Abstract
Atrial septal defects (ASD) divert flow from systemic to pulmonary circulation, and some degree of plasma volume expansion and neurohormonal activation are necessary to maintain the effective circulatory volume. The aim of the present study was to understand the patterns of neurohormonal activation in ASD patients. 16 ASD patients and 10 controls were enrolled. Fasting blood samples were collected prior to procedure and 48 h after defect closure. At baseline, renin (185.0(79.0—437.0 vs. 79.4(60.8—110.0), p = 0.04), aldosterone (20.2 ± 7.6 vs. 11.7 ± 2.8, p < 0.001), copeptin (43.6 ± 27.5 vs. 16.4 ± 8.7, p = 0.002) and both natriuretic peptides were higher in ASD patients, while noradrenaline (113.0 ± 61.3 vs. 178.0 ± 49.4, p = 0.009) and endothelin-1 (2.93 ± 2.00 vs. 5.06 ± 1.25, p = 0.006) were higher in controls. After ASD closure, only NT-proBNP reduced significantly (p = 0.02). There were negative correlations between defect area with noradrenaline (r=-0.73, p = 0.002) and with endothelin-1 (r=-0.59, p = 0.02). Present findings suggest that in patients with an ASD, there is an increase in neurohormones that are related to regulation of plasma volume (aldosterone and arginine vasopressin) with simultaneous reductions in neurohormones related to vasoconstriction in systemic and pulmonary beds (noradrenaline and endothelin-1).
Similar content being viewed by others
Introduction
Atrial septal defect (ASD) is the most common type of congenital heart disease (CHD) seen in adults and constitute up to 30% of all grown-up CHDs1. The defect itself can be repaired with relative ease with either surgical or transcatheter methods, and both options offer excellent durability and safety2,3. However, patients treated at an older age are at risk of arrhythmias, heart failure and pulmonary hypertension (PH) even after the closure of the ASD, particularly if the defect was large before the closure4,5. More recent data from a Danish nationwide cohort have found that patients an ASD had more frequent cardiovascular events and higher mortality compared to population, even after the defect is closed6,7. Although an ASD is conceptually a simple defect with foreseeable natural course, it is clear that the pathophysiology of ASD involves more than the shunt itself and not all consequences of the disease could be prevented by closing the defect.
Heart failure is characterized by the activation of compensatory neurohormonal mechanisms, which are initially useful to maintain homeostasis in the short term but have detrimental effects on disease progression and survival in the long term8,9. Reduced effective arterial blood volume are sensed by the baroreceptors located in the aorta and the carotid arch that leads to an increase in sympathetic outflow, while reduced blood flow to the juxtaglomerular cells located in the kidneys increase renin secretion to retain salt and water. Compensatory neurohormonal activation is associated with late-onset adverse events related to heart failure, including arrhythmias, cardiac remodeling, pathologic left ventricular hypertrophy, pump failure and ultimately death9.
Since flow is diverted away from the systemic circulation, patients with a left-to-right shunt need plasma volume expansion to maintain an adequate effective arterial blood volume10. The exact mechanisms underlying this volume expansion is not entirely clear, but it is reasonable to consider that activation of neurohormonal mechanisms may be associated with the plasma volume increase. Indeed, there are some evidence suggesting that several neurohormonal biomarkers are elevated in patients with left-to-right shunts or in other congenital heart disorders, but available evidence is rather scant and incomplete11,12,13,14.
The primary aim of the present study was to understand the degree of neurohormonal activation in ASD patients undergoing percutaneous defect closure and the changes in these hormones immediately following closure. As secondary aims, we sought to understand the relationship of neurohormonal activation with echocardiographic measures of ASD, right-sided chamber dimensions and pulmonary artery pressure, as well as the relationships between neurohormones.
