (BMI), and poverty income ratio (PIR) were taken into account. Majority of myopia appears during childhood and stabilizes by the age of 18 years19,20. Since visual acuity data of individuals aged 12 years or older were collected during NHANES21, it was not able to determine the relationship between DII and myopia in the children’s group. Therefore, we enrolled people aged 12–18 years, which seemed to be adequate. Furthermore, myopia in adolescents or young adults can be divided into early and late myopia using the age of 15 years as a cut-off point22,23. Then, all subjects were grouped into low age (12 to 15 years) and high age (16 to 18 years) groups. Race were divided into five categories: non-Hispanic white, non-Hispanic black, other Hispanic, Mexican-American and other ethnicities. The levels of education were designated as < 9th grade, 9-11th grade, high school grade, some college or above24. Three categories of PIR were identified: ≤ 1.3, 1.3–3.5, and > 3.5 25. BMI levels were classified as < 25 kg/m2, 25 to 30 kg/m2 or > 30 kg/m2 26.
Statistical analysis
Continuous variables were expressed as means with standard deviations (SDs), and categorical variables as frequencies and percentages based on the presence of myopia. To detect differences in baseline characteristics between myopia and non-myopia participants, variables were compared using Student’s t-test and chi-square test. DII scores were divided into quartiles from lowest (Q1) to highest (Q4), with the first quartile (Q1) being designated as the reference quartile. Multivariable logistic regression analyses were conducted to explore the risk of myopia with DII. The results were presented as odd ratios (OR) and 95% confidence intervals (95% CI). We estimated three models. Model 1 was a crude model adjusted for no covariate. Model 2 was a minimally adjusted model in which age and gender were first introduced (Model 2−1), and then age, gender, PIR, education level (Model 2–2). Model 3 was adjusted for age, PIR, education level, BMI, gender, race. Tests for trend were also performed with logistic regression. Additionally, subgroup analyses in terms of gender, age, race, education level, PIR, BMI were conducted to examine the presence of significant interactions of these covariates with the association between DII and myopia. Meanwhile, a smooth curve analysis was performed to elucidate the trend of the association between DII and myopia, where the DII score was treated as a continuous variable. DecisionLinnc software (https://fast.statsape.com) and GraphPad Prism were used for statistical analysis and figure design. Statistical significance was identified as a two-sided P < 0.05.
Results
Baseline characteristics of the study participants
The characteristics of participants are shown in Table 1. A total of 3624 (50.40%) of the participants were males, and the other 3567 (49.60%) were females. The prevalence of myopia among males and females were 45.08% (1634/3624) and 48.58% (1733/3567), respectively. A total of 2369 out of 7191 (32.94%) were Mexican American, 370 (5.15%) were other Hispanic, and 1951 (27.13%) were non-Hispanic white, 2185 (30.39%) were non-Hispanic black, and 316 (4.39%) were other races. More than half of the participants were under 9th grade, while those who suffered from myopia accounted for 46.15% (1692/3666). People with higher education were more likely to become myopic, and prevalence of myopia in per group were 47.03% (1356/2883), 48.37% (237/490), 53.95% (82/152) for 9-11th grade, high school grade, some college or above, respectively. This trend was consistent with previous study27. In myopia group, the median (standard error) DII values were 2.05 (1.54). Noteworthily, there was statistically disparity in DII between emmetropes and myopia group. Similarly, significant differences were observed between two group regarding gender, PIR, BMI, spherical equivalent of right eye.
Logistic regression analysis of adolescent myopia risk
As shown in Table 2, multivariate logistic regression analysis was performed to illustrate the association between DII and myopia. In the non-adjusted (Model 1) and minimally adjusted model (Model 2–2), a higher DII was correlated with myopia. After adjustment of age, gender, education levels, PIR, the ORs with 95% CIs for myopia in increasing quartiles of DII were 1.09 (0.96, 1.23), 1.12 (0.98, 1.28), and 1.15 (1.01, 1.32), compared to the lowest quartile, respectively. It indicated that participants in quartile 4 had an association with 15.00% increased risk of myopia (P for trend < 0.050). In Model 3, the positive association between DII and myopia became insignificant in the whole population. Subsequently, stratified analyses were further performed.
