Abstract
Heat shock protein 90 (Hsp90), a molecular chaperone, contributes to the preservation of folding, structure, stability, and function proteins. In this study, novel compounds comprising isoxazole structure were designed, synthesized and their potential ability as Hsp90 inhibitors was validated through docking studies. The active site-based compounds were prepared through a multi-step synthesis process and their chemical structures were characterized employing FT-IR, NMR, and mass spectrometry analysis. Cytotoxic and Hsp90 inhibition activities of synthesized compounds were assessed by MTT assay and ELISA kit, respectively. Based on the obtained results, compound 5 exhibited the highest cytotoxicity (IC50; 14 µM) against cancer cells and reduced Hsp90 expression from 5.54 ng/mL in untreated (normal cells) to 1.56 ng/mL in cancer cells. Moreover, molecular dynamics (MD) simulation results indicated its high affinity to target protein and approved its excellent stability which is essential for exerting an inhibitory effect on cancer cell proliferation.
Similar content being viewed by others
Introduction
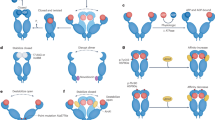
Heat shock proteins (Hsps) function as molecular chaperones, facilitating the proper folding and functionality of proteins within biological organisms1,2,3,4,5. Based on their molecular weights, Hsps are primarily classified into two categories: small Hsps and large Hsps, which encompass Hsp40, Hsp60, Hsp70, Hsp90, and Hsp1106. Hsp90 plays a critical role in maintaining proteostasis under both normal physiological conditions and during various stressors, including hypoxia, elevated temperatures, and exposure to radiation7,8,9. The Hsp90 chaperone system is integral to the folding and activation of numerous signaling proteins10 and is also involved in the degradation of misfolded proteins11. The basic structure of Hsp90 has been illustrated in Fig. 112,13,14.
In neoplastic cells, the expression levels of Hsp90 are observed to range from 4 to 6%, which is significantly elevated compared to the expression rates of 1–2% typically found in non-cancerous cells15. This notable overexpression presents a promising target for oncological therapies utilizing Hsp90 inhibitors16. N-terminal Hsp90 inhibitors are categorized into natural and synthetic compounds with promising therapeutic results in preclinical or clinical studies17,18,19. The first generation of natural Hsp90 inhibitors includes Geldanamycin (GM) and its analogues (17-AAG and 17-DMAG), Novobiocin, and Radicicol20,21. Moreover, several synthesized compounds act as Hsp90 inhibitors22; these organic compounds include PU3, pyrazole, indazole, aminoquinoline, triazine, and isoxazole scaffolds23,24,25,26,27.
Figure 2 depicts the chemical structures of several potent synthetic and natural inhibitors of Hsp90 that are presently undergoing evaluation in clinical trials to assess their therapeutic efficacy. Among these, isoxazole-based organic compounds are known as promising potential Hsp90 inhibitors such as Luminespib, named also as VER52296 or NVP-AUY922. This compound has an IC50 of approximately 21 nM and a Kd of 1.7 nM. Its impressive potency has led to its being valued in a phase II clinical trial28,29. Inspired by the therapeutic potential of isoxazole-based organic molecules30,31,32,33, and our previous successful experiences34,35, a new series of 3, 4-disubstituted isoxazole scaffolds were designed and synthesized through several steps. The efficacy of the synthesized molecules was evaluated by employing the MTT assay and Hsp90 molecular chaperone inhibition activity. MD simulations were also applied to assess the stability of the optimal combination within the active site of the target protein.
Experimental
Computational studies
Molecular docking
Molecular docking studies were conducted utilizing AutoDock 4.2 to evaluate the binding interactions of 3, 4-disubstituted isoxazole derivatives as inhibitors of Hsp90 in this research36. The protein structure was obtained from the Protein Data Bank (PDB: 3OWD)37. Prior to analysis, the structure was prepared by removing all water molecules, ions, and ligands, with the exception of those water molecules essential for facilitating protein–ligand interactions. Atomic charges and hydrogens were added and computed using the Kollman process. The prepared file was saved in the pdbqt format. A grid box size of 90 × 90 × 90 Å, centered on the protein binding pocket, was employed with a grid spacing of 0.375 nm. The Gasteiger-Marsili procedure was applied to calculate the partial atomic charges of the ligands. This calculation is significant as partial charges play a critical role in molecular binding by determining the macromolecule and ligand’s interactions38.
The Lamarckian evolution was performed through 100 runs, initiating with a populace of 150 and setting the maximum number of energy evaluations to 25 × 105. Furthermore, parameters related to water molecules were incorporated into the AD4-parameter and AD4-bound files. The 3D ligands’ structures were sketched using the Marvin Sketch v5.7 software and saved in pdb format. All runs were classified on the basis of the lowest binding energy, and an in-depth analysis was conducted to identify the optimal conformation and ligand orientation within the protein’s active site. The docking process was scrutinized and visualized using both Pymol version 1 and Discovery Studio Visualizer version 17.239,40.
