Abstract
The co-occurrence of drought and heat significantly hampers plant productivity. Although their impacts are well studied, these studies have been based on the effects of individual stressors rather than their combined influence. Okra is crucial for food and nutritional security and livelihoods in many regions, yet it remains under-researched and unimproved. Okra has been proven to be sensitive to both drought and heat stress. This study employed a cost-effective phenotyping method to assess key traits characterising the diversity of okra morphophysiological responses to independent and interactive heat-drought stresses. This study aimed to understand okra responses to stress, identify stress-resilient traits, and characterise okra genotypes. We also addressed the need to examine interactive stress effects, which mirror real-world scenarios more accurately than single-stress studies. Sixty-three okra genotypes were subjected to heat, drought, or concurrent heat-drought stress at the seedling stage in improvised climate-controlled chambers. The germplasm exhibited significant variations in response to the various stresses. The broad-sense heritability was high (> 0.60) for traits such as chlorophyll content, plant biomass, performance indices, electrolyte leakage, and total leaf area. Drought stress alone had a more pronounced effect than heat stress alone, and the adverse impact was worsened under combined heat and drought stress. The interactive impact of drought and heat was more likely additive than antagonistic or synergistic. A positive and strong relationship was observed between photosynthetic efficiency parameters such as the Fv/Fm ratio, chlorophyll content, relative water index, and biomass parameters such as dry shoot weight. The 63 okra genotypes were classified into three distinct clusters, suggesting potential for future breeding efforts. Okra genotype considered to be tolerant or climate resilient (such as GH170, V1060831, GH174, V1060874, and GH106) to drought and heat, maintained enhanced photosynthetic efficiency and high internal water potential, possibly reducing osmotic and oxidative damage. This study revealed some mechanisms underlying the adaptation of okra genotypes to independent and combined heat and drought stress. The results provide a basis for breeding efforts to develop climate-resilient okra varieties.
Similar content being viewed by others
Introduction
Okra is an important nutritional and socioeconomic fruit vegetable 1 valued for its essential nutrients and minerals (fibres, vitamins, protein, magnesium, zinc, calcium, etc.), phenolic, antimicrobial, antioxidant, and phytochemical properties 2. Economically, okra cultivation is a paramount source of income and employment in rural and peri-urban African regions 3. In many jurisdictions, okra is thus vital for attaining several Sustainable Development Goals (SDGs), particularly SDGs 1 and 2, focused on zero hunger and reduced poverty. Even so, the plant is labelled underutilised 2. A yield gap has been reported between an actual yield of 2.5 t/ha and a potential yield of 8.8 t/ha 4. The low productivity and decreased yield are attributed to biotic stresses and the complex interactions of abiotic stressors, particularly heat and drought 2. Additionally, using genetically inferior and unimproved varieties, which lack effective adaptation mechanisms under suboptimal conditions, further contributes to this problem 5.
Climate change remains a prominent contributor to the increasing trend of higher temperatures and erratic rainfall episodes 6, significantly threatening overall crop productivity and yield 7. Both heat and drought have been reported to trigger a reduction in plant growth and development and alter physiological traits 8. In most crops, including okra 9, wheat 10, and Amaranthus species 11, high temperatures and drought have been reported to reduce photosynthetic efficiency, chlorophyll content, chlorophyll inflorescence, membrane water content, stomatal conductance, increased cell damage and electrolyte leakage. As sessile organisms, plants have evolved strategies involving diverse morphological, cellular, physiological, and biochemical responses to adapt under suboptimal conditions 12. Even so, these mechanisms strongly depend on the severity of stress, plant species, genotype and developmental stage 13. Therefore, conducting a comprehensive screening for broad phenotypic and genetic diversity in the abiotic stress adaptation mechanisms within okra germplasm is crucial. This approach will facilitate rapid crop breeding, focusing on abiotic stress tolerance and increased yields.
Previous studies have explored adaptive mechanisms to heat and drought among okra genotypes 14. Ahmed and El-Sayed 15 investigated physiological traits under drought stress in crossed okra varieties, while Ayub et al. 16and Adejumo et al. 17 examined the responses of different okra cultivars to water deficit conditions, highlighting critical growth stages and physiological mechanisms involved in drought resilience. Mkhabela et al. 18 showed phenotypic responses under drought-stressed and nonstressed conditions. On the other hand, studies addressing heat stress, such as Hussain et al. 19, Hayamanesh et al. 9, and Khan et al. 20, have explored osmotic balance, antioxidant activities, and molecular screening for high-temperature tolerance emphasising the physiological and biochemical adaptations that confer heat tolerance in okra. Despite these and other studies, there is still a gap in the literature regarding the combined effects of drought and heat stress on okra.
Many studies often consider the effects of heat or drought stress alone on okra morphophysiology. However, these stressors typically occur concurrently 12. There is a significant gap in the scientific literature regarding the mechanisms and traits that enable okra germplasm to adapt to the combined stress of heat and drought. In addition to their effects, these stresses can be severely detrimental or even fatal to plants. Multiple stresses occurring in tandem may have contrasting or interactive effects that cannot be extrapolated from the impacts of individual stress 21. Therefore, plant responses to individual abiotic factors cannot fully explain the unique reactions that occur when multiple abiotic factors are combined concurrently 22.
The existence of genetic variability among genotypes in stress responses is a critical component of successful breeding for tolerance. Moreover, complex and time-consuming techniques measure many abiotic tolerance-related traits 23. The ability to screen for variation in tolerance traits using simple techniques, such as measuring relative leaf water content (as a quotient of the difference between fresh or turgid leaf disc weight and dry leaf disc weight), chlorophyll fluorescence with a portable chlorophyll meter, and electrolyte leakage assays with a conductivity meter, and in the early stages of growth, would reduce the duration of progeny trials and speed up improvement programmes of crop plants, provided these traits do not have significant interactions with ontogeny. The present study utilised a simple and adaptable phenotyping protocol to assess the responses of okra to single and combined drought and heat stress. We aimed to understand okra responses at multiple levels, identify stress-resilient traits, and characterise valuable genetic resources. This study addresses the need to examine interactive stress effects, which mirror real-world scenarios more accurately than single-stress studies. Our findings aim to improve breeding programs by integrating identified resilient traits and diverse germplasms, ultimately contributing to developing high-yielding okra varieties and resilience to climate change.