Methods
Patient selection
For the present study, patients with secundum type atrial septal defects that were undergoing percutaneous closure were prospectively screened at three study centers between January 2022 and December 2022. Patients under 16 years old, those with a left-to-right defect other than secundum type ASD (including other types of ASDs) or those with additional congenital heart disease were excluded. Other exclusion criteria included cardiomyopathy of any type, significant valvular disease (defined as any degree of valvular stenosis, more than trace mitral or aortic regurgitation or more than moderate tricuspid or pulmonary regurgitation), hypertension, diabetes, acute or chronic kidney or liver disease of any severity, and arrhythmias other than infrequent extra systolic beats. In addition, patients with significant pulmonary hypertension that precluded defect closure were excluded. All patients underwent a screening examination, electrocardiography and transthoracic echocardiography to exclude aforementioned criteria. A total of 22 patients were screened and 16 patients that fulfilled these criteria were included in the final analysis. 10 age and gender matched volunteers without any known medical conditions were included as controls. All controls underwent the same screening procedure with the patients to ensure the absence of aforementioned exclusion criteria, as well as the absence of pulmonary hypertension.
The study was conducted according to the principles outlined in the 1975 Helsinki Declaration and its subsequent revisions, and all participants and parents/legal guardians for patients below 18 years old gave their written informed consent prior to enrollment. An ethics approval was obtained from an ethics committee prior to the commencement (Document No: HNEAH-KAEK 2022/247–4034).
Echocardiography
All patients underwent transthoracic and transesophageal echocardiography prior to ASD closure, and available echocardiography platforms and probes were used for the procedures at each institution. The dimensions of the right-sided heart chambers and pulmonary artery systolic pressure were measured according to the available guidelines15,16. The ratio of pulmonary to systemic flow was calculated by multiplying the cross-sectional area of right and left ventricular outflow tracts with time-velocity integrals obtained from the respective locations17. The minimum and maximum dimensions of the ASD, as well as ASD surface area were measured using 3-D transesophageal echocardiography prior to defect closure. In all centers, a cardiologist experienced in both transthoracic and transesophageal echocardiographic assessment of the ASDs did perform the procedures.
Laboratory analysis
After overnight fasting, blood was withdrawn in the morning from the antecubital fossa using a 20-gauze needle and collected samples were transferred to the local biochemistry laboratory within 30 min of collection. A second set of samples were obtained from ASD patients 48 h after closure of the defect. For the measurement of renin, angiotensin II, aldosterone, endothelin, N-terminal pro-B-type natriuretic peptide (NT-proBNP), mid-regional atrial natriuretic peptide (ANP), noradrenaline, normetaepinephrine, and copeptin, samples were stored in -80°C and transferred to the central laboratory in dry ice. In the central laboratory, tubes containing samples were centrifuged at 1500 g for 5 min and the serum parts of them were aliquoted. The aliquots prepared for use in the analyses were stored at -80 °C, until laboratory analyses for determination of biochemical parameters. Before starting the analysis, frozen samples were thawed at room temperature, and re-thawing-freezing processes were avoided. All samples and used kits were brought to room temperature (18–26 °C) before starting the study. The concentrations of ALD, REN, NT-proBNP, NMN, NA/NE, MR-proANP, ET-1, ANG II and CPP were determined using enzyme-linked immunosorbent assay (ELISA) kits (Fine Test cat.no; EU2580, EH0268, EH0350, EU0255, EU2565, EH4063, EU0205, EH2627, EH2880). According to the manufacturer’s instructions, a colored product is formed in proportion to the amount of biochemical parameter present in the sample or standard. The reaction is terminated by addition of acid and absorbance is measured at 450 nm. A standard curve is prepared from standard dilutions for each parameter and sample concentration determined. Remaining biochemical analyses were done in local laboratories using conventional methods.