Molecular dynamics simulation
In this investigation, the most favorable docking structure of the Hsp90 complex with the ligand was chosen as the starting point for MD simulations. The GROMACS-2021.5 package41 was utilized for the MD simulations. The protein topology parameters, which dictate the connectivity and interaction potentials of the atoms in the protein, were assigned using the Amber99sb force field42. Additionally, the ACPYPE (AnteChamber Python Parser Interface)43 was utilized to determine the topology parameters of the ligand, allowing for accurate representation of its interactions with the protein. To replicate physiological conditions, the Hsp90-ligand complex was placed in a dodecahedron box contains TIP3P water molecules. Counter ions (Na+ or Cl−) were introduced as needed to ensure overall charge neutrality, crucial for maintaining simulation stability. After energy minimization, a two-step approach was implemented to ensure the accuracy and reliability of the MD simulation. The initial phase involved equilibrating the system under NVT conditions, ensuring a constant temperature of 300 K, a constant pressure of 1 bar, and a constant number of particles. This initial simulation was carried out for duration of 500 ps. In the second step, the system was optimized under NPT conditions for 500 ps. Following system stabilization, the primary simulation was conducted at a temperature of 300 K over a time span of 100 ns. After the simulation, an analysis of the results encompassing RMSF (root mean square fluctuations), RMSD (root mean square deviation), and Rg (radius of gyration) was undertaken and the findings were reported.
Synthetic procedure
Materials and methods
All chemical compounds used in the experiment were obtained from Sigma-Aldrich and/or Merck companies and used without more purification as they were already of high quality and met the requirements of the experiment. TLC (thin layer chromatography) was employed to verify the accuracy and purity of the compounds throughout the experiment. An Electrothermal 9200 melting point device was used to determine the compounds’ melting points. Infrared spectra, using JASCO FT-IR, were captured in KBr disks, allowing for the analysis of the compounds’ molecular structure and identification of their functional groups. The compounds were subjected to analysis via 1H NMR and 13C NMR spectroscopy, conducted using a Bruker Ultrashield spectrometer operating at 400 MHz and 125 MHz, respectively, with CDCl3 and DMSO-d6 employed as solvents. Mass spectra were acquired utilizing a Shimadzu QP2010 S-type spectrometer (Japan), employing electron impact (EI) method for ionization.
Synthetic procedures for E/Z-2, 4-dimethoxybenzaldehydeoxime (B)
A mixture of 2, 4-dimethoxy benzaldehyde (A; 1.0 mmol, 0.166 g), sodium hydroxyl amine (1.8 mmol, 0.125 g), and pyridine (0.45 mL) was stirred at room temperature in a 50 mL flask. Rotary vacuum evaporation was used to remove the ethanol when the reaction was completed, which was observed by TLC. To help the mixture form aldoxime crystals, 10 mL of distilled water was added while it was in the ice bath44. This resulted in an off-white solid with an 85% yield. m.p.:103–105 °C. FT-IR (KBr, cm− 1): 3251, 1610, 1591, 1110, 956, 839. 1HNMR (400 MHz, CDCl3, δ, ppm): δ 9.16 (s, 1H), 8.40 (s, 1H), 7.58 (d, J = 8.5 Hz, 1H), 6.52 (m, 2 H), 3.86 (d, J = 5.8 Hz, 6 H). 13CNMR (125 MHz, DMSO): δ 162.17, 158.58, 143.70, 126.90, 114.18, 106.54, 98.78, 56.18, 55.81. Mass ESI (+) m/z, 182 [M+].
Synthetic procedures for N-hydroxy-2, 4-dimethoxybenzimidoyl chloride (C)
2, 4-dimethoxybenzaldehydeoxime (B; 1 mmol, 0.181 g), and NCS (2.5 mmol, 0.334 g) in DMF (5 mL) were stirred at room temperature for 24 h. The reaction progress was checked by TLC. The mixture was neutralized using crushed ice, filtrated, and washed44. The obtained product (75% yield), a white solid, was then characterized. m.p.: 123–125 °C. FT-IR (KBr, cm− 1): 3400, 3241, 2291, 1606, 1207. 1HNMR (400 MHz, CDCl3, δ, ppm): δ 9.05 (s, 1H), 7.59 (s, 1H), 6.55 (s, 2 H), 3.97 (s, 6 H). 13CNMR (125 MHz, DMSO): δ 158.82, 158.51, 131.34, 117.06, 107.29, 98.40, 57.30, 57.09. Mass ESI (+) m/z, 216 [M+].
