Abstract
In this study, we aimed to investigate the nationwide utilization of antipsychotic drugs (APDs) during radiotherapy and evaluate their association with survival in patients with breast cancer. This retrospective cohort study used the National Health Insurance Service database in Korea and included patients diagnosed with breast cancer from 2010 to 2020 who received radiotherapy. The APDs included in the analysis were aripiprazole, quetiapine, olanzapine, risperidone, haloperidol, and chlorpromazine, and the APD prescription details included prescription time, dosage, and duration. Among 170,226 patients with breast cancer treated with radiotherapy, 3361 (1.97%) received APD during radiotherapy. Use of APDs was significantly associated with higher mortality in all patients and in a subgroup of patients excluding those with metastasis or other cancers. Among patients taking APD during radiotherapy, those with accompanied psychiatric history and long-term APD use for ≥ 3 months were associated with lower mortality, whereas patients who started APD during radiotherapy had higher mortality than those who started APD before radiotherapy. The high mortality observed in breast cancer patients using APDs during radiotherapy could be influenced by the underlying conditions that necessitated APD use. Further studies are needed to determine the effects of APDs during radiotherapy in patients with breast cancer.
Similar content being viewed by others
Introduction
Radiotherapy plays a crucial role in the treatment of breast cancer. Previous meta-analyses have demonstrated the overall survival benefit of adjuvant radiotherapy for invasive cancers not only after breast-conserving surgery but also after total mastectomy for advanced-stage breast cancer1,2. Moreover, radiotherapy has been widely applied in palliative care for local lesions in metastatic breast cancer. Based on the treatment plan, the radiotherapy schedule generally lasts for 3–9 weeks, and drugs being taken for other medical conditions, including psychiatric drugs, should be concurrently used during the radiotherapy period.
Antipsychotic drugs (APDs) are used in various clinical settings and for major clinical conditions, such as schizophrenia, bipolar disorder, other psychoses, and dementia. However, in recent decades, the off-label use of APDs has shown an increasing trend worldwide3,4. Despite their more tolerable safety profile5, the use of second-generation APDs has been associated with metabolic and endocrine abnormalities6. A particularly common side effect of APD is elevated prolactin levels, which occurs secondary to the direct blockade of the D2 dopamine receptors in the pituitary gland7. APDs may transiently increase prolactin release; however, first-generation APDs and some second-generation APDs (e.g., risperidone and amisulpride) have been shown to prolong the elevation of prolactin levels, leading to osteoporosis, galactorrhea, and sexual dysfunction8,9. Furthermore, prolactin may be associated with both etiology and progression of breast cancer.
Breast cancer occurs approximately 25% more frequently in women with schizophrenia than in the general female population10. Although hyper-prolactinemia increases the risk of breast cancer in women, the association of APD with breast cancer remains relatively unelucidated11,12, despite reports stating that APD use increases the risk of breast cancer13. However, few studies have investigated the effects of APDs on survival during breast cancer treatment.
Therefore, in this study, we aimed to investigate the effects of APD use during radiotherapy on the survival of patients with breast cancer. We explored real-world situations in the Republic of Korea by using data from a nationwide healthcare database managed by the National Health Insurance Service (NHIS).
Results
Frequency of APD use and baseline patient characteristics
From the NHIS database, we identified a total of 725,897 patients with cancer who underwent radiotherapy, and among them, 170,226 patients with breast cancer were enrolled in this analysis. Among patients who received radiotherapy for breast cancer, 12,401 (7.29%) received APDs throughout the study period, whereas 3,361 (1.97%) received APDs during radiotherapy. A flowchart of the patient-selection process is shown in Fig. 1.
Table 1 presents the baseline characteristics and proportion of APD use according to each characteristic. The proportion of APD use was calculated as the percentage of patients with APD use among all participants evaluated for each variable. Based on whether an APD was used, statistically significant differences were noted in all variables (p < 0.0001).