Statistical analysis
Continuous data is given as mean ± SD or as median and interquartile range, while categorical data is given as percentages. The patterns of distribution for continuous data is analyzed with visual inspection of histograms and QQ plots and with Shapiro-Wilk test. Differences between groups were analyzed using Student’s T test (with Welch correction when necessary) and with Mann-Whitney U test as appropriate. For paired tests, either T test for dependent variables or Wilcoxon ranked sign tests were used. Linear correlation analyses were done with Pearson product moment correlation or with Spearman’s rho. Additional exploratory analyses were done with quadratic and cubic transformations. Bayesian analyses with independent samples T test and paired T tests were done to evaluate the strength of evidence favoring null or alternative hypotheses. For frequentist analyses, a p value below 0.05 was accepted as significant, while for Bayesian analyses a BF10 value of > 30, 10–30 and 3–10 indicated very strong, strong and moderate evidence, respectively. A BF10 value < 0.3 was considered evidence favoring null hypothesis, while the remaining values showed no or anectodal evidence. All statistical analyses were performed with Jamovi (The jamovi project (2024). jamovi Version 2.3 for Mac. Retrieved from https://www.jamovi.org) statistical software.
Results
Baseline characteristics of ASD patients included in the study were given in Table 1. Compared to controls, there were no significant differences in ASD patients in terms of age, gender, height or weight (p > 0.05 for all).
Comparison of neurohormones between ASD patients and controls prior to defect closure
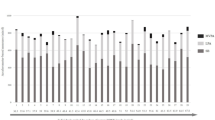
Table 2 summarizes concentrations of neurohormones in ASD and control groups. Patients with ASD had higher plasma renin, aldosterone, NT-proBNP, mid-regional ANP and copeptin concentrations compared to controls, while plasma concentrations of noradrenaline, normetanephrine and endothelin-1 were all lower in the ASD group (Figs. 1 and 2).
Changes in concentrations of neurohormones after defect closure
Only NT-proBNP reduced significantly following ASD closure, but there were numerical increases in both noradrenaline and endothelin-1 acutely after the closure (Table 3). When post-closure concentrations were compared to the initial values obtained from the controls, neither noradrenaline (p = 0.09) nor endothelin-1 (p = 0.17) were significantly different between the groups. Apart from these two neurohormones and and angiotensin-II, all other markers of neurohumoral activation (including normetaneprine) remained significantly different between controls and ASD patients post closure (p < 0.05 for all).
Correlations between echocardiographic characteristics and concentrations of neurohormones in ASD patients prior to defect closure
There were significant negative correlations between ASD size and both noradrenaline and endothelin-1 concentrations (Fig. 3). There was a trend towards a positive correlation between copeptin concentration and pulmonary artery systolic pressure (r = 0.46, p = 0.07). Finally, there were significant nonlinear correlations between angiotensin-II concentrations and indexed RV minor diameter and indexed right atrial area, with the relationship being quadratic.
Correlations between neurohormone concentrations in ASD patients prior to defect closure
Apart from the expected correlation between angiotensin-II and aldosterone (r = 0.54, p = 0.03), both natriuretic peptides showed positive correlations with aldosterone (r = 0.54, p = 0.03 for NT-proBNP and r = 0.58, p = 0.02 for mid regional ANP), and there was a significant negative correlation between noradrenaline and aldosterone (r=-0.58, p = 0.02). Midregional ANP (but not NT-proBNP) showed significant negative correlations with noradrenaline (r=-0.66, p = 0.007), normetanephrine (r=-0.69, p = 0.004) and endothelin-1 (r=-0.65, p = 0.008), while both noradrenaline and normetanephrine showed significant positive correlations with endothelin-1 (Fig. 4).
Bayesian analyses
There were strong evidence supporting a difference between groups for aldosterone, NT-proBNP, midregional ANP and copeptin while there were moderate evidence supporting a difference between groups for noradrenaline and endothelin-1. Evidence supporting a difference between the groups for renin was weak, while there was evidence supporting a lack of difference between groups for angiotensin-II. Other than NT-proBNP, there were no evidence supporting an acute change in neurohormones following closure and the evidence available for NT-proBNP was weak to moderate. As summary of all Bayesian analyses were given in Supplementary Table 1.