Synthetic procedures for 5-amino-(2, 4-dimethoxyphenyl) isoxazole-4-carbonitrile (1)
Malonitrile (1 mmol, 0.066 g) was added into a sodium methoxide solution (2 mmol, 0.108 g) in ethanol (3 mL) at 0 °C for 20 min, stirred for 30 min at room temperature. Then, N-hydroxy-2, 4-dimethoxybenzimidoyl chloride (C; 1 mmol, 0.215 g) was added and stirred for 24 h. The progression of the reaction was monitored by TLC. H2O/ice was used to neutralize, and after stirring for 5 min, an orange solid was yielded which was characterized by filtering, washing, and drying45. The obtained white solid with a yield of 64%. m. p.: 160–162 °C. FT-IR (KBr, cm − 1):3384, 2934, 2228, 1625, 1211. 1HNMR (400 MHz, DMSO, δ, ppm): δ 8.42 (s, 2 H), 7.43 (s, 1H), 6.94 (s, 2 H), 3.98 (s, 3 H), 3.91 (s, 3 H). 13CNMR (125 MHz, DMSO): δ 173.40, 159.94, 157.85, 157.81, 131.35, 130.44, 113.70, 112.86, 109.11, 98.58, 57.04, 56.84. Mass ESI (+) m/z, 246 [M].
Synthetic procedures for N-(4-cyano-3(2, 4-dimethoxyphenyl) isoxazol-5-yl) Arylamide (2–8)
5-Amino-(2, 4-dimethoxyphenyl) isoxazole-4-carbonitrile (1; 1 mmol, 0.185 g), triethylamine (2 mmol, 0.275 mL) was dissolved in THF. Derivative of Benzoyl chloride (1 mmol) in THF (1 mL) was gradually added dropwise and stirred overnight. After filtration, methanol was added to induce precipitate formation, which was recrystallized using hexane–EtOAc (1:1)46. Synthesized compounds were characterized using FT-IR, 1HNMR, 13CNMR and Mass spectroscopy.
Biology
Cytotoxic activity assay
The cytotoxicity of the synthesized compounds was evaluated through MTT assay43. The cell lines utilized in this study were obtained from the Pasteur Institute of Iran (Tehran, Iran). HeLa cells were maintained in DMEM, while Human Umbilical Vein Endothelial Cells (HUVECs) and MCF-7 breast cancer cells were cultured in RPMI 1640 medium. Both media were supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin, and incubated at 37 °C in a humidified atmosphere containing 5% CO2. For the experimental setup, cancer cells were plated in a 96-well plate at a density of 5,000 cells per well. After a 24-hour incubation period to allow for cell adherence, the cells were treated with varying concentrations of the synthesized compounds (0.1, 1, 10, and 100 µM). Stock solutions (10 mM) of the compounds were prepared in DMSO and subsequently diluted with the respective culture medium to achieve the desired concentrations. Following treatment, cells were incubated for an additional 72 h. Doxorubicin was employed as a positive control for comparison. To assess cell viability, 20 µL of MTT solution (5 mg/mL) was added to each well and incubated for an additional 3 h. The resulting formazan crystals were solubilized by adding 150 µL of DMSO to each well. Absorbance was measured using an ELISA plate reader. The percentage of cell survival was calculated using the following equation:
% cell survival = [(Abs of sample - Abs of blank)/(Abs of control- Abs of blank)] × 100.
Hsp90 protein level assay
The impact of cytotoxic substances on the expression level of Hsp90 was assessed using the Hsp90 expression level kit (Cusabio, USA), following the instructions provided by the manufacturer. Standards and samples were introduced into the well and placed in an incubator at a temperature of 37 °C for duration of 2 h. Following incubation, the supernatant was replaced with 100 µL of biotin antibody and incubated for a further 1 h at 37 °C. The solution from each well was then removed and washed three times with the kit wash solution (3 × 400 µL). In the next step, 100 µL of HRP-avidin (horseradish peroxidase Avidin) was added to the well and incubated for 1 h at 37 °C, followed by the washing procedure described above. Next, 90 µL of TMB (3, 3’, 5, 5’-Tetramethylbenzidine) medium was introduced into the well and allowed to incubate for 15–30 min at a temperature of 37 °C. After adding the stop solution, the sample absorbance was measured with an ELISA plate reader at 450 nm. The detailed approaches of these tests have also been presented in the supplemental data file.