Only 0.21% of male patients with breast cancer received radiotherapy. Among patients with APD use, 34.39%, 21.04%, 9.43% and 2.86% were in their 50s, 60s, 70s, and ≥ 80 years, respectively. The proportions of APD use in each age group were as follows: 2.04%, 2.37%, 3.03% and 6.73% in their 50s, 60s, 70s, and ≥ 80, respectively. Charlson comorbidity index (CCI) ≥ 3 was recorded in 59.63% of APD patients and in 47.16% of non-APD patients. In APD patients during radiotherapy, 2,690 (80.04%) had psychiatric history; the proportion of patients with bipolar disorder, psychotic disorder, depressive disorder, delirium, and other psychiatric disorders was 27.93%, 29.19%, 52.51%, 2.13%, and 16.01%, respectively. In patients with bipolar disorder, psychotic disorder, depressive disorder, disorder, delirium, and other psychiatric disorders, the proportion of APD patients was 41.22%, 61.78%, 6.95%, 79.59%, and 1.64%, respectively. In non-APD patients, the proportion of patients with other cancers or metastases was 3.25% and 8.11%, respectively, whereas in APD patients, the proportion was 18.35% and 37.96%, respectively.
Prescription of APD
The details of APD prescription were confirmed for all patients who received radiotherapy for breast cancer during the study period (2010–2020) or during radiotherapy. During the total study period, 12,401 (7.2%) participants were prescribed APD, and the most frequently prescribed APDs were quetiapine (58.04%), followed by olanzapine (22.84%), risperidone (12.07%), chlorpromazine (3.83%), aripiprazole (0.18%), and haloperidol (0.04%) on duplicate-allowed prescription basis. For duplicate-allowed prescriptions, the proportion of APDs prescribed during radiotherapy was as follows: quetiapine (62.97%), risperidone (18.15%), olanzapine (15.3%), chlorpromazine (3.44%), and aripiprazole (0.14%). For single prescriptions, the APDs that were most frequently prescribed during radiotherapy were quetiapine (66.66%), risperidone (15.57%), olanzapine (14.93%), chlorpromazine (2.72%), and aripiprazole (0.13%). Overall, 6.9% of patients received complex prescriptions for APD during radiotherapy, as shown in Table 2.
Among the patients who were prescribed APDs, 53.91% and 46.09% were prescribed APDs before and during radiotherapy, respectively. Moreover, 37.52% of these patients were prescribed APD after radiotherapy, and 52% of the patients who used APD after radiotherapy were prescribed APD even 1 year after radiotherapy. Among the APD-prescribed patients, 88% and 12% were prescribed low-dose and high-dose APDs, respectively. Among the patients who were prescribed APDs, 55% had a prescription duration < 3 months, whereas 45% had prescription durations ≥ 3 months. The APD prescription times based on radiotherapy and APD doses are presented in Table 3.
Survival stratified by APD use
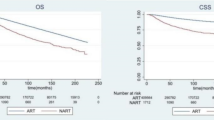
Table 4 presents the survival analyses based on APD use during radiotherapy. Using Cox proportional hazard regression analysis, we estimated the risk of mortality without adjustment and with adjustment by sex, age, CCI, medical history, and psychiatric history. These analyses were performed on all patients and after excluding those with metastases or other cancers. Among the entire study cohort, APD use during radiotherapy was significantly associated with higher mortality in the unadjusted and adjusted analyses (unadjusted HR: 8.62, 95% CI: 8.19–9.07; adjusted HR: 11.07, 95% CI: 10.42–11.77; p < 0.0001; Fig. 2a). Among patients who used APDs during radiotherapy, those with a psychiatric history exhibited lower mortality than those without (adjusted HR: 035, 95% CI: 0.27–0.45, p < 0.001). Within the subgroup of patients with a psychiatric history who used APDs during radiotherapy, those without delirium had lower mortality rates than those with delirium, although the difference was not statistically significant. (adjusted HR: 0.85, 95% CI: 0.61–1.20, p = 0.3609). Among patients who used APDs during radiotherapy, the mortality of patients treated with high-dose APD was lower than that of patients treated with low-dose APD in the unadjusted analysis (unadjusted HR: 0.48, 95% CI: 0.40–0.58, p < 0.0001); however, no significant intergroup difference was noted in the adjusted analysis (adjusted HR: 0.83, 95% CI: 0.67–1.02, p = 0.0721). Analysis based on first prescription timing showed a higher mortality in patients who were prescribed APD during radiotherapy than in patients who were prescribed APD prior to radiotherapy (adjusted HR: 7.78, 95% CI: 6.87–8.81, p < 0.0001). Analysis based on prescription duration showed a lower mortality in patients who were prescribed APDs for ≥ 3 months than in those prescribed APD for < 3 months (adjusted HR: 0.7, 95% CI: 0.6–0.81, p < 0.0001). Supplementary Table S1 presents the results of the sex-stratified analysis of APD use during radiotherapy in the entire study cohort. In both male and female participants, APD use during radiotherapy was significantly associated with higher mortality in the adjusted analyses (HR in men: 10.01, 95% CI: 5.65–17.74; HR in women: 11.15, 95% CI: 10.49–11.85; p < 0.0001). In the subgroup analysis based on the age cutoff of 65 years, the mortality rate of male patients with breast cancer who used APD during radiotherapy was similarly high in both groups.