Subgroup analyses
Univariate subgroup analysis as well as interaction effect analysis were performed to evaluate the potential modification of the association between DII and myopia (Table 3). Only education levels and age might influence the association between DII and myopia (P for interaction < 0.05). Other covariates, such as gender, race and BMI levels did not impact myopia prevalence. In addition, in participants aged at 16 to 18 years old or in 9-11th grade, the risk of myopia increased significantly with increased DII (Table 4), while this association was not observed for those aged at 12 to 15 years or under 9th grade. Further logistic regression analysis in the 16 to 18 years old group showed no variables that might modify the association between DII and myopia (Table 5). Additionally, the smooth curve fitting model confirmed that higher DII scores were associated with higher risk of myopia (Figs. 2 and 3).
Discussion
In this cross-sectional study utilizing data from five consecutive NHANES 2-year cycle (1999–2008), the prevalence of adolescent myopia reached 46.82%. This was similar to other study16. The individuals with myopia had a significantly higher mean DII score than those with emmetropes. Then, our results showed an increasing trend in the adjusted prevalence of myopia with a higher DII level in the population aged 12–18 years old. Importantly, in the 16–18 years old group and 9-11th grade group, each unit increase in DII was associated with an 6.00% increase in the incidence of myopia. When DII was in the fourth quantile, the risk of myopia was 31.00% and 27.00%, respectively. The smooth curve fitting results indicated and further confirmed the positive correlation between DII and myopia in the two group. For these patients, limiting daily pro-inflammatory diet is vital for lowering myopia risk. Overall, this study is the pioneering investigation of the correction between DII and myopia.
Some findings10,28,29 have suggested that dietary shifts in population undergoing rapid economic transitions were partly responsible for the rapid intergenerational rise in myopia prevalence. For example, daily gavage of omega-3 polyunsaturated fatty acids (ω-3 PUFAs), known for their anti-inflammatory effects, could significantly attenuate the development of myopia in animal study10. Likewise, human study suggested that oral administration of ω-3 PUFAs partially alleviated decreases in choroidal blood perfusion (ChBP), a risk factor for myopia10. In addition, the Mediterranean diet is considered one of the healthiest dietary patterns worldwide, thanks to a combination of foods rich mainly in anti-inflammatory nutrients30. It was identified that special dietary patterns, similar to Mediterranean diet31, had a protective effect against the risk of myopia in Chinese children aged 10–11 years32. These observations are consistent with growing evidence that inflammation has a pathological influence on myopia3,4. Our study combined multiple dietary data instead of a single diet or the special dietary pattern, thereby preserved the predictive value for diet-related inflammation of DII. That better represented dietary inflammatory potential, which was crucial for understanding relationship between diet and myopia.
On the other hand, from a systemic perspective, a pro-inflammatory diet contributes to higher values of systemic inflammation21. In fact, the DII is a valuable tool to reflect the level of six inflammatory makers (IL-1β, IL-4, IL-6, IL-10, TNF-α and CRP). These indicators are more or less associated with systemic inflammatory diseases. Other studies showed that a higher incidence of myopia among patients with inflammatory diseases such as type 1 diabetes mellitus, allergic conjunctivitis, uveitis3,4. In this sense, our observation was consistent with prior results. Consequently, this finding supports the importance of using a comprehensive tool as DII in epidemiological and clinical settings to investigate relationship between diet and myopia.
Our findings suggested that DII was positively associated with a high risk of myopia in US adolescents aged in 16 to 18 years or with 9-11th grade educational level, but not in those aged 12 to 15 years or with lower educational level. The reason for this phenomenon could be the following. Primarily, the assessment of dietary data was subjective, which may be more likely to introduce self-report and recall bias into the analysis and omit some dietary risk factors in the younger or lower grade groups. Concurrently, people in lower age group or lower grade lack of decision-making ability to plan and choose foods based on taste preferences under parents’ concern. This makes them more likely to keep away from unhealthy meals. In addition, as genetic factors greatly contribute to the early development of myopia34, the effect of dietary factors may be limited. We suspect a large sample size may lead to a statistically significant result.