Results and discussion
Molecular docking study
Based on our previous research studies47, focusing on the effective and essential pharmacophore functional groups, numerous 3, 4-disubstituted isoxazole heterocyclic compounds have been designed. The interaction of proposed structures in Hsp90 protein space was investigated by docking studies as the most popular way to predict their activities. For this purpose, docking interaction of Hsp90 protein and designed ligands were conducted. The chemical structures of most interacted designed ligands have been depicted in Fig. 3 and their docking outcomes are presented in Table 1. Through docking results, selected compounds were positioned in the active site with suitable corresponding binding energies within the hydrophobic parts, Ala55, Met98, Ile96, Phe138, Leu107, and Val150, along with structural water molecules, were identified as key residues in the Hsp90 protein pocket. In addition, within the hydrophilic part, Asp93, Asn51, and Thr184 played significant roles.
All compounds were considered by the SwissADME web server for their physicochemical properties48. The calculated physicochemical properties, including molecular weight, log P, and quantities of H-bond donors and H-bond acceptors were documented in Table 2, adhering to Lipinski’s Rule of Five. The hydrogen bonds of compound 1 with Hsp90 amino acids of Asp93 and Thr184, pi-alkyl interactions of the ligand with Leu48, Met98, Phe138, and Val186, and formation of pi sigma interaction between Ala55 and the oxygen atom of isoxazole ring have been depicted in Table 3. The carboxamide group of compound 4 acts as adenine mimics due to the creation of hydrogen bonds with Leu48, Asn51, and Asp93 and pi interaction with Met9849,50. Two methoxy groups of the dimethoxyphenylisoxazole moiety form hydrogen bonds with Asn106, Ile110, Lys112, and Tyr139 and form Pi alkyls with Ile26, Leu110, and Lys112, and isoxazole’ oxygen forms an alkyl interaction with Leu107. The carboxamide group of compound 5 establishes hydrogen bonds with the Hsp90 amino acids Asp93 and Asn51 and it engages in pi interactions with Ala55 and Met98. The two methoxy groups of the dimethoxyphenylisoxazole moiety form carbon-hydrogen bonds with Ile110, Asn106, and Tyr139 and interactions with Ile26, Ile110, and Lys112 and isoxazole’ oxygen form pi sigma with Leu107. 2D and 3D analyses of the interaction of other new inhibitors are displayed in the supplementary data file.
Chemistry
Encouraged by molecular docking results, eight new heterocyclic compounds were synthesized in several steps through novel economic synthesis strategies. The synthesis process of 3, 4-disubstituted isoxazole compounds has been illustrated in Scheme 1. In the first step, 2, 4-dimethoxybenzaldehydeoxime (B) was synthesized via condensation of 2, 4-dimethoxybenzaldehyde (A) and sodium hydroxylamine with ethanol as solvent and pyridine as catalyst. Compound (B) on further reaction with solution of DMF and NCS afforded N-hydroxy-2, 4-dimethoxybenzimidoyl chloride (C) via γ-elimination, it finally converted to its nitrile oxide form44. Later, compound (C) and malonitrile in the presence of sodium methoxide in ethanol afforded 5-amino-(2, 4-dimethoxyphenyl)-isoxazole-4-carbonitrile (1) via 1, 3-dipolar cycloadditions45. Compound (1) was N-substituted by benzoyl chloride in the presence of Et3N and THF to give the corresponding derivatives (2–8)46. The structure of the synthesized compounds was verified through both physical and spectroscopic analyses. Comprehensive characterization data of the products are given in the supplementary data.
Cytotoxic activity
MTT assay
MTT assay was applied to investigate the cytotoxicity of prepared compounds against cancer cell lines of HeLa and MCF-7 and normal cell line of HUVEC. IC50 and R² values were calculated based on the obtained results. Herein, doxorubicin was selected as positive control. The Tanespimycin (17-AAG, InvivoGen, USA) compound was acquired for use as a reference standard. The cancer cytotoxicity of most tested compounds (≥ 10 µM) was approved. MCF-7 and HeLa cells are overexpressed Hsp90 and used as negative control models for anti-Hsp90 agent activities. Consequently, the MCF-7 and HeLa cell lines, which exhibit overexpression of Hsp90, provided an appropriate model for investigating the cytotoxic effects of these compounds51,52.