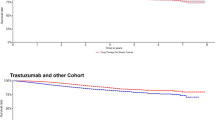
In the analysis conducted after excluding patients with metastasis or other cancers, APD use during radiotherapy was significantly associated with higher mortality (adjusted HR: 5.83, 95% CI: 5.22–6.53, p < 0.0001; Fig. 2b). The mortality in both low- and high-dose APD patients was lower than that in non-APD patients (adjusted HR: 5.65 and 4, 95% CI: 5.03–6.34 and 2.98–5.38, p < 0.0001), and no significant intergroup difference was noted in the analysis based on APD dose (adjusted HR: 0.88, 95% CI: 0.64–1.21, p = 0.0822). Analysis based on first prescription timing showed a higher mortality in patients who were prescribed APD during radiotherapy than in patients prescribed APD prior to radiotherapy (adjusted HR: 5.84, 95% CI: 4.76–7.17, p < 0.0001). Analysis based on prescription duration showed a lower mortality in patients prescribed APD for ≥ 3 months than in those prescribed APD for < 3 months (adjusted HR: 0.74, 95% CI: 0.58–0.94, p = 0.0153). Survival curves based on APD prescription details in patients, excluding those with metastasis and other cancers, are shown in Fig. 3.
Overall survival curves stratified according to the (a) prescription timing of antipsychotic drug (pre-radiotherapy vs. during radiotherapy); (b) prescription dosage (low-dose vs. high-dose); and (c) prescription duration (< 6 months vs. ≥6 months) in patients taking antipsychotic drugs during radiotherapy.
Prognostic factors for overall survival
Table 5 presents the results of univariate and multivariable analyses of prognostic factors for overall survival. These analyses were performed in all patients, after excluding those with metastasis or other cancers. In univariate and multivariable analyses, APD use during radiotherapy, older age, higher CCI, and delirium were statistically significant poor prognostic factors. Bipolar disorder and psychotic disorders were poor prognostic factors in the univariate analyses but good prognostic factors in the multivariable analyses. Depression and other psychotic disorders were statistically significant poor prognostic factors in the univariate analysis; however, no statistical significance was noted in the multivariable analysis.
Discussion
This study evaluated the effects of using APDs during radiotherapy on the survival of patients with breast cancer. Some studies have reported an increased incidence of breast cancer associated with APD use14,15. However, to the best of our knowledge, no study to date has reported the effects of APD use during cancer treatment on breast cancer survival. To the best of our knowledge, this is the first study to report the effects of APD use during radiotherapy on the survival of patients with breast cancer.
Compared to the general population, women with schizophrenia and bipolar disorder have been shown to have a higher risk of breast cancer, and APD use has the potential to account for at least some of this increased risk16,17. This aligns with the widely accepted working hypothesis regarding the hyperprolactinemia-inducing properties of some APDs6,18. Prolactin-elevating APDs include paliperidone, risperidone, amisulpride, and haloperidol, whereas prolactin-sparing antipsychotics include aripiprazole, brexpiprazole, cariprazine, and quetiapine19. Two recent epidemiological studies have found an association between prolactin-elevating APDs and increased risk of breast cancer10,20. These findings were mainly confirmed in patients who received long-term and high-dose APD therapy. In a population-based cohort of patients with breast cancer, APD use was associated with increased breast cancer-specific mortality. However, there was no dose–response relationship, and, importantly, no association was observed in patients with severe mental illness. The authors suggested that the observed association was likely due to confounding by the indication21. In our study, we confirmed the effect of APD use during radiotherapy on mortality, which differs from the results of previous studies in the target patient population.