Materials and methods
Environmental conditions and genetic materials
The study was conducted from November to January 2021/2022 and was repeated from February to March 2022 at the University of Cape Coast (UCC) in Ghana. This study utilised 63 okra genotypes sourced from ten African countries. Many of these genotypes were from Ghana. The distribution of genotypes across these countries is detailed in Supplementary Table S1. Eighteen genotypes were obtained from the World Vegetable Center Genebank (https://genebank.worldveg.org/), where we specifically selected materials sourced from countries in West Africa, including Cameroon, Togo, Niger, Benin, Malawi, and Mali. The Council of Scientific and Industrial Research – Crop Research Institute (CSIR-CRI) in Ghana provided the remaining forty-five genotypes. The 63 okra genotypes (Table S1) were screened using a conventional sawtooth plastic greenhouse (temperature: 19 to 30 °C; relative humidity: 70 to 90%) and two locally constructed growth chambers, as described in Opoku et al. 10. Natural sunlight was the light source in the greenhouse, so the light intensity was uncontrolled.
Experimental design, treatments, sowing and management
We adapted the treatments and experimental design described in Opoku et al. 12. The study was conducted at the A.G. Carson Technology Centre, School of Agriculture, University of Cape Coast, located at 5.1155°N latitude and 1.2909°W longitude in the Central Region of Ghana. This study employed an alpha lattice design with three screening batches comprising 21 genotypes. We included a check genotype (GH131) in each batch and accounted for any variation between experimental runs as a cofactor. Thus, each batch included all 21 of the 63 genotypes, including the check genotype (GH131), and was designed to handle the experimental load and account for potential variations between experimental runs. The alpha lattice design allowed us to screen the genotypes in batches while controlling for batch-specific effects by including them as cofactors in our analysis. Each treatment was replicated two times, involving 16 plants per genotype per replicate, resulting in 36 plants per genotype for each treatment. Replications were systematically rotated within the greenhouse and growth chambers to minimise environmental variation.
Seedlings of each okra genotype were initially grown in four nursery trays per genotype in a conventional greenhouse for five weeks, during which the plants reached the 6–8 leaf stage. Seedlings of each genotype were raised in nursery trays (128 cells; 28 × 54 cm with cells; 5 × 3 cm) filled with Fertiplus potting mix (Dizengoff Ghana, https://www.baltoncp.com/) in the conventional greenhouse. One seedling per cell remained after postgermination thinning at seven days after nursing. Poly-Feed™ was applied (2.5 g/L) two times before the imposition of the stress treatments, and the mixture was discontinued every two weeks before data collection. Nursery trays containing the potting mix were placed in a water-filled basin, allowing the soil to soak through capillary action for approximately one hour before draining. In the conventional greenhouse, nursery trays were systematically rotated to mitigate potential temperature, light, and humidity fluctuations.
Treatment application
From the conventional greenhouse, the plants were transferred to a controlled climate growth chamber upon reaching the 6–8 leaf stage. The plants were allocated to one of two environments in the growth chamber: an open ambient chamber set at 28/19 °C day/night with 70% relative humidity and an enclosed heat chamber set at 41/32 °C with 50% relative humidity. Thus, a temperature approximately 10 °C above the ambient temperature was maintained in the heating chamber. The seedlings were divided into two treatment groups within the two growth chambers. One group received continuous watering as a control in the ambient chamber, while the other group underwent progressive drought stress. For this purpose, nursery trays were placed in water-filled basins, initially allowing the potting mix to saturate by capillarity. The water level in the basins gradually decreased over time until watering completely ceased to impose progressive drought. This reduction in water height and subsequent termination of watering decreased the capillary rise, decreasing plant moisture availability. The water level was adjusted every six hours, and watering was completed on the second day of drought treatment. One group was continuously watered in the heat chamber to impose heat stress only. In contrast, as described above, the other group experienced heat and progressive drought stress, combined with high temperatures and gradually reduced water availability. This experimental design allowed us to assess heat and drought stress’s individual and combined effects on okra genotypes. The nursery trays were fully saturated for approximately one hour before stress imposition to ensure uniform moisture content across all the cells. The drought and heat stress screenings lasted five days within the chambers, with both the stressed and nonstressed trays systematically rotated to avoid potential gradients in temperature and humidity.
Data collection
Morphophysiological data from stressed and nonstressed plants were collected on the 1st, 2nd, and 4th days after stress initiation. The data were gathered from four randomly selected plants for each sampling day and genotype. The total leaf area was calculated as a product of the length and width of fully opened leaves and a factor of 0.75 12. After the shoot fresh weight was determined, the samples were oven-dried at 80 °C until a constant weight was reached, after which the dry shoot weight was determined using an electronic scale. The leaf chlorophyll content was measured nondestructively using a SPAD-502 Plus Chlorophyll Meter (Spectrum Technologies, Inc., Aurora, IL, USA). For chlorophyll fluorescence measurements, the 3rd fully expanded leaves were dark-adapted for 30 min before the variable-to-maximum fluorescence ratio (Fv/Fm ratio) and performance index were assessed with a PEA portable fluorometer (Hansatech Instrument Ltd.), which emits 650 nm wavelength light at an intensity of 3500 μmol m−2 s−1 over a 1-s detection time 24. Two young leaves were taken from each sampled plant, and four leaf discs were cut with a number 9 cork borer (1.9 cm diameter) to measure the relative leaf water content. The fresh weight of the discs was recorded, after which the plants were floated in a petri dish filled with water for 24 h at room temperature. The turgid weight of the plants was recorded after blotting the discs dry with a paper towel. The discs were then oven-dried at 80 °C for 24 h to obtain their dry weight. The relative leaf water content was calculated using Eq. 110.