The compounds 5, 3, 1, 4, and 7 displayed cytotoxicity against HeLa cells, with IC50 values of 2.06, 3.61, 7.91, and 19.41 µM, respectively, as illustrated in Fig. 4a. Compounds such as 8, 6 and 2 displayed moderate activity level at the concentrations of 31.92, 75.77 and > 100 µM, respectively. The cytotoxicity of the synthesized compounds against the MCF-7 cancer cell line is illustrated in Fig. 4b. As shown in this figure, all tested derivatives were cytotoxic against MCF-7 cells at a concentration of ≥ 100µM except for compounds 1 and 5, which showed IC50 values of 0.31 and 14.74, respectively. Similar to HeLa cells, compounds 2 and 6 showed the lowest cytotoxic activity against MCF-7 with IC50 of ≥ 100µM (p < 0.05). Compounds including 4, 8, 3 and 7 exhibited moderate activity level at the concentrations of 50.60, 54.12, 68.30 and 70.01 µM, respectively. 17-AAG was cytotoxic against MCF-7 cells with IC50 values of 0.1 µM. The cytotoxic activity of the synthesized compounds was tested against HUVEC as a normal cell line. The results are shown in Fig. 4c, in which most of the compounds were not cytotoxic against this cell line; on the other hand, most IC50 values were > 100 µM. Overall, compounds 1, 4, and 5 demonstrated concentration-dependent cytotoxic effects on both HeLa and MCF-7 cell lines. Compounds 2, 6, and 8 showed mild cytotoxic effects against the two experienced cancer cell lines at the highest concentrations. Consequently, compounds 1, 4, and 5 have been recognized as promising isoxazolyl derivatives for further investigation.
Hsp90 protein level assay
We used the ELISA kit to detect the intracellular protein levels of Hsp90 in order to look into whether the synthesized compounds would reduce Hsp90 protein levels. The most cytotoxic substances (1, 4, and 5) were employed for this purpose, and the level of Hsp90 protein in cancer cells was measured and compared with untreated cells. The outcomes showed that the protein levels in the cells after treatments with substances 1, 4, and 5 were 4.85, 2.81, and 1.56 ng/mL, respectively; whereas protein level in control cells was detected as 5.54 ng/mL (Fig. 5). According to Fig. 5, compound 5 were the most effective among the synthesized derivatives, showing promising activity at a concentration of 10 µM in MCF-7 cells. Therefore, the potential exhibited by compound 5 introduces it as a valuable candidate for further refinement and advancement in the development of Hsp90 inhibitors.
Molecular dynamics simulation
After undergoing a 100-ns simulation, an analysis of the time-dependent behavior of RMSD, RMSF, and Rg was conducted to evaluate the stability of Hsp90 throughout the simulation period. The RMSD of the protein in complex with compounds 1, 4, and 5 was evaluated. Figure 6a illustrates the RMSD diagram of the protein backbone complexed with compounds 1, 4, and 5. As observed, when compound 5 was complexed with the protein, the RMSD changes during 100 ns was observed less than 0.1 nm, indicating the stability of compound 5 in the active site of the protein. The RMSD changes in compound 4 after the first 30 ns are similar to those of compound 5, approximately 0.1 nm. The deviation of the RMSD diagram during 100 ns for compound 1 is close to 0.2 nm.
Figure 6b displays RMSF results, where the lowest points represent the most stable amino acids during the simulation. The amino acids located within the protein’s active site play a pivotal role in its interactions, and ideally, stability in these residues is essential. Notably, these crucial amino acids encompass Asn51, Met98, Ala55, Asp93, Leu107, Phe138, Asn106, and Thr184. It is evident that when compound 5 is complexed with the protein, the amino acids are in the most stable state. In interaction of compound 4, amino acids 62–75 exhibited significant instability, even though these amino acids are not part of the enzyme’s active site.
The Rg plot is revealed in Fig. 6c. The Rg values for compounds 4 and 5 over 100 ns are nearly identical. However, when compound 1 is situated in the active site of the protein, it exhibits more fluctuation. Overally, the simulation suggests that compound 5 is better positioned in the active site than the other compounds.
Conclusions
In this research, a novel series of 3, 4-disubstituted isoxazole compounds was designed and synthesized as potential Hsp90 inhibitors. Molecular docking investigations were employed to assess and predict the binding capabilities of these isoxazole structures within the Hsp90 pocket. The findings demonstrated that Thr184, Asn51, Asp93, Asp58, Met98, and Val150 were among the amino acid residues in the Hsp90 pocket that the aforementioned compounds effectively interacted with. The MTT assay was employed to investigate the cytotoxicity of the compounds, and a thorough screening process was conducted against various cell lines, including MCF-7, HeLa, and normal HUVEC cell lines. The most effective synthesized molecule, which had three fluoromethyl and fluoro pharmacophore groups, showed a strong affinity for Hsp90 and caused its expression level to drop to 1.56 ng/mL. Furthermore, with an average IC50 of roughly 14 µM, it successfully stopped the growth of several human cancer cell lines (HeLa, MCF-7). In addition, in MD simulations, these molecules showed stability in the Hsp90 protein’s ATPase active site. Collectively, these results introduced a new heterocyclic compound as a promising candidate for further studies in anticancer agents which was synthesized through green and facile synthesis approaches. Further studies on comprehensive assessments of its safety and efficacy may be undertaken.
Data availability
All relevant data are within the manuscript and its additional files. The data are available from the corresponding author on reasonable request.