Among the patients who received radiotherapy for breast cancer, only 1.97% received APD during radiotherapy, which was comparable to the proportion of APD use in patients with cancer reported in China (1.4%) and Brazil (1.67%)22,23. The prevalence of APD use increased with age, with 6.73% of patients being > 80 years of age. CCI was higher in patients with APD use, and the proportion of patients taking APD increased as CCI increased. Compared to those not using APDs, patients using APDs were more likely to have a psychiatric history, metastasis, and other cancers. We attempted to identify psychiatric diagnostic codes in the target patients to determine the reason for APD use during radiotherapy; however, 20% of patients who used APD during radiotherapy were not accompanied by psychiatric history. Patients who underwent APD without a psychiatric diagnosis may have been incident users of APD and may have had accompanying fatal diagnoses such as delirium. In our study, fewer patients using APDs during radiotherapy were diagnosed with delirium, and they did not show statistically significant higher mortality compared to those with other psychiatric diagnoses. However, among patients who used APDs during radiotherapy, patients with a psychiatric history had lower mortality rates than those without. We can speculate that incident users of APDs without a psychiatric history were accompanied by diagnoses such as delirium, which is frequently observed in patients with terminal cancer24, and that this condition may be related to the high mortality rate observed.
The data used in this study included those of male patients with breast cancer, and we included them in the study’s results to provide information on male patients who received radiotherapy. We performed a sex-stratified analysis, and the results were similar to those of the entire cohort analysis. Although male patients with breast cancer accounted for a very small proportion of the overall breast cancer patient population, we confirmed that their APD prescription rate was higher than that of female patients. In addition, we confirmed that the APD prescription rate tended to increase with age. Thus, we conducted age- and sex-stratified analyses, and patients with breast cancer who used APD during radiotherapy had increased mortality in both the older and younger groups and both sexes. The stratified analysis of the effect of APD use during radiotherapy in male patients with breast cancer was attempted but was difficult to perform because of their very small sample size. Moreover, the 95% confidence intervals in the results were very wide, which indicated very low statistical reliability. As the results of the analysis of male patients alone seemed uncertain, a more detailed analysis was not conducted. Further large-sample studies are needed after obtaining specific information on male patients with breast cancer.
Our findings revealed a significant association between APD use during radiotherapy and high mortality. Patients with APD use had a significantly higher mortality than those who did not use APDs (HR, 11.07). In these patients, excluding those with metastasis or other cancers, using APD during radiotherapy was also significantly associated with higher mortality (HR 5.83), but the HR was lower than that in all patients. This decreased mortality risk is attributed to the exclusion of the risk of motility due to metastasis or other cancers. In our study, mortality was very high, even after excluding those with metastasis or other cancers, which was higher than the increased breast cancer-specific mortality (HR 2.25) in APD used breast cancer patients reported by Hicks et al.21. This difference can be attributed to the fact that this other study analyzed patients newly diagnosed with primary breast cancer, whereas our study focused on patients who underwent radiotherapy. Among patients with APD use, long-term use (≥ 3 months) resulted in lower mortality. In contrast, patients who initiated APDs during radiotherapy had higher mortality than those who initiated APD before radiotherapy. Taken together, it can be inferred that patients who should have started APD during radiotherapy and those who were unavailable or did not use it for a long period of time had a higher mortality rate. Here, we can speculate that the underlying status of patients, such as old age or delirium, who had to start APD during radiotherapy and had to use APD for a short period, may be related to the high mortality observed in these patients.
In our analysis, we also analyzed prognostic factors for overall survival. We performed an analysis that excluded patients with metastasis or other cancers to exclude the effect of metastasis or other cancers on survival. Age; CCI; accompanying hypertension, myocardial infarction, and delirium; and APD use during radiotherapy were identified as poor prognostic factors. In contrast, accompanying bipolar disorder and psychotic disorder are considered good prognostic factors. This result also makes it possible to infer that underlying conditions such as delirium or old age in patients taking APDs may be associated with high mortality in APD patients. Delirium is the most common neurological condition in patients with cancer and is observed more commonly in older patients. Studies show that 22‒44% of patients with cancer develop delirium and that this incidence increases to 87% in the final days of life24. Therefore, it is possible that the older age of patients with delirium included in this study contributed to the high mortality rate of APD patients.