Three leaves from each seedling were rinsed with deionised water and cut into 5.5 mm long disks, which were subsequently placed in 10 mL of deionised water and incubated overnight at room temperature. An Eutech PC 450 conductivity meter was used to measure the initial conductivity of the water after the tubes containing 10 mL of deionised water had been placed in a boiling water bath at 100 °C for 30 min. The final conductivity was then measured following cooling at room temperature. The percentage of electrolyte leakage was calculated as the initial and final conductivity quotient 10.
To determine whether the interactive effects of drought and heat stress on okra physiology were additive, synergistic, or antagonistic, we calculated the mean trait values for each group as follows: TC for the control treatment, TD for drought stress, TH for heat stress, and TD + H for combined stress. The expected combined effect under the additivity assumption (ED + H) was calculated using Eq. 2.
We compared the observed combined stress effect (TD + H) with the expected value to assess the nature of the combined stress effect. If TD + H was approximately equal to ED + H, the effect was considered additive. A decrease in TD + H compared with ED + H indicated a synergistic effect; if TD + H was greater than ED + H, it indicated an antagonistic effect.
Statistical analysis
Analysis of variance (ANOVA) was also conducted to assess the variations among the treatments. Principal component analysis (PCA) was used to identify morphophysiological traits that significantly contributed to variations, employing the Kaiser criterion to retain components with an eigenvalue of ≥ 1. The data were found to be normally distributed and did not require transformation. ANOVA, PCA, correlation, and broad-sense heritability (which measures the proportion of total variance attributable to genotype variance) were performed using the prcomp function of the built-in R statistics package 25. The results were visualized with the factoextra package 26. Cluster analysis was conducted using Ward’s hierarchical method, which employs the minimum variance linking method with Euclidean distance 27. The optimum number of clusters was determined using the ‘elbow criterion’, which evaluates the sum of squared differences for various cluster solutions 28. Broad-sense heritability (H2) was calculated using Eq. 329, and phenotypic variance was estimated using Eq. 4 as described by 12, where r represents the replication, n denotes the number of experiments, and \(\delta_{{g^{2 \times t } }}\) represents the variance in the interaction between the genotype and the experimental run.
Results
Descriptive statistics of evaluated traits
The coefficient of variation (CoV) across all treatments ranged from 2.1% for the Fv/Fm ratio to 34.8% for electrolyte leakage (Table 1). Broad-sense heritability (H2) estimates ranged from 0.21 to 0.98. H2 was high (> 0.60) for electrolyte leakage, leaf area, shoot fresh weight, chlorophyll content and performance index; intermediate (0.30 – 0.60) for relative water content and shoot dry weight; and low (< 30) for the Fv/Fm ratio (Table 1). The leaf area ranged from 20.4 to 48.8 cm2, with a mean of 29 cm2. The shoot dry weight ranged from 0.22 to 0.48 g, and the mean weight was 0.34 g (Table 1).
Differences in morphophysiology among okra genotypes
There was a significant effect (p < 0.001) of genotype on all the morphophysiological parameters measured (Table 2) across all the treatments. The trial significantly (p < 0.001) affected several morphophysiological traits, including the Fv/Fm ratio, total leaf area, performance index, relative water content, shoot dry weight, shoot fresh weight, and chlorophyll content. However, the trial did not significantly impact electrolyte leakage (Table 2). Sampling time significantly (p < 0.001) affected all the measured traits. Significant interactions (p < 0.001) between genotype and stress factors, as well as between stress factors and trial, were observed for all the measured traits (Table 2). The three-way interaction effect of genotype, stress, and experimental trial was significant (p < 0.001) for the majority of the measured traits, except for Fv/Fm, shoot biomass, and electrolyte leakage, which had no significant effect (Table 2). Supplementary Table S2 presents the mean performance of each genotype across all treatments, providing a comparative overview of genotypic differences irrespective of stress conditions. For instance, there was a onefold increase in leaf area between the top ten and last ten genotypes, and the relative leaf water content of the top 15 genotypes was 15% greater than that of the last 15 (Supplementary Table S2). The five topmost genotypes obtained 82% more biomass than the last five, and there was a twofold difference in electrolyte leakage between the top 10 genotypes and the last 10 (Supplementary Table S2).
Single and concurrent effects of heat and drought on morphophysiological traits
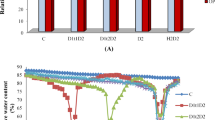
There was a significant (p < 0.001 or p < 0.05) effect of imposed stress on the morphophysiology of okra genotypes (Table 2). We observed that for photosynthetic efficiency parameters such as the Fv/Fm ratio, heat and drought stress alone caused approximately 4 and 16% reductions, respectively. However, the combined effect of drought and heat stress resulted in an approximately 31% reduction (Fig. 1A). Compared to that under heat stress alone, a 10% decrease in chlorophyll content was observed under both heat and drought stress. In comparison, a 4% decrease in chlorophyll content was observed under combined heat and drought stress compared to that under single drought stress (Fig. 1B). Shoot biomass decreased under both stress conditions compared to that of the control (Fig. 1C,D), with an approximately 67% reduction in SDW when heat and drought stress were combined and 9% and 41% reductions, respectively, when heat and drought were imposed independently (Fig. 1C). In general, an 11% reduction in relative water yield was recorded under drought stress alone, while combined heat and drought caused an approximately 23% reduction compared to that in the control treatment. Electrolyte leakage was significantly greater for the genotypes cultivated under combined heat drought (44.0%) than for those cultivated under drought (42%) or heat stress (36%). Most of the genotypes exhibited low electrolyte leakage under the control treatment; however, V1060833, GH115, GH116, GH132, GH133 and GH175 had lower electrolyte leakage under heat stress than under the control and other stress levels (Supplementary Table S3). Most of the physiological traits assessed exhibited additive effects (Table 3). For example, the combined stress effect on the chlorophyll content (TD + H = 27.6) was approximately equal to the expected additive effect (ED + H = 27.8). Other traits that exhibited additive effects were dry shoot biomass, the Fv‒Fm ratio, the performance index, and total leaf area. Electrolyte leakage and relative water content had antagonistic and synergistic effects, respectively (Table 3).