References
Whitesell, L. & Lindquist, S. L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 5, 761–772. https://doi.org/10.1038/nrc1716 (2005).
Trepel, J., Mollapour, M., Giaccone, G. & Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10, 537–549. https://doi.org/10.1038/nrc2887 (2010).
Neckers, L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 8, S61. https://doi.org/10.1016/S1471-4914(02)02316-X (2002).
Liu, J., Shu, H., Xia, Q., You, Q. & Wang, L. Recent developments of HSP90 inhibitors: an updated patent review (2020-present). Expert Opin. Ther. Pat. 34, 1–15. https://doi.org/10.1080/13543776.2024.2327295 (2024).
Gupta, S. D., Bommaka, M. K. & Banerjee, A. Inhibiting protein-protein interactions of Hsp90 as a novel approach for targeting cancer. Eur. J. Med. Chem. 178, 48–63. https://doi.org/10.1016/j.ejmech.2019.05.073 (2019).
Hoter, A., El-Sabban, M. E. & Naim, H. Y. The Hsp90 family: structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 19, 2560. https://doi.org/10.3390/ijms19092560 (2018).
Whitesell, L., Bagatell, R. & Falsey, R. The stress response: implications for the clinical development of hsp90 inhibitors. Curr. Cancer Drug Targets. 3, 349–358. https://doi.org/10.2174/1568009033481787 (2003).
Hsieh, Y. Y., Hung, P. H. & Leu, J. Y. Hsp90 regulates nongenetic variation in response to environmental stress. Mol. Cell 50, 82–92. https://doi.org/10.1016/j.molcel.2013.01.026 (2013).
Jarosz, D. F. & Lindquist, S. Hsp90 and environmental stress transform the adaptive value of natural genetic variation. Science 330, 1820–1824. https://doi.org/10.1126/science.1195487 (2010).
Zhang, H. & Burrows, F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J. Mol. Med. 82, 488–499. https://doi.org/10.1007/s00109-004-0549-9 (2004).
Eisele, F. et al. An Hsp90 co-chaperone links protein folding and degradation and is part of a conserved protein quality control. Cell Rep. 35 https://doi.org/10.1016/j.celrep.2021.109328 (2021).
Ardestani, M. et al. Heterocyclic compounds as Hsp90 inhibitors: a perspective on Anticancer Applications. Pharmaceutics 14, 2220. https://doi.org/10.3390/pharmaceutics14102220 (2022).
Garg, G., Khandelwal, A. & Blagg, B. S. Anticancer inhibitors of Hsp90 function: beyond the usual suspects. Adv. Cancer Res. 129, 51–88. https://doi.org/10.1016/bs.acr.2015.12.001 (2016).
Jackson, S. E. Hsp90: structure and function. Top. Curr. Chem. 155–240. https://doi.org/10.1007/128_2012_356 (2013).
Dernovšek, J. & Tomašič, T. Following the design path of isoform-selective Hsp90 inhibitors: small differences, great opportunities. Pharmacol. Ther. 108396. https://doi.org/10.1016/j.pharmthera.2023.108396 (2023).
Zagouri, F., Bournakis, E., Koutsoukos, K. & Papadimitriou, C. A. Heat shock protein 90 (Hsp90) expression and breast cancer. Pharm 5 (9), 1008–1020. https://doi.org/10.3390/ph5091008 (2012).
Almehmadi, S. J. et al. Novel tropane analogues as Hsp90 inhibitors targeting colon cancer: synthesis, biological estimation, and molecular docking study. Bioorg. Chem. 4, 107497. https://doi.org/10.1016/j.bioorg.2024.107497 (2024).
Li, L., Chen, N. N., You, Q. D. & Xu, X. L. An updated patent review of anticancer Hsp90 inhibitors (2013-present). Expert Opin. Ther. Pat. 31, 67–80. https://doi.org/10.1080/13543776.2021.1829595 (2021).
Nitzsche, B., Höpfner, M. & Biersack, B. Synthetic small molecule modulators of Hsp70 and Hsp40 chaperones as promising anticancer agents. Int. J. Mol. Sci. 24, 4083. https://doi.org/10.3390/ijms24044083 (2023).
Liew, H. Y., Tan, X. Y., Chan, H. H., Khaw, K. Y. & Ong, Y. S. Natural HSP90 inhibitors as a potential therapeutic intervention in treating cancers: a comprehensive review. Pharmacol. Res. 106260. https://doi.org/10.1016/j.phrs.2022.106260 (2022).
Hadden, K., Lubbers, D. J. & Blagg, B. S. J. Geldanamycin, radicicol, and chimeric inhibitors of the Hsp90 N-terminal ATP binding site. Curr. Top. Med. Chem. 6, 1173–1182. https://doi.org/10.2174/156802606777812031 (2006).