The mechanism of myopia is complex. Several reports have proposed a role for inflammation in myopia susceptibility35,36. The weakening of scleral connective tissue as a result of chronic inflammation contribute to the higher incidence of myopia37. A cohort study showed that patients with autoimmune diseases, such type 1 diabetes mellitus, uveitis, and systemic lupus erythematosus have higher risks of myopia compared to those without autoimmune diseases4. The involvement of inflammatory responses in myopia and treatment with anti-inflammatory agents can effectively inhibit myopia progression4. It is commonly agreed that DII scores is related to the pro-inflammatory and anti-inflammatory capacity of the diet. Hence, when the intake of pro-inflammatory diets exceeds the body load, a higher DII score could reflect a negative effect against myopia as described in our results. The potential underlying mechanism could be multifaceted, as shown in Fig. 4. First, immune systemic chronic and sustained activation develop into mild inflammation due to pro-inflammatory diet38. This resulting inflammation may exacerbate myopia, which even lead to a vicious cycle. Second, with activated immune cells, reactive oxygen species (ROS) can be produced excessively. ROS are one of the major contributors of oxidative stress and may alter proteins, deleterious peroxidation of lipids and DNA cleavage39. In status of hypoxia, oxidative stress damage the retina40. This could be one of the key aspects to explain oxidative stress and myopia. Another explanation could be ascribed to the influence of dietary intake on the gut-eye axis. Microecological disturbance induced by diet can activate immune cells or penetrate mucosa41. Then, released endotoxins weaken intestinal barrier and produce pro-inflammatory cytokines that mediate local and systemic inflammation. This gut microbiota disorders, particularly its connection with inflammation may affect myopia42,43.
To the best of our knowledge, this is the first study to investigate the relationship between DII and myopia, and we found that the higher DII score contributes to the higher prevalence of myopia in adolescents, especially in the population aged in 16 to 18 years old and those with 9-11th grade educational level. However, there are several limitations in this study. First, due to the cross-sectional design in the NHANES database, it seems to be difficult to assess the causal relationship between DII and myopia using the logistic regression model. Second, dietary assessments were based on self-reported 24-h dietary records, rather than real-time and time-series surveys, which may introduce dietary recall bias. Third, we used the NHANES database to investigate the positive association between DII and myopia, but myopia can be influenced by many factors, including genetic factors. Therefore, our assessment may not be comprehensive enough. Further large-scale prospective cohort of dietary intervention studies are warranted to examine the effect of DII on myopia.
Conclusions
In summary, this finding showed an increasing trend in the prevalence of myopia with increased DII score in adolescents. Particularly for attention, DII was positively associated with the risk of myopia among the population aged in 16 to 18 years old and with 9-11th grade educational level. Adherence to pro-inflammatory diet patterns should be a risk factor for myopia in adolescents, especially in late adolescents and those with higher levels of education. The results may be helpful to public policy makers in developing healthy and anti-inflammatory dietary recommendations, in order to better prevent and control adolescent myopia.
Data availability
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. All relevant data are within its Supporting Information files. The datasets in this study are freely accessible on the NHANES website (https://www.cdc.gov/nchs/nhanes).
References
Holden, B. A. et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 123, 1036–1042 (2016).
Xu, R., Zheng, J., Liu, L. Q. & Zhang, W. Q. Effects of inflammation on myopia: Evidence and potential mechanisms. Front. Immunol. 14, 1260592 (2023).
Wei, C. C. et al. Allergic conjunctivitis-induced retinal inflammation promotes myopia progression. EBioMedicine 28, 274–286 (2018).
Lin, H. J. et al. Role of chronic inflammation in myopia progression: Clinical evidence and experimental validation. Ebiomedicine. 10, 269–281 (2016).
Esposito, K. et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. Jama-J Am. Med. Assoc. 292, 1440–1446 (2004).