This study has some limitations. First, there are inherent limitations because of the observational retrospective design. Although we established an association between APD use during radiotherapy and survival, we cannot definitively prove that APD use during radiotherapy directly results in a high mortality in patients with breast cancer. Second, as this study was based on medical claims and not a medical chart review, non-claims-related information affecting survival, such as stage, was excluded from the analysis. Finally, the limited identification of psychotic diagnostic codes in patients conferred limitations for analyzing the effects of APD during radiotherapy. A previous study21 showed that restricting the cohort to patients with severe mental illness attenuated the association between APD use and breast cancer-specific mortality and that the effect of APD use during radiotherapy on survival rates may differ in patients who receive APD for severe mental illness other than delirium.
In conclusion, only 1.97% of patients who underwent radiotherapy received APD simultaneously during radiotherapy for breast cancer. The high mortality observed in breast cancer patients using APDs during radiotherapy could be influenced by the underlying conditions that necessitated APD use. Further studies are needed to determine the effects of APDs during radiotherapy in patients with breast cancer.
Methods
Data sources and data selection
In this retrospective cohort study, we utilized data obtained from the NHIS database, which encompasses medical expense claim data for the entire population of South Korea. From the NHIS database, we obtained the claims data of patients who received radiotherapy for breast cancer (diagnostic code C50, according to the International Classification of Diseases, 10th Edition [ICD-10]) from 2010 to 2020. The claims data included prescriptions for APDs, such as aripiprazole, quetiapine, olanzapine, risperidone, haloperidol, and chlorpromazine, as well as prescriptions for radiotherapy. The claims data included age, sex, CCI, and diagnostic codes for chronic diseases and psychiatric disorders. The diagnostic codes included in the analysis were hypertension (I10–I15), diabetes (E10–E14), dyslipidemia (E78), chronic renal disease (N17–N19), stroke (I60–I69), peripheral vascular disease (I70–I79), myocardial infarction (I21 and I22), anemia (D50–53, D55–59), bipolar disorder (F30 and F31), psychotic disorder (F20–F29), depressive disorder (F32–F34), delirium (F05), and other psychiatric disorders (F00–F99). Medical history included all diagnosis made throughout the study period, whereas psychiatric history encompassed diagnosis made either before or during radiotherapy. In this study, we used NHIS research data, which were analyzed by statistical experts with extensive experience in data analysis.
Operational definitions
In this study, the total study duration was from 2010 to 2020 when data were provided by the NHIS. Patients with radiotherapy-related procedure codes were defined as those who underwent radiotherapy. In all patients with claims for radiotherapy, APDs were considered prescribed and taken if the APD was claimed at least once. If the radiotherapy period overlapped with the APD claim period, we considered that the APD was taken during radiotherapy. Patients who received two or more sessions of interrupted radiotherapy and with the same diagnostic code were counted as one patient. Patients who received APD during radiotherapy were categorized as “APD patients,” whereas those who did not receive APD were categorized as “non-APD patients.”
Depending on the dose prescribed in monotherapy (simple prescription), APD patients were further stratified into high-dose and low-dose groups. Based on the equivalent dose of chlorpromazine (100 mg/day), the equivalent dose was expressed as follows: quetiapine 60 mg/day, olanzapine 3 mg/day, risperidone 0.8 mg/day, aripiprazole 4 mg/day, and haloperidol 1.6 mg/day. The previously reported drug-specific criteria by Leucht et al.25 for high-dose treatment were as follows: chlorpromazine 250 mg/day, quetiapine 150 mg/day, olanzapine 7.5 mg/day, risperidone 2 mg/day, aripiprazole 10 mg/day, and haloperidol 4 g/day.
Ethics
This research was conducted in accordance with the Declaration of Helsinki. This study was approved by the Institutional Review Board of Chung-Ang University Hospital, and with the approval number CAUH IRB No. 2202-022-19407. The requirement for informed consent was waived owing to the retrospective nature of the study. Data were provided with an anonymous identification code, which made it impossible for the researchers to identify the patients.