Kitson, R. R. & Moody, C. J. Learning from nature: advances in geldanamycin-and radicicol-based inhibitors of Hsp90. J. Org. Chem. 78, 5117–5141. https://doi.org/10.1021/jo4002849 (2013).
Xiao, Y. & Liu, Y. Recent advances in the discovery of novel HSP90 inhibitors: an update from 2014. Curr. Drug Targets 21, 302–317. https://doi.org/10.2174/1389450120666190829162544 (2020).
Taldone, T. et al. Design, synthesis, and evaluation of small molecule Hsp90 probes. Bioorg. Med. Chem. 19, 2603–2614. https://doi.org/10.1016/j.bmc.2011.03.013 (2011).
Hadden, M. K., Hill, S. A., Davenport, J., Matts, R. L. & Blagg, B. S. J. Synthesis and evaluation of Hsp90 inhibitors that contain the 1,4-naphthoquinone scaffold. Bioorg. Med. Chem. 17, 634–640. https://doi.org/10.1016/j.bmc.2008.11.064 (2009).
Cherfaoui, B. et al. Synthesis and evaluation of 4-(2-hydroxypropyl) piperazin-1-yl) derivatives as Hsp90 inhibitors. Bioorg. Med. Chem. 24, 2423–2432. https://doi.org/10.1016/j.bmc.2016.03.049 (2016).
Wright, L. et al. Structure-activity relationships in purine-based inhibitor binding to HSP90 isoforms. Chem. Biol. 11, 775–785. https://doi.org/10.1016/j.chembiol.2004.03.033 (2004).
Brough, P. et al. 4,5-Diarylisoxazole Hsp90 chaperone inhibitors: potential therapeutic agents for the treatment of Cancer. J. Med. Chem. 51, 196–218. https://doi.org/10.1021/jm701018h (2008).
Sharp, S. Y. et al. Inhibition of the heat shock protein 90 molecular chaperone in vitro and in vivo by novel, synthetic, potent resorcinylic pyrazole/isoxazole amide analogues. Mol. Cancer Ther. 6, 1198–1211. https://doi.org/10.1021/ct200560w (2007).
Trivedi, J. et al. Discovery of 2-isoxazol-3-yl-acetamide analogues as heat shock protein 90 (HSP90) inhibitors with significant anti-HIV activity. Eur. J. Med. Chem. 183, 111699. https://doi.org/10.1016/j.ejmech.2019.111699 (2019).
Chen, D. et al. Discovery of Potent N-(Isoxazol-5-yl) amides as Hsp90 inhibitors. Eur. J. Med. Chem. 87, 765–781. https://doi.org/10.1016/j.ejmech.2014.09.065 (2014).
Bargiotti, L. et al. Isoxazolo (aza) naphthoquinones: a new class of cytotoxic Hsp90 inhibitors. Eur. J. Med. Chem. 53, 64–75. https://doi.org/10.1016/j.ejmech.2012.03.036 (2012).
Sun, J. et al. Synthesis and biological evaluation of 3,5-Disubstituted-4-Alkynylisoxazoles as a Novel Class of Hsp90 inhibitors. Bioorg. Med. Chem. Lett. 25, 3129–3134. https://doi.org/10.1016/j.bmcl.2015.06.009 (2015).
Abbasi, M., Sadeghi-Aliabadi, H. & Amanlou, M. 3D-QSAR, Molecular Docking, and molecular dynamic simulations for prediction of new Hsp90 inhibitors based on Isoxazole Scaffold. J. Biomol. Struct. Dyn. 36, 1463–1478. https://doi.org/10.1080/07391102.2017.1323655 (2018).
Abbasi, M. et al. New heat shock protein (Hsp90) inhibitors, designed by pharmacophore Modeling and virtual screening: synthesis, biological evaluation and molecular dynamics studies. J. Biomol. Struct. Dyn. 38, 3462–3473. https://doi.org/10.1080/07391102.2019.1660216 (2020).
Morris, G. M. et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30 (16), 2785–2791. https://doi.org/10.1002/jcc.21256 (2009).
Bruncko, M. et al. N-arylbenzimidazolones as novel small molecule HSP90 inhibitors. Bioorg. Med. Chem. Lett. 20 (24), 7503–7506. https://doi.org/10.1016/j.bmcl.2010.10.010 (2010).
Azizian, H., Bahrami, H., Pasalar, P. & Amanlou, M. Molecular modeling of Helicobacter pylori Arginase and the inhibitor coordination interactions. J. Mol. Graph Model. 28 (7), 626–635. https://doi.org/10.1016/j.jmgm.2009.12.007 (2010).