Discussion
Present findings demonstrate that although there is a neurohormonal activation in patients with ASD, the pattern of this activation deviates significantly from those with heart failure. The main takeaways from the present work are as follows: (i) Neurohormones that play a major role in volume regulation, namely renin, aldosterone, arginine vasopressin and natriuretic peptides are increased in patients with an ASD; (ii) in contrast, neurohomones that are mainly responsible for setting the arterial tone in systemic or pulmonary beds, such as noradrenaline or endothelin-1 (but not angiotensin-II) were reduced in ASD patients as compared to controls, although both neurohormones tend to increase following defect closure; (iii) both noradrenaline and endothelin-1 were lower in those with a larger ASD, but the degree of activation in aldosterone, arginine vasopressin and natriuretic peptides were unrelated to the defect size. Other notable findings include similar angiotensin-II concentrations in ASD and controls and the rather counterintuitive negative correlation between noradrenaline and aldosterone.
Activation of renin-angiotensin-aldosterone system is the hallmark compensatory mechanism in patients with heart failure and it allows maintenance of cardiac output at rest18,19. Since all components of this system activate each other in a downstream manner, starting with increased renin secretion due to reduced perfusion of the juxtaglomerular cells, all three neurohormones are increased more or less simultaneously in heart failure. However, each individual component of the system have its own set of regulators in addition to the positive and negative feedback relationships that exist between individual components20,21. For example, increased β1 adrenergic stimulation enhances renin(but not aldosterone) secretion, while potassium concentration and adrenocorticotrophic hormone level affect aldosterone synthesis and secretion independent of renin20,22. These additional regulatory mechanisms may help explaining why there was a strong increase in aldosterone concentrations in ASD patients despite relatively mild increase in renin concentration, with no increase in angiotensin-II concentration at all. Both aldosterone and arginine vasopressin act to expand plasma volume, and therefore a concomitant increase in both neurohormones (copeptin is the stable cleavage product of the prohormone for arginine vasopressin) is probably necessary for the volume expansion necessary to maintain effective circulatory volume within the systemic circulation as some of the flow is diverted to the pulmonary circulation.
A reduced endothelin-1 concentration in ASD patients appears to be counterintuitive at first glance, given that congenital shunt defects are associated with an increased pulmonary vascular resistance in the long term23. Endothelin-1 is a potent vasoconstrictor that plays a major role in the development of pulmonary hypertension in patients with pulmonary arterial hypertension24. At least two previous studies in patients with ASDs, as well as one study that included children with congenital heart defects and pulmonary hypertension suggested an increase in endothelin-125,26,27. However, all these studies either included patients with known pulmonary hypertension or endothelin-1 concentrations were increased only in the subgroup of patients with an elevated pulmonary artery pressure. In the absence of pulmonary hypertension, pulmonary compliance is increased in shunt lesions to accommodate for the increased blood flow and pulmonary vascular resistance should be below that what is expected from normal individuals without a shunt lesion28. Also, at least one study showed that plasma endothelin-1 concentration is independent from pulmonary blood flow in patients with shunt lesions29. Finally, present data also suggests a negative correlation between defect size and endothelin-1 concentration and an increase in endothelin-1 following defect closure, with both findings being what to be expected when there is a true reduction in endothelin-1 in ASD patients. As such, it is reasonable to consider that endothelin-1 remains low in the earlier stages of disease course where pulmonary compliance is high and pulmonary vascular disease is not present, while it gradually increases as pulmonary arterial hypertension ensues. A more direct evidence could be obtained by correlating pulmonary vascular resistance with endothelin-1 concentrations in future studies, since catheterization data was not available in the present work.
Similar to endothelin-1, both noradrenaline and normetanephrine concentrations were found to be lower in ASD patients. Available evidence suggests presence of sympathetic activation (and hence increased adrenaline and/or noradrenaline concentrations) in patients with pulmonary hypertension, but there is little data in patients with left-to-right shunt lesions in the absence of pulmonary hypertension or right ventricular failure30. Present results suggest that sympathetic activation is lower than the controls in ASD patients, and this reduction in noradrenaline correlate well with plasma endothelin-1 concentration. As with the pulmonary arterial bed, a reduced noradrenaline concentration would increase the compliance within the systemic vasculature to divert the flow away from the ASD. Again, lack of hemodynamic data in the present study limits interpreting the potential consequences of a low noradrenaline concentration, as systemic vascular resistance could not be calculated.