Statistical analysis
Descriptive statistics, chi-square tests, and independent t-tests were employed for basic statistical analyses to identify the demographic characteristics of the participants. The results of the analysis are expressed as follows: categorical variables are expressed as frequencies and percentages and continuous variables as mean ± standard deviation. Drugs were counted for patients who received a single prescription or multiple prescriptions. Using the Cox proportional hazards regression analysis, we estimated the risk of mortality without adjustment and with adjustment. Hazard ratio (HR) and 95% confidence interval (CI) were adjusted for age, sex, CCI, and medical history26. We conducted a survival analysis of the prognostic factors that potentially affected overall survival27. The starting date of the survival analysis was defined as the date of the first concurrent administration of radiotherapy and APD in patients with breast cancer from 2010 to 2020. In the group of patients who were not prescribed APD, the date of the first radiotherapy of the patient with breast cancer was considered the starting point. The endpoint was defined as the date of the patient’s death or December 31, 2020 for patients who survived. Attrition occurred when patients were no longer available for follow-up due to death, emigration, or loss of National Health Insurance eligibility; thus, data up to the last observation point were included in the analysis. The analysis included APD use, age, hypertension, myocardial infarction, psychiatric history (bipolar disorder, psychotic disorder, depression, delirium, and other psychotic disorders), and CCI. Using univariate analysis, we intuitively identified the impact of each variable on survival. When multiple variables were included, multivariable analysis was applied to identify their association with survival, and the important variables were identified. Kaplan–Meier survival curves were used to verify the proportional hazards assumption, and the number of patients at risk for each 2-year interval was confirmed. All data were analyzed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) and R (version 4.1.3), with a significance level of alpha = 0.05.
Data availability
This study was based on information extracted from the National Health Insurance Service (NHIS) database, which encompasses the medical expense claims data for the entire South Korean population. The authors do not own these data, and therefore, are not permitted to share these data in the original form. Qualifying researchers may apply to access a minimal dataset by contacting Professor Dae Ryong Kang. Entire data can be obtained by applying to the National Health Insurance Service homepage (https://nhiss.nhis.or.kr) through appropriate procedures.
References
Early Breast Cancer Trialists’ et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 378, 1707–1716. https://doi.org/10.1016/S0140-6736(11)61629-2 (2011).
Ebctcg et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 383, 2127–2135. https://doi.org/10.1016/S0140-6736(14)60488-8 (2014).
Halfdanarson, O. et al. International trends in antipsychotic use: a study in 16 countries, 2005–2014. Eur. Neuropsychopharmacol. 27, 1064–1076. https://doi.org/10.1016/j.euroneuro.2017.07.001 (2017).
Ng, V. W. S. et al. Bipolar disorder prevalence and psychotropic medication utilisation in Hong Kong and the United Kingdom. Pharmacoepidemiol Drug Saf. 30, 1588–1600. https://doi.org/10.1002/pds.5318 (2021).
Herrmann, N., Mamdani, M. & Lanctot, K. L. Atypical antipsychotics and risk of cerebrovascular accidents. Am. J. Psychiatry. 161, 1113–1115. https://doi.org/10.1176/appi.ajp.161.6.1113 (2004).
De Hert, M., Detraux, J., van Winkel, R., Yu, W. & Correll, C. U. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 8, 114–126. https://doi.org/10.1038/nrendo.2011.156 (2011).
Bushe, C., Shaw, M. & Peveler, R. C. A review of the association between antipsychotic use and hyperprolactinaemia. J. Psychopharmacol. 22, 46–55. https://doi.org/10.1177/0269881107088435 (2008).
Bostwick, J. R., Guthrie, S. K. & Ellingrod, V. L. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy. 29, 64–73. https://doi.org/10.1592/phco.29.1.64 (2009).
Peuskens, J., Pani, L., Detraux, J. & De Hert, M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 28, 421–453. https://doi.org/10.1007/s40263-014-0157-3 (2014).
Taipale, H. et al. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry. 8, 883–891. https://doi.org/10.1016/S2215-0366(21)00241-8 (2021).
Aranha, A. F. et al. Impact of the prolactin levels in breast cancer: a systematic review and meta-analysis. Gynecol. Endocrinol. 38, 385–390. https://doi.org/10.1080/09513590.2022.2047173 (2022).
Wang, M., Wu, X., Chai, F., Zhang, Y. & Jiang, J. Plasma prolactin and breast cancer risk: a meta- analysis. Sci. Rep. 6, 25998. https://doi.org/10.1038/srep25998 (2016).