Makarewicz, T. & Kaźmierkiewicz, R. Molecular dynamics simulation by GROMACS using GUI Plugin for PyMOL. J. Chem. Inf. Model. 53, 1229–1234. https://doi.org/10.1021/ci400071x (2013).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 1, 19–25. https://doi.org/10.1016/j.softx.2015.06.001 (2015).
Pronk, S. et al. GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29 (7), 845–854. https://doi.org/10.1093/bioinformatics/btt055 (2013).
Cornell, W. D. et al. A second Generation Force Field for the Simulation of Proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 117 (19), 5179–5197. https://doi.org/10.1021/ja00124a002 (1995).
Abbasi, M., Amanlou, M., Aghaei, M., Hassanzadeh, F. & Sadeghi-Aliabadi, H. Identification of new Hsp90 inhibitors: structure based virtual screening, molecular dynamic simulation, synthesis and biological evaluation. Anti-Cancer Agents Med. Chem. 21 (18), 2583–2591. https://doi.org/10.2174/1871520621666210201101818 (2021).
Liu, K. C., Shelton, B. R. & Howe, R. K. A particularly convenient preparation of benzohydroximinoyl chlorides (nitrile oxide precursors). J. Org. Chem. 45 (19), 3916–3918. https://doi.org/10.1021/jo01307a039 (1980).
Gaikwad, N. B. et al. Design, synthesis, and Biological evaluation of N-(4-Substituted)-3-Phenylisoxazolo [5, 4–d] pyrimidin-4-Amine derivatives as apoptosis-inducing cytotoxic agents. Bioorg. Med. Chem. Lett. 49, 128294. https://doi.org/10.1016/j.bmcl.2021.128294 (2021).
Bousfield, T. W., Pearce, K. P., Nyamini, S. B., Angelis-Dimakis, A. & Camp, J. E. Synthesis of amides from acid chlorides and amines in the Bio-based solvent cyrene™. Green. Chem. 21 (13), 3675–3681. https://doi.org/10.1039/C9GC01180C (2019).
Abbasi, M., Sadeghi-Aliabadi, H. & Amanlou, M. Prediction of new Hsp90 inhibitors based on 3,4-Isoxazolediamide scaffold using QSAR study, molecular docking and molecular dynamic simulation. Daru J. Pharm. Sci. 25 (1), 1–16. https://doi.org/10.1186/s40199-017-0182-0 (2017).
SwissADME Accessed http://www.swissadme.ch/index.php (2023).
Janke, E. M. B. & Weisz, K. Switching binding sites: low-temperature NMR studies on Adenosine – aspartic acid interactions. J. Phys. Chem. A. 111 (48), 12136–12140. https://doi.org/10.1021/jp076429v (2007).
Jiang, H. et al. Synthesis and evaluation of the antitumor activity of 2-amino-4-tetrahydroindazole-substituted benzamide derivatives as HSP90 inhibitors. J. Mol. Struct. 1300, 137266. https://doi.org/10.1016/j.molstruc.2023.137266 (2024).
Xu, X. L. et al. CPUY201112, a Novel Synthetic small-molecule compound and inhibitor of heat shock protein Hsp90, induces p53-Mediated apoptosis in MCF-7 cells. Sci. Rep. 6 (1), 1–16. https://doi.org/10.1038/srep19004 (2016).
Du, X., Ruoran, M., Quanxin, Q., Ye, Q. & Tianfu, Y. Effects of Geldanamycin on expression of Bcl-2 in human cervical Cancer HeLa cells. Chin. Clin. Oncol. 5, 113–117. https://doi.org/10.1007/s11805-008-0113-4 (2008).
Acknowledgements
We express our gratitude to Hormozgan University of Medical Sciences, Bandar Abbas, Iran for providing computing facilities. Special thanks to Prof. Afshin Zarghi and Prof. Mehran Ghiaci for their valuable guidance.
Funding
This work was supported by the National Institute for Medical Research Development (Nimad) (Project No. 988767) and the research assistantship provided by Isfahan Pharmaceutical Sciences Research Center (Project No. 298121).
Author information
Authors and Affiliations
Contributions
F.K.: Methodology, Conceptualization, Data curation, Writing – original draft, M.A.: Software, Validation Z.K.: Writing – review & editing, Formal Analysis, M.A. Conceptualization of methodology analysis, H.S.-A: Supervision, Project management, Visualization, Formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The project was found to be in accordance to the ethical principle and national norms and standards for conducting medical research in Iran (Approval ID IR.MUI.RESEARCH.REC.1398.425 and Approval date 2019-11-02).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Keshavarzipour, F., Abbasi, M., Khorsandi, Z. et al. Design, synthesis and biological studies of new isoxazole compounds as potent Hsp90 inhibitors. Sci Rep 14, 28017 (2024). https://doi.org/10.1038/s41598-024-79051-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-79051-5