Phillips, C. M. et al. Dietary inflammatory index and non-communicable disease risk: A narrative review. Nutrients. 11, 1873 (2019).
Hochrein, S. M. et al. The glucose transporter GLUT3 controls T helper 17 cell responses through glycolytic-epigenetic reprogramming. Cell. Metab. 34, 516–532 (2022).
Lin, X. L. et al. Augmentation of scleral glycolysis promotes myopia through histone lactylation. Cell. Metab. 36, 511–525 (2024).
Xu, H. et al. A proinflammatory diet is associated with systemic inflammation and reduced kidney function in elderly adults. J. Nutr. 145, 729–735 (2015).
Pan, M. Z. et al. Dietary ω-3 polyunsaturated fatty acids are protective for myopia. Proc. Natl. Acad. Sci. USA 118, e2104689118 (2021).
Shivappa, N., Steck, S. E., Hurley, T. G., Hussey, J. R. & Hebert, J. R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public. Health Nutr. 17, 1689–1696 (2014).
Vieujean, S. et al. Impact of the exposome on the epigenome in inflammatory bowel disease patients and animal models. Int. J. Mol. Sci. 23, 7611 (2022).
Hariharan, R. et al. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 23, e13349 (2022).
Wu, L. D. et al. Estimated pulse wave velocity is associated with all-cause mortality and cardiovascular mortality among adults with diabetes. Front. Cardiovasc. Med. 10, 1157163 (2023).
Chen, N., Sheng, Y., Wang, G. & Liu, J. Association between physical indicators and myopia in American adolescents: National Health and Nutrition Examination Survey 1999–2008. Am. J. Ophthalmol. 260, 132–139 (2024).
Zhang, R. H. et al. Association between vitamin D and myopia in adolescents and young adults: Evidence of national cross-sectional study. Eur. J. Ophthalmol. 33, 1883–1891 (2023).
Sandhu, R. K., Munoz, B. E., Swenor, B. K. & West, S. K. Refractive error and visual function difficulty in a latino population. Ophthalmology 119, 1731–1736 (2012).
Shi, X. H., Dong, L., Zhang, R. H. & Wei, W. B. Association between weight-adjusted waist index and myopia in adolescents and young adults: Results from NHANES 1999–2008. BMC Ophthalmol. 24, 14 (2024).
Bullimore, M. A. et al. IMI-Onset and progression of myopia in young adults. Invest. Ophth Vis. Sci. 64, 2 (2023).
Dong, L. M. et al. Myopia stabilization and associated factors among participants in the correction of myopia evaluation trial (COMET). Invest. Ophth Vis. Sci. 54, 7871–7884 (2013).
Giugliano, D., Ceriello, A. & Esposito, K. The effects of diet on inflammation emphasis on the metabolic syndrome. J. Am. Coll.Cardiol. 48, 677–685 (2006).
Morgan, I. G. & Rose, K. A. Myopia and international educational performance. Ophthal. Physl. Opt. 33, 329–338 (2013).
Ostadimoghaddam, H. et al. Prevalence of the refractive errors by age and gender: The Mashhad eye study of Iran. Clin. Exp. Ophthalmol. 39, 743–751 (2011).
Lei, X., Xu, Z. X. & Chen, W. W. Association of oxidative balance score with sleep quality: NHANES 2007–2014. J. Affect. Disord. 339, 435–442 (2023).
Zhang, Y. Y. et al. Association between overactive bladder and depression in American adults: A cross-sectional study from NHANES 2005–2018. J. Affect. Disorders. 356, 545–553 (2024).
Wu, J. Y. et al. The association of blood metals with latent tuberculosis infection among adults and adolescents. Front. Nutr. 10, 1259902 (2023).
Williams, K. M. et al. Increasing prevalence of myopia in Europe and the impact of education. Ophthalmology 122, 1489–1497 (2015).
Liu, Z. Z. et al. Association between whole-grain intake and myopia in Chinese children: A cross-sectional epidemiological study. BMC Ophthalmol. 23, 1 (2023).