Although present results suggest that there is indeed neurohormonal activation in ASD patients, the pattern of this activation appears to be significantly different from what is seen in heart failure patients in whom virtually all neurohormonal systems were activated in concert9,31. Indeed, our findings suggest that there is a simultaneous activation of neurohormones predominantly related to volume expansion (aldosterone and arginine vasopressin) together with suppression of vasoconstrictor peptides (noradrenaline and endothelin-1) to ensure a compliant pulmonary and vascular bed. Both renin and angiotensin-II appears to act differently from other neurohormones, since their concentrations were either slightly increased (in case of renin) or similar to controls (in case of angiotensin-II). The exact cause of this difference is not immediately clear, but both neurohormones have concomitant effects on volume regulation and vascular tonus that favor vasoconstriction and modulation of their secretion by different factors - such as feedback loops related to other neurohormones - may provide a possible explanation32.
Given that ASD’s amenable to closure are closed soon after the diagnosis, and neurohormonal activation probably abates in months following the closure, present results have little relevance to guide the treatment decisions in ASD patients. However, these patients are subjected to the potentially harmful effects of several neurohormones with profibrotic actions - such as aldosterone - until the defects are closed, which may explain why these patients remain at lifelong risk for atrial fibrillation and heart failure even after defect closure. The applicability of this data to the two other subgroups, namely patients with severe pulmonary hypertension precluding closure and those with defects that are too small to cause hemodynamic effects, is limited as the pattern of neurohormonal activation may differ in the presence of pulmonary hypertension or very small defects. Based on the present data, it can be expected that both noradrenaline and endothelin-1 concentrations would be closer to normal in those with a smaller defect as there was a relatively strong negative correlation between defect size and the concentration of both neurohormones. For other neurohormones, particularly for aldosterone and antidiuretic hormone, uncertainty is greater as these did not show a correlation (either linear or non-linear) with ASD area. Given that aldosterone is involved in the pathophysiology of both heart failure and atrial fibrillation, an increased aldosterone concentration may explain the relatively unexpected increase in the incidence of both conditions in patients with unrepaired restrictive defects33,34. These patients may theoretically benefit from aldosterone antagonism provided that they would have a similar pattern of neurohormonal activation, but this assumption needs to be validated in future studies.
As the structural alterations caused by shunt lesions accumulate over time, it can be expected that elder patients would have a different neurohormonal activation pattern as compared to those diagnosed at a younger age. We did not find any evidence for a correlation between age (or age - defect area product) with baseline neurohormone concentrations or with the changes in neurohormones following closure (details not provided in the results section), but the present study sample was too small to make dedicated analyses on different subgroups. However, it is possible that a neurohormonal pattern persisting over lifetime (i.e. continuously high aldosterone concentration) could cause structural alterations without any changes in the pattern itself. To note, present study included patients amenable for defect closure with excluding those with established pulmonary hypertension, who may have a neurohormonal pattern changing through disease course (i.e. an increasing ET-1 causing pulmonary vascular disease) as suggested by others25,26,27.
Present study had several limitations that should be mentioned. Although the patients were recruited from multiple centers, the sample size was small due to limitations caused by restrictions in funding and resources. As multiple neurohormones were studied, it would not be practical to perform a power analysis prior to the study as a different sample size would be required for each parameter studied. Thus, a limited sample size increases the risk of both Type I and Type II statistical errors. Strengths of evidence calculated using Bayesian tests suggested that a larger study would be unlikely to give a different result for most neurohormones. For other neurohormones, particularly for renin, evidence favoring a difference was not particularly strong and different results might be obtained in larger studies. As none of the centers perform routine cardiac catheterization prior to defect closure in patients and all patients were deemed eligible for closure based on noninvasive imaging, hemodynamic data could not be obtained. Although present work provides insights for possible patterns of neurohormonal activation in patients undergoing ASD closure, it provides little actionable data as these patients were already scheduled for definitive treatment. Data from other groups, such as those with so-called restrictive ASDs or those with significant pulmonary vascular disease, knowledge on the pattern of neurohormonal activation could be more valuable to guide patients management. Repeat measurements were obtained soon after the closure to avoid missing follow-up data, and although present trends provide clues on how ASD closure may affect neurohormonal activation, they do not provide definitive evidence for a complete normalization. Data from others suggest that most biomarkers, including endothelin-1, reverts back to normal months after defect closure, but more data is needed to ascertain whether all neurohormonal activity subsides after defect closure11,14.