Responses of okra genotypes to drought, heat or a combination of heat and drought at the seedling stage. (A) Fv/Fm ratio; (B) chlorophyll content; (C) shoot dry weight; (D) shoot fresh weight; (E) relative water content; (F) performance index; (G) electrolyte leakage; and (H) total leaf area. Error bars indicating the standard error of the mean (SEM) show differences between stress factors and the interaction between stress and day, which was established using ANOVA. These differences are represented by l.s.d. (P < 0.05) bars.
Multivariate analysis
Correlations between measured traits for the pooled treatment data and contrasting stress conditions
Positive or negative relationships existed between traits for the pooled data for all treatments (Fig. 2). The RWC had a positive correlation (ranging from r = 0.48 to 0.96, p < 0.001) with several other traits, including the performance index, shoot biomass (SFW and SDW), and chlorophyll content. However, the relative leaf water content was negatively correlated (r = − 0.64, p < 0.001) with the electrolyte concentration. Electrolyte leakage had a strong but negative relationship with SFW and SDW (r = − 0.52; − 0.73, p < 0.001) and with chlorophyll content (r = − 0.72, p < 0.001) and a weak but significant negative relation with the Fv/Fm ratio (r = − 0.28, p < 0.01) and total leaf area (r = − 0.30, p < 0.01) (Fig. 2). A positive and strong significant correlation existed between chlorophyll content and shoot biomass traits; SFW (r = 0.96, p < 0.001) and SDW (r = 0.78, p < 0.001); and photosynthetic efficiency traits, including the Fv/Fm ratio (r = 0.56, p < 0.001) and performance index (r = 0.51, p < 0.001) (Fig. 2).
Correlations between traits observed in okra germplasm screened under heat and drought conditions. The analysis was conducted on pooled data for all treatments, and the traits listed on the diagonal of the matrix are as follows: Fv. Fm (Fv/Fm ratio), TLA (total leaf area), EL (electrolyte leakage), PI (performance index), RWC (relative leaf water content), SDW (shoot dry weight), SFW (shoot fresh weight) and CC (chlorophyll content).
Under control or non-stress condition, electrolyte leakage had a strong and negative significant (r = − 0.73 to − 0.92, p = 0.05 to < 0.001) with CC, SFW, SDW and RWC but has no association with Fv/Fm ratio, leaf area and performance index (Supplementary Fig. 1A). A strong and positive significant correlation existed between chllorophyll content and biomass traits; SFW (r = 0.95, p < 0.001) and SDW (r = 0.76, p < 0.05) (Supplementary Fig. 1A). Simialrly, under heat stress condition, chlorophyll content had a positive and strong significant correlation with biomass traits; SFW (r = 0.91, p < 0.01) and SDW (r = 0.79, p < 0.05); PI (r = 0.83, p < 0.05) and RWC (r = 0.71, p < 0.05) (Supplementary Fig. 1B). Electrolyte leakage had a strong and negative significant correlation with biomass traits; SFW and SDW (r = − 0.75 and – 0.91, p < 0.01) and with other traits such as RWC, PI and CC (Supplementary Fig. 1B). Under drought condition, RWC had positive strong and significant correlation with traits such as SDW, SFW and CC (r = 0.71 – 0.78, p < 0.05) whereas electrolyte leakage showed a signifcant negative (r = − 0.80 to – 0.93, p < 0.05 − < 0.001) with most of traits except Fv/Fm ratio and TLA (Supplementary Fig. 1C). Under combined heat and drought, electrolyte leakage showed a signifcant negative and strong association (r = − 0.76 to – 0.94, p < 0.05 − < 0.001) with most of traits except Fv/Fm ratio and TLA (Supplementary Fig. 1D). Fv/Fm ratio and TLA had no association with measured traits. The chlorophyll content was associated positively and significantly with biomass traits: SFW (r = 0.98, p < 0.001) and SDW (r = 0.92, p < 0.01) (Supplementary Fig. 1D).
Principal component and cluster analysis for pooled treatment data
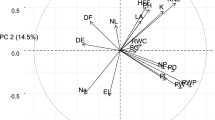
The PCA resulted in a two-factor solution explaining approximately 72% of the variance on pooled data for all treatments (Fig. 3A). The two significant PCs accounted for 61.8% and 15% of the variance, respectively. Six (6) distinct traits were found in the first dimension, including shoot fresh and dry weight, chlorophyll content, performance index, relative water content, and electrolyte leakage (Fig. 3B). The groups that accounted for the variability in the PCs and their contributions are shown in Fig. 3C. Five out of the eight measured traits correlated with the first. The traits associated with PC1 included shoot fresh and dry weight, chlorophyll content, performance indices, relative water content and electrolyte leakage (Fig. 3C). Approximately 63% of the eight measured morphophysiological traits exceeded the average cutoff point, contributing significantly to the variability observed in PC1. The highest contributing traits included chlorophyll content and shoot dry weight (Fig. 3C).
(A) Principal component plot showing relationships between all variables. The analysis was conducted on pooled data for all treatments, and the variables are colour-coded based on their quality of representation on the factor map, with the adjacent scale indicating the cos2 values of the corresponding variables; (B) Loading scores of variable groups for the first four dimensions, including the two significant ones; and (C) Contribution of variable groups to the first dimension (as determined by PCA). Trait abbreviations in the figure are as follows: Fv/Fm (Fv/Fm ratio), TLA (total leaf area), EL (electrolyte leakage), PI (performance index), RWC (relative leaf water content), SDW (shoot dry weight), SFW (shoot fresh weight) and CC (chlorophyll content).
The dendrogram generated from a cluster analysis indicated the presence of three distinct clusters (Fig. 4A). The Cluster one memberships included the following genotypes: GH112, GH113, GH114, GH117, GH,131, GH133, GH136, GH144, GH148, GH151, GH153, GH175, V1063912 and V1060833. The okra genotypes that belonged to cluster two included GH102, GH103, GH111, GH115, GH116, GH118, GH119, GH128, GH132, GH135, GH145, GH157, V1060830, V1063900, V10663947, V1062547, V1060686, V1059458, V1060871, and V1060844. The individuals who were clustered into the third group included GH106, GH121, GH108, GH170, GH172, V106392, V1060874, V1060831, V1063895 and V1060691. Overlaying the genotypes on the PC map suggested that cluster one was largely positive for PC1 and that cluster 2 was largely negative for PC1 and PC2; cluster three was amorphous, straddling both the positive and negative axes of PC2 (Fig. 4B).
(A) Dendrogram illustrating the clustering patterns of measured traits among okra genotypes screened for heat and drought tolerance at the seedling stage. (B) Okra genotypes, displayed on the principal component map, are grouped and colour-coded according to the clusters assigned to them according to the cluster analysis. The analysis was conducted using pooled data for all treatments.
Discussion
Genetic variation in response to single and costress drought and heat
The present study assessed drought and heat stress’s independent and combined effects on okra growth and physiology. We identified focal phenotypic traits useful for characterizing diversity in the morphophysiological response of okra to single and combined heat-drought stress. This study presents an essential prebreeding step toward developing climate-resilient okra genotypes. This study revealed significant genotypic variation in the morphophysiology of 63 okra genotypes screened under single and costressive drought and heat conditions. Even though the impacts of heat and drought and their co-occurrence were observed in all the cases, they were not uniform for all the genotypes, with some being more resilient than others. Genotypes such as GH148, GH136, GH131, GH147, GH111, GH116, GH112, GH133, GH148, GH150, GH144, GH153, V1063947, and V1063912 appeared more sensitive than others. The reduction in relative water content was relatively lower in some genotypes, including GH170, GH172, V1063895, GH174, V1060831, V1060874, GH106, V1060691, and V1063894 (Supplementary Table S2), indicating the ability of these genotypes to maintain internal water balance in response to heat and drought stress as well as costress 30. A higher relative water content could have accounted for the high photosynthetic efficiency traits, such as the Fv/Fm ratio, chlorophyll content and performance indices, observed among these genotypes. This is further justified by the significant positive association observed between relative water content and photosynthetic traits, as indicated in Fig. 2. Thus, genotypes considered to be tolerant may employ an avoidance mechanism via enhanced tissue and membrane water content in response to stress conditions 31.
Biomass was significantly greater for the genotypes considered tolerant, such as GH170, GH172, and V1063895, in response to stress conditions (Supplementary Table S3), as has been reported for wheat, eggplant, and potato genotypes 32,33, which maintained greater biomass under single and combined heat and drought conditions. The contrasting responses to heat and drought illustrate the genetic diversity of okra germplasm, which could be leveraged for selection and building resilience for improved okra production 2. The results of the present study revealed that morphophysiological metrics such as relative water content, electrolyte leakage, biomass, and chlorophyll content might be the main putative factors accounting for genotypic differences in okra responses to abiotic stress. These traits could thus be exploited to select abiotic stress resilience for crops.
Drought stress had a predominant effect on okra morphophysiology compared with heat stress
Drought and heat stress have been reported to impact the morphology and physiology of crop plants. However, this depends on the growth stage, stress intensity, plant species and traits measured 34. Our results indicate that both stresses individually impair the morphophysiological parameters of okra, with drought stress exerting a more pronounced negative impact than heat stress. Indeed, heat stress alone also adversely affects okra growth, albeit to a lesser extent than drought stress. High temperatures disrupt enzymatic activities and membrane stability, impairing photosynthetic processes. Specifically, we recorded a 4% reduction in photosynthetic efficiency parameters, such as the Fv/Fm ratio, under heat stress, indicating thermal damage to the photosynthetic machinery.
Drought stress had a more pronounced effect on the measured morphophysiological traits than heat stress (Fig. 1A–H). Drought stress resulted in a 20% increase in electrolyte leakage compared to heat stress, indicating cell damage due to elevated oxidative stress under drought conditions. Compared with those under heat stress, electrolyte leakage under drought conditions is reportedly greater than that under heat stress 12,34. Similarly, the single effect of drought resulted in a significant reduction in photosynthetic traits, such as the Fv/Fm ratio, chlorophyll content, and performance indices (Fig. 1). Unlike heat-stress plants, plants reduce their stomatal aperture to reduce transpirational water loss. However, this increase in internal temperature disrupts the photosynthetic apparatus due to PSII damage 35, which could account for the intense magnitude of the effect of drought observed in the present study. Drought stress may be more dominant because an enlarged stomatal aperture during high temperatures is likely to aggravate drought stress, given that the bulk of water absorbed is mediated by transpiration.
An increased effect of drought stress on morphophysiology compared to heat stress has been reported in African eggplants, tomatoes, potatoes, peppers, etc. 12,36. A reduced water content causes stomata to close, causing photorespiration. This process results in the production of reactive oxygen species that are responsible for several destructive cellular processes 37. Water deficit conditions severely limit cell expansion and photosynthetic efficiency, leading to stunted growth and reduced productivity in various crops, including okra. Water scarcity disrupts the turgor pressure necessary for cell enlargement and division, directly impacting leaf development and stem elongation. The results of the present study indicated that drought stress more significantly impacts relative water content than heat (Fig. 1E), and these results agree with previous findings 38. The greater reduction in relative water content observed under drought stress than under heat stress could have accounted for the lower biomass accumulation under drought stress (Fig. 1C–E). Additionally, the effect of drought stress on photosynthetic traits (Fig. 1A,B) could have reduced overall photosynthesis and carbon accumulation. Our research corroborates earlier studies that observed a marked reduction in shoot biomass in tomato cultivars under drought conditions, as opposed to heat and control treatments 38.
Costress from drought and heat had greater effects on morphophysiology than single-stress
The interaction effect of drought and heat stress revealed complex dynamics. The measured morphophysiological traits were affected by both the single and combined effects of heat and drought but increased markedly further under the combined stress (Fig. 1A–H). Under costress, the effects can be additive (combined stress equals the sum of individual stresses), synergistic (combined stress is greater than the sum of individual stresses), or antagonistic (combined stress is less than the sum of individual stresses) 39. In the present study, when both stresses were imposed concurrently, the cumulative effect on okra morphophysiology was mostly an additive detrimental effect, exacerbating the decline in measured parameters more than stress alone (Table 3). Thus, the present results suggest that the combined impact of drought and heat stress on the overall performance of okra may be simply the sum of their individual effects. For example, the combined effect of drought and heat stress on relative water availability, shoot dry weight, chlorophyll content, performance index and total leaf area was approximately the sum of the effects of both stressors applied individually (Fig. 1; Table 3). Here, the combined stresses on the chlorophyll content and Fv/Fm were also additive, suggesting that these stresses reduced photosynthetic efficiency without interacting. Thus, there are possible independent impairments of the photosynthetic machinery by each stress type, which do not interact in a manner that compounds or alleviates the damage. The additive effect is likely due to the compounded strain affecting the plant’s physiological mechanisms, which concurrently combat dehydration and thermal stress.
The additive interaction effect of drought and heat stress on okra morphophysiology is consistent with the findings of other reports. For example, in wheat, concurrent exposure to heat and drought stress had an additive negative effect on various aspects of plant phenology, physiology, and grain yield 40. Similarly, in soybeans, the effects of these stresses, individually and in combination, on yield depend upon the phenological stage at which the stress occurs. Combined stresses additively reduce seed yield during pod formation by impairing leaf performance and carbon assimilation, leading to decreased pod setting and smaller seed size 41.
Machado and Paulsen 42 noted that although responses to drought and heat stress share some common mechanisms, other physiological processes are antagonistic. Here, an antagonistic reaction was recorded for electrolyte leakage; compared with that of the control, a 27% increase in electrolyte leakage was observed under combined heat and drought stress, and the observed combined stress effect (TD + H = 34) was slightly greater than the expected additive effect (ED + H = 33). Here, heat stress-induced upregulation of certain stress-responsive genes might confer some tolerance to drought stress or vice versa. Thus, the cross-tolerance mechanisms activated in response to one type of stress may provide a degree of protection, albeit inadequate, against the other, leading to an antagonistic interaction. Similarly, the relative water content recorded a synergistic reaction, but we have to question the conclusiveness of this observation based on the arguments above. This highlights the need for further investigations to confirm the nature of these interactions. Notably, antagonistic reactions have been reported in several previous studies for many crops, including African eggplant, tomato, maize, and wheat 43,44, where antagonistic effects of combined heat and drought have been reported for traits such as biomass, relative water content, gas exchange, and electrolyte leakage.
While the observed antagonistic effect is plausible, the margin between the observed and expected values here is quite small, raising questions about the conclusiveness of this result. Statistical analysis would provide stronger evidence for the nature of the interactions between drought and heat stress on the physiological traits of okra plants. Given the lack of replication and statistical analysis between the observed and expected values to validate this difference, the interactive effects recorded here may be inconclusive. For example, according to these findings, there may be no significant difference between TD + H and ED + H; thus, the observed effect may not be truly antagonistic but rather additive. However, the data here highlights observable trends that can help understand how these stresses interact. While statistical validation would be ideal for more robust conclusions, the current data still reflects meaningful patterns, such as additive or synergistic effects consistent with physiological expectations. These trends offer a basis for hypothesis generation and further experimentation, and the nature of effects indicated in Table 3 serves as an initial interpretation.
Broad-sense heritability and the relationship between measured traits and genotypes
The phenotyping approach was rapid and robust, leading to a low to moderate coefficient of variation (CoV; 2.1–36.1%; Table 1) for all the traits assessed. Low CoV values are indicative of precise trait estimates. Usually, large values of CoV for given traits are attributable to vagaries in experimental conditions on those traits. In plant breeding, broad-sense heritability (H2), which measures the ratio of phenotypic to genetic variance, is useful as a descriptive metric for evaluating the accuracy of experimental results. Here, H2 was variable among the traits, being high (> 0.60) for electrolyte leakage, chlorophyll content, total leaf area, performance index, and shoot fresh weight; intermediate 45 for dry shoot weight and relative water content; and lower (< 0.30) for the Fv/Fm ratio (Table 1). The low heritability of specific traits, including the Fv/Fm ratio, could indicate stronger environmental influences on these traits than on the other traits 46. Therefore, using these characteristics directly as criteria for selection may be difficult; in that case, identifying molecular markers for screening purposes may be a more reliable method 46. On the other hand, the high heritability observed among traits such as electrolyte leakage, chlorophyll content, and biomass indicate that environmental vagaries were less impactful. These traits could be phenotypes with fewer replications 29.
A strong positive relationship was observed among photosynthetic parameters such as the Fv/Fm ratio, performance indices, chlorophyll content and biomass parameters such as shoot fresh and dry weight (Fig. 2). The PCA further confirmed the correlation observed among the morphophysiological traits (Fig. 3A). The PC revealed a positive association between the Fv/Fm ratio, performance index, chlorophyll content and biomass parameters. However, these traits had a negative association with electrolyte leakage. These factors were among the essential characteristics for explaining the variability in a given dataset (Fig. 3A). These positive correlations may be due to a common mechanism for stress tolerance among okra genotypes. It could also offer a route to selection based on proxy traits. The 63 okra genotypes were classified into three (3) distinct clusters (Fig. 4A), suggesting potential for future breeding efforts to support climate-resilient vegetable production, especially for heat and drought stress. The results suggest wide diversity among the genotypes. The genotypes in cluster 1 might be explored for drought and heat stress tolerance.
Conclusions
Global warming and climate change have worsened abiotic stress conditions for crop plants, especially those vulnerable to environmental stressors, including vegetables. The morphophysiology of horticultural crops is severely affected by heat and drought but markedly worsens under the simultaneous occurrence of these stresses. Although plants possess an inherent ability to adapt to environmental stress, natural processes of plant adaptation cannot catch up with rapid climate change and its effects. It is, therefore, imperative to leverage crop breeding to develop stress-resistant or tolerant species. However, crop improvement programmes dedicated to developing climate-resilient crops have mainly focused on cereal and staple crops, which form a substantial proportion of global food demands, neglecting essential crops such as vegetables. Okra is one of the most neglected vegetables, albeit vital to food and nutrition security and livelihoods to many in the tropics, subtropics, and warm temperate regions. The present study hypothesized that genetic diversity exists in the morphophysiology of okra in response to single heat and drought stresses and in combination. Both single and simultaneous drought and heat stress significantly altered okra genotype morphology and physiological traits; however, the genotypes exhibited contrasting responses. The tolerant genotypes, such as GH170, V1060831, GH174, V1060874, and GH106, employ avoidance mechanisms such as high relative water content to maintain internal water balance and cell turgor under drought stress. These genotypes produced greater biomasses under single and combined stresses due to their ability to protect photosynthetic traits, such as the Fv/Fm ratio, chlorophyll content and performance indices, major drivers of carbon assimilation and accumulation under stress conditions. The combined effects of these stresses were additive, reflecting the significant impact of drought and heat stress on the morphophysiology of okra, with drought stress being particularly detrimental. This study revealed that morphophysiological traits such as the Fv/Fm ratio, chlorophyll content, electrolyte leakage, relative water content and biomass could be important for selecting stress tolerance in okra germplasm. These results highlight the necessity for integrated stress management strategies and the development of crop varieties with enhanced tolerance to multiple stresses. Assessing the molecular and genetic basis of stress tolerance will be vital to better equip okra with the increasingly variable climatic conditions predicted under global climate change scenarios. Even so, if the results of the present study are confirmed under field conditions, the genotypes identified here as drought- and heat-stress-tolerant could be exploited in breeding climate-resilient okra varieties.
Data availability
This article and the supplementary information provide all the data supporting the findings of this study. For further queries, the corresponding author was contacted.
References
Schafleitner, R. et al. The World Vegetable Center Okra (Abelmoschus esculentus) core collection as a source for flooding stress tolerance traits for breeding. Agriculture 11, 66 (2021).
Mkhabela, S. S., Shimelis, H., Gerrano, A. S. & Mashilo, J. Phenotypic and genotypic divergence in Okra [Abelmoschus esculentus (L.) Moench] and implications for drought tolerance breeding: A review. S. Afr. J. Bot. 145, 56–64 (2022).
Ibitoye, D. O. & Kolawole, A. O. Farmers’ appraisal on Okra [Abelmoschus esculentus (L.)] production and phenotypic characterization: A synergistic approach for improvement. Front. Plant. Sci. 13, 66 (2022).
Mekouar, M. A. 15. Food and Agriculture Organization of the United Nations (FAO). Yearb. Int. Environ. Law 29, 448–68. https://doi.org/10.1093/yiel/yvz057 (2018).
Alake, C. O. Genetic variability and diversity in okra landraces using agromorphological traits and seed elemental minerals. Int. J. Veg. Sci. 26(2), 127–149. https://doi.org/10.1080/19315260.2019.1610926 (2020).
Konapala, G., Mishra, A. K., Wada, Y. & Mann, M. E. Climate change will affect global water availability through compounding changes in seasonal precipitation and evaporation. Nat. Commun. 11(1), 3044. https://doi.org/10.1038/s41467-020-16757-w (2020).
Arnell, N. W., Lowe, J. A., Challinor, A. J. & Osborn, T. J. Global and regional impacts of climate change at different levels of global temperature increase. Clim. Change 155(3), 377–391. https://doi.org/10.1007/s10584-019-02464-z (2019).
Bhusal, N. et al. Photosynthetic traits and plant hydraulic dynamics in Gamhong apple cultivar under drought, waterlogging, and stress recovery periods. Sci. Hortic. 321, 112276 (2023).
Hayamanesh, S., Trethowan, R., Mahmood, T., Ahmad, N. & Keitel, C. Physiological and molecular screening of high temperature tolerance in Okra [Abelmoschus esculentus (L.) Moench]. Horticulturae 9, 66 (2023).
Urban, O. et al. Combined effects of drought and high temperature on photosynthetic characteristics in four winter wheat genotypes. F Crop. Res. 223, 137–149 (2018).
Netshimbupfe, M. H., Berner, J. & Gouws, C. The interactive effects of drought and heat stress on photosynthetic efficiency and biochemical defense mechanisms of Amaranthus species. Plant-Environ. Interact. 3(5), 212–225 (2022).
Opoku, V. A. et al. Rapid and low-cost screening for single and combined effects of drought and heat stress on the morpho-physiological traits of African eggplant (Solanum aethiopicum) germplasm. PLoS ONE 19(1), e0295512. https://doi.org/10.1371/journal.pone.0295512 (2024).
Jampoh, E. A. et al. Morpho-anatomical, physiological and biochemical adjustments in response to heat and drought co-stress in winter barley. Plants 12, 66 (2023).
Abd El-Fattah, B. E. S., Haridy, A. G. & Abbas, H. S. Response to planting date, stress tolerance and genetic diversity analysis among okra (Abelmoschus esculentus (L.) Moench.) varieties. Genet. Resour. Crop Evol. 67, 831–51 (2020).
Ahmed, Z. G. & El-Sayed, M. A. Influence of drought stress on physiological traits of crossed okra varieties. Jordan J. Biol. Sci. 14(2), 66 (2021).
Ayub, Q. et al. Responses of different okra (Abelmoschus esculentus) cultivars to water deficit conditions. J. Hortic. Sci. 16(1), 53–63 (2021).
Adejumo, S. A., Ezeh, O. S. & Mur, L. A. J. Okra growth and drought tolerance when exposed to water regimes at different growth stages. Int. J. Veg. Sci. 25(3), 226–258 (2019).
Mkhabela, S. S., Shimelis, H., Gerrano, A. S. & Mashilo, J. Drought tolerance assessment of Okra (Abelmoschus esculentus [L.] Moench) accessions based on leaf gas exchange and chlorophyll fluorescence. Life 13(3), 682 (2023).