Zhou, Z. X. et al. Association of n-3 polyunsaturated fatty acid intakes with juvenile myopia: A cross-sectional study based on the NHANES database. Front. Pediatr. 11, 1122773 (2023).
Angelidi, A. M. et al. Mediterranean diet as a nutritional approach for COVID-19. Metabolism 114, 154407 (2021).
Serra-Majem, L. et al. Food, youth and the mediterranean diet in Spain: Development of KIDMED, Mediterranean diet quality index in children and adolescents. Public. Health Nutr. 7, 931–935 (2004).
Yin, C. J. et al. Dietary patterns and associations with myopia in Chinese children. Nutrients 15, 1946 (2023).
Jee, D., Morgan, I. G. & Kim, E. C. Inverse relationship between sleep duration and myopia. Acta Ophthalmol. 94, E204–E210 (2016).
Chua, S. Y. L. et al. Relative contribution of risk factors for early-onset myopia in young Asian children. Invest. Ophth Vis. Sci. 56, 8101–8107 (2015).
Kung, Y. J. et al. Kawasaki disease increases the incidence of myopia. Biomed Res. Int 2017, 2657913 (2017).
Herbort, C. P., Papadia, M. & Neri, P. Myopia and inflammation. J. Ophthalm. Vis. Res. 6, 270–283 (2011).
Fledelius, H., Zak, M. & Pedersen, F. K. Refraction in juvenile chronic arthritis: A long-term follow-up study, with emphasis on myopia. Acta Ophthalmol. 79, 237–239 (2001).
Pavillard, L. E., Marín-Aguilar, F., Bullon, P. & Cordero, M. D. Cardiovascular diseases, NLRP3 inflammasome, and western dietary patterns. Pharmacol. Res. 131, 44–50 (2018).
Zandalinas, S. I. & Mittler, R. ROS-induced ROS release in plant and animal cells. Free Radic. Biol. Med. 122, 21–27 (2018).
Li, M. et al. Retinal microvascular network and microcirculation assessments in high myopia. Am. J. Ophthalmol. 174, 56–67 (2017).
Perler, B. K., Friedman, E. S. & Wu, G. D. The role of the gut microbiota in the relationship between diet and human health. Annu. Rev. Physiol. 85, 449–468 (2023).
Li, H. et al. Gut microbiota-derived indole-3-acetic acid suppresses high myopia progression by promoting type I collagen synthesis. Cell. Discov. 10, 89 (2024).
Omar, W. E. W., Singh, G., McBain, A. J., Cruickshank, F. & Radhakrishnan, H. Gut microbiota profiles in myopes and nonmyopes. Invest. Ophth Vis. Sci. 65, 2 (2024).
Acknowledgements
The authors acknowledge NHANES database for providing free platforms and appreciate all participants in our present study.
Funding
This work is supported by Central High-Level Traditional Chinese Medicine Hospital Project of eye Hospital China Academy of Chinese medical science (GSP4-01), and the Postdoctoral Fellowship Program of CPSF (GZC20242026).
Author information
Authors and Affiliations
Contributions
Z.F.K. obtained funding. S.S.Y. and Z.F.K designed the study. S.S.Y. and X.Y.H. collected the data. Y.P.S. and K.S. analyzed the data. S.S.Y., Y.P.S and L.L.W. drafted the manuscript. Z.F.K. was responsible for checking and verifying the data. Z.F.K. and S.S.Y. participated in the research design and editor of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study protocol was approved by the NHANES Institutional Review Board, and was conducted in accordance with the local legislation and institutional requirement. All participants provided signed informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, S., Hou, X., Song, K. et al. Association between dietary inflammatory index and adolescent myopia based on the National Health and Nutrition Examination Survey. Sci Rep 14, 28048 (2024). https://doi.org/10.1038/s41598-024-78629-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78629-3
Keywords
This article is cited by
-
Environmental exposure to perchlorate, nitrate, and thiocyanate in relation to myopia in adolescents: a cross-sectional NHANES study
European Journal of Medical Research (2026)