Conclusions
Present findings suggest the presence of neurohormonal activation in patients with ASDs scheduled for defect closure, but the pattern of this activation deviated significantly from what is observed in patients with heart failure. Lifelong exposure to some neurohormones with detrimental effects on myocardium, such as aldosterone, may explain why these patients remain at risk for atrial fibrillation or heart failure even after closure of the defect. Other findings, such as a decrease in endothelin-1 concentration in ASD patients - may explain the increased compliance of the pulmonary vascular bed in these patients until true pulmonary vascular disease develops. However, more data is needed to understand whether the present pattern of neurohormonal activation extends to patients with smaller ASDs or those with significant pulmonary vascular disease, or whether pharmacologic interventions to alter this neurohormonal activation would be clinically useful to prevent long term cardiovascular adverse events - particularly atrial fibrillation and heart failure - linked to ASDs.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Lindsey, J. B. & Hillis, L. D. Clinical update: Atrial septal defect in adults. Lancet 369, 1244–1246 (2007).
Hopkins, R. A., Bert, A. A., Buchholz, B., Guarino, K. & Meyers, M. Surgical patch closure of atrial septal defects. Ann. Thorac. Surg. 77, 2144–2149 (2004).
Butera, G. et al. Percutaneous versus surgical closure of secundum atrial septal defects: A systematic review and meta-analysis of currently available clinical evidence. EuroIntervention 7, 377–385 (2011).
Murphy, J. G. et al. Long-term outcome after surgical repair of isolated atrial septal defect. Follow-up at 27 to 32 years. N. Engl. J. Med. 323, 1645–1650 (1990).
Attie, F. et al. Surgical treatment for secundum atrial septal defects in patients >40 years old. A randomized clinical trial. J. Am. Coll. Cardiol. 38, 2035–2042 (2001).
Nyboe, C., Olsen, M. S., Nielsen-Kudsk, J. E. & Hjortdal, V. E. Atrial fibrillation and stroke in adult patients with atrial septal defect and the long-term effect of closure. Heart 101, 706–711 (2015).
Nyboe, C., Karunanithi, Z., Nielsen-Kudsk, J. E. & Hjortdal, V. E. Long-term mortality in patients with atrial septal defect: A nationwide cohort-study. Eur. Heart J. 39, 993–998 (2018).
Eriksson, S. V., Eneroth, P., Kjekshus, J., Offstad, J. & Swedberg, K. Neuroendocrine activation in relation to left ventricular function in chronic severe congestive heart failure: A subgroup analysis from the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Clin. Cardiol. 17, 603–606 (1994).
Hartupee, J. & Mann, D. L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat. Rev. Cardiol. 14, 30–38 (2017).
Long, R. T. The total blood volume in patients with left-to-right cardiac shunts. Ann. Surg. 160, 897–900 (1964).
Weber, M. et al. Left ventricular adaptation after atrial septal defect closure assessed by increased concentrations of N-terminal pro-brain natriuretic peptide and cardiac magnetic resonance imaging in adult patients. Heart 92, 671–675 (2006).
Heng, E. L. et al. Neurohormonal activation and its relation to outcomes late after repair of tetralogy of Fallot. Heart 101, 447–454 (2015).
Bolger, A. P. et al. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation 106, 92–99 (2002).
Geenen, L. W. et al. Evolution of blood biomarker levels following percutaneous atrial septal defect closure in adults. Int. J. Cardiol. Heart Vasc. 30, 100582. https://doi.org/10.1016/j.ijcha.2020.100582 (2020).