Wang, P. S. et al. Dopamine antagonists and the development of breast cancer. Arch. Gen. Psychiatry. 59, 1147–1154. https://doi.org/10.1001/archpsyc.59.12.1147 (2002).
Dalton, S. O. et al. Cancer risk among users of neuroleptic medication: a population-based cohort study. Br. J. Cancer. 95, 934–939. https://doi.org/10.1038/sj.bjc.6603259 (2006).
Nielsen, R. E., Lolk, A., Rodrigo-Domingo, M., Valentin, J. B. & Andersen, K. Antipsychotic treatment effects on cardiovascular, cancer, infection, and intentional self-harm as cause of death in patients with Alzheimer’s dementia. Eur. Psychiatry. 42, 14–23. https://doi.org/10.1016/j.eurpsy.2016.11.013 (2017).
Wu Chou, A. I., Wang, Y. C., Lin, C. L. & Kao, C. H. Female schizophrenia patients and risk of breast cancer: a population-based cohort study. Schizophr Res. 188, 165–171. https://doi.org/10.1016/j.schres.2017.01.019 (2017).
Anmella, G. et al. Risk of cancer in bipolar disorder and the potential role of lithium: International collaborative systematic review and meta-analyses. Neurosci. Biobehav Rev. 126, 529–541. https://doi.org/10.1016/j.neubiorev.2021.03.034 (2021).
Johnston, A. N. et al. Hyperprolactinemia-inducing antipsychotics increase breast cancer risk by activating JAK-STAT5 in precancerous lesions. Breast Cancer Res. https://doi.org/10.1186/s13058-018-0969-z (2018).
Hope, J. D., Keks, N. A. & Copolov, D. L. Association between long-term use of prolactin-elevating antipsychotics in women and the risk of breast cancer: what are the clinical implications? Australas Psychiatry. 31, 205–208. https://doi.org/10.1177/10398562231158925 (2023).
Rahman, T. et al. Risk of breast Cancer with Prolactin elevating antipsychotic drugs: an observational study of US women (ages 18–64 years). J. Clin. Psychopharmacol. 42, 7–16. https://doi.org/10.1097/JCP.0000000000001513 (2022).
Hicks, B. M. et al. Post-diagnostic antipsychotic use and cancer mortality: a population based cohort study. BMC Cancer. 20, 804. https://doi.org/10.1186/s12885-020-07320-3 (2020).
Ng, C. G., Boks, M. P., Smeets, H. M. & Zainal, N. Z. De Wit, N. J. Prescription patterns for psychotropic drugs in cancer patients; a large population study in the Netherlands. Psychooncology. 22, 762–767. https://doi.org/10.1002/pon.3056 (2013).
Shirama, F. H. et al. Factors associated with common mental disorders and use of psychiatric drugs in cancer outpatients. Arch. Psychiatr Nurs. 33, 88–93. https://doi.org/10.1016/j.apnu.2019.06.001 (2019).
El Majzoub, I., Abunafeesa, H., Cheaito, R., Cheaito, M. A. & Elsayem, A. F. Management of altered mental status and delirium in cancer patients. Ann. Palliat. Med. 8, 728–739. https://doi.org/10.21037/apm.2019.09.14 (2019).
Leucht, S. et al. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 40, 314–326. https://doi.org/10.1093/schbul/sbu001 (2014).
Cox, D. R. Regression models and life-tables. J. R Stat. Soc. Ser. B Stat. Methodol. 34, 187–120 (1972).
Kaplan, E. L. & Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53, 457–481 (1958).
Funding
This work was supported by the VSPharm Tech Co., Ltd., Republic of Korea.
Author information
Authors and Affiliations
Contributions
IGH and JHC wrote the manuscript and participated in all study-related processes. JHC and IGH conceived and designed the study. SMK presented and interpreted the criteria for clinical analysis. DRK, THG, and SGH analyzed, validated, and visualized the data. SYP and HL participated in the research planning and acquired funds. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hwang, I., Kim, S., Kang, D. et al. Effects of antipsychotic drugs during radiotherapy in breast cancer in South Korea: a retrospective cohort study. Sci Rep 14, 27138 (2024). https://doi.org/10.1038/s41598-024-78698-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78698-4