Hussain, R. et al. Regulation of osmotic balance and increased antioxidant activities under heat stress in Abelmoschus esculentus L. triggered by exogenous proline application. Agronomy 11(4), 685 (2021).
Khan, M. W., Hussain, Z. & Farooq, M. Maintenance of tissue water status, osmoregulation, and antioxidant defence system improves heat tolerance in okra genotypes with contrast heat tolerance. J. Soil Sci. Plant Nutr. 22(4), 4273–4281. https://doi.org/10.1007/s42729-022-01026-0 (2022).
Mukamuhirwa, A. et al. Concurrent drought and temperature stress in rice—A possible result of the predicted climate change: effects on yield attributes, eating characteristics, and health promoting compounds. Int. J. Environ. Res. Public Health 16(6), 1043 (2019).
Martinez, V. et al. Tolerance to stress combination in tomato plants: New insights in the protective role of melatonin. Molecules 23(3), 535 (2018).
Marin, M. et al. Significance of root hairs for plant performance under contrasting field conditions and water deficit. Ann. Bot. 128(1), 1–16 (2021).
Guo, Y. Y., Yu, H. Y., Kong, D. S., Yan, F. & Zhang, Y. J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica 54(4), 524–31 (2016).
Team R. R A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2020) [cited 2024 Feb 12]. Available from: https://cir.nii.ac.jp/crid/1370298755636824325.bib?lang=en
Kassambara, A. Practical Guide To Principal Component Methods in R: PCA, M (CA), FAMD, MFA, HCPC, factoextra. vol. 2 (STHDA, 2017).
Strauss, T. & von Maltitz, M. J. Generalising Ward’s method for use with Manhattan distances. PLoS ONE 12(1), e0168288. https://doi.org/10.1371/journal.pone.0168288 (2017).
Esmaeilzadeh, S. et al. Multiscale modeling of compartmentalized reservoirs using a hybrid clustering-based non-local approach. J. Pet. Sci. Eng. 184, 106485 (2020).
Adu, M. O. et al. A scanner system for high-resolution quantification of variation in root growth dynamics of Brassica rapa genotypes. J. Exp. Bot. 65(8), 2039–2048 (2014).
Udpuay, S. et al. Drought tolerance screening of okra genotypes in relation to growth and physio–biochemical traits at the vegetative stage. Genet. Resour. Crop Evol. 71(3), 1271–90. https://doi.org/10.1007/s10722-023-01689-3 (2024).
Ilyas, M. et al. Drought tolerance strategies in plants: A mechanistic approach. J. Plant Growth Regul. 40(3), 926–944. https://doi.org/10.1007/s00344-020-10174-5 (2021).
Mathew, I. et al. Selection of wheat genotypes for biomass allocation to improve drought tolerance and carbon sequestration into soils. J. Agron. Crop Sci. 205(4), 385–400. https://doi.org/10.1111/jac.12332 (2019).
Nasir, M. W. & Toth, Z. Response of different potato genotypes to drought stress. Agriculture 11(8), 763 (2021).
dos Santos, T. B., Ribas, A. F., de Souza, S. G. H., Budzinski, I. G. F. & Domingues, D. S. Physiological responses to drought, salinity, and heat stress in plants: A review. Stresses 2(1), 113–135 (2022).
Abdelhakim, L. O. A., Zhou, R. & Ottosen, C. O. Physiological responses of plants to combined drought and heat under elevated CO2. Agronomy 12(10), 2526 (2022).
Handayani, T. & Watanabe, K. The combination of drought and heat stress has a greater effect on potato plants than single stresses. Plant Soil Environ. 66(4), 175–182 (2020).
Qaseem, M. F., Qureshi, R. & Shaheen, H. Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci. Rep. 9(1), 6955 (2019).
Zhou, R. et al. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 17(1), 1–13 (2017).
Zandalinas, S. I. & Mittler, R. Plant responses to multifactorial stress combination. New Phytol. 234(4), 1161–1167 (2022).
Tricker, P. J., Elhabti, A., Schmidt, J. & Fleury, D. The physiological and genetic basis of combined drought and heat tolerance in wheat. J. Exp. Bot. 69(13), 3195–3210 (2018).
Soba, D., Arrese-Igor, C. & Aranjuelo, I. Additive effects of heatwave and water stresses on soybean seed yield is caused by impaired carbon assimilation at pod formation but not at flowering. Plant Sci. 321, 111320 (2022).
Giordano, M., Petropoulos, S. A. & Rouphael, Y. Response and defence mechanisms of vegetable crops against drought, heat and salinity stress. Agriculture 11(5), 463 (2021).
Zhou, R. et al. Physiological response of tomatoes at drought, heat and their combination followed by recovery. Physiol. Plant. 165(2), 144–154 (2019).
Hussain, H. A. et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 9(1), 3890 (2019).
Zandalinas, S. I., Mittler, R., Balfagón, D., Arbona, V. & Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 162(1), 2–12 (2018).
Karbstein, K., Prinz, K., Hellwig, F. & Römermann, C. Plant intraspecific functional trait variation is related to within-habitat heterogeneity and genetic diversity in Trifolium montanum L. Ecol. Evol. 10(11), 5015–5033. https://doi.org/10.1002/ece3.6255 (2020).
Acknowledgements
We are grateful to the Council of Scientific and Industrial Research—Crop Research Institute (CSIR-CRI) of Ghana and the World Vegetable Center Genebank for supplying the genetic material used in this work. We value the internal evaluation and advice Prof. J. P. Tetteh provided about the study and article, and we express special gratitude to Samuel Banafo, Solomon Amamu, Rosemond Appiah, Joseph Baidoo and Azure Sanleri for their assistance with the laboratory work.
Funding
This research was funded by the Ministry of Foreign Affairs of Denmark through the project “Building vegetable farmers’ resilience to climate change, Fruitbunch”, DFC project no: 19–04-AU.
Author information
Authors and Affiliations
Contributions
Conceptualization: Michael O. Adu, Paul A. Asare, Mathias N. Andersen. Methodology: Justice Asante, Godswil Hygienous, Vincent A. Opoku, Michael O