Rudski, L. G. et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 23, 685–713 (2010).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28, 1–39 (2015).
Sanders, S. P., Yeager, S. & Williams, R. G. Measurement of systemic and pulmonary blood flow and QP/QS ratio using Doppler and two-dimensional echocardiography. Am. J. Cardiol. 51, 952–956 (1983).
Schrier, R. W. & Abraham, W. T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 341, 577–585 (1999).
Mentz, R. J. et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart Fail. 3, 97–107 (2015).
Schweda, F. & Kurtz, A. Regulation of renin release by local and systemic factors. Rev. Physiol. Biochem. Pharmacol. 161, 1–44 (2011).
Mulrow, P. J. Angiotensin II and aldosterone regulation. Regul. Pept. 80, 27–32 (1999).
Quinn, S. J. & Williams, G. H. Regulation of aldosterone secretion. Annu. Rev. Physiol. 50, 409–426 (1988).
D’Alto, M. & Mahadevan, V. S. Pulmonary arterial hypertension associated with congenital heart disease. Eur. Respir. Rev. 21, 328–337 (2012).
Galié, N., Manes, A. & Branzi, A. The endothelin system in pulmonary arterial hypertension. Cardiovasc. Res. 61, 227–237 (2004).
Komar, M. et al. Elevated level of plasma endothelin-1 in patients with atrial septal defect. Cardiovasc. Ultrasound. 12, 31. https://doi.org/10.1186/1476-7120-12-31 (2014).
Dinarti, L. K. et al. Profile of endothelin-1, nitric oxide, and prostacyclin levels in pulmonary arterial hypertension related to uncorrected atrial septal defect: Results from a single center study in Indonesia. Cardiol. Res. Pract. 2020, 7526508. https://doi.org/10.1155/2020/7526508 (2020).
Yoshibayashi, M. et al. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects. Evidence for increased production of endothelin in pulmonary circulation. Circulation 84, 2280–2285 (1991).
Kulik, T. J. Pulmonary blood flow and pulmonary hypertension: Is the pulmonary circulation flowophobic or flowophilic?. Pulm Circ. 2, 327–339 (2012).
Gorenflo, M. et al. Plasma endothelin-1 in patients with left-to-right shunt. Am. Heart J. 130, 537–542 (1995).
Velez-Roa, S. et al. Increased sympathetic nerve activity in pulmonary artery hypertension. Circulation 110, 1308–1312 (2004).
Zannad, F. Aldosterone and heart failure. Eur. Heart J. 16, 98–102 (1995).
Triebel, H. & Castrop, H. The renin angiotensin aldosterone system. Pflugers Arch. 476, 705–713 (2024).
Reil, J. C. et al. Aldosterone promotes atrial fibrillation. Eur. Heart J. 33, 2098–2108 (2012).
Iravanian, S. & Dudley, S. C. Jr. The renin-angiotensin-aldosterone system (RAAS) and cardiac arrhythmias. Heart Rhythm. 5, S12–S17 (2008).
Funding
This study was funded by a research grant from Istanbul Okan University (Document No: 24.02.2022/5).
Author information
Authors and Affiliations
Contributions
RÇG, SKA, TSG, MBÇ and MÇ designed the study, RÇG, FBÇ, EPO, AAA, HG, NÖ and MÇ collected data, SKA performed the laboratory analyses, TSG performed data curation and statistical analyses, RÇG, TSG, FBÇ, EPO, AAA, HG wrote the manuscript, NÖ, MBÇ and MÇ supervised the project and provided critical preview of the draft, and all authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Çetin Güvenç, R., Koç Ada, S., Güvenç, T.S. et al. Neurohormonal activation pattern in patients with atrial septal defect. Sci Rep 14, 29980 (2024). https://doi.org/10.1038/s41598-024-78950-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-78950-x
Keywords
This article is cited by
-
Defining the Molecular Landscape of Cardiac Injury in Congenital Heart Disease: A Comprehensive Review of the Current Literature
Current Treatment Options in Cardiovascular Medicine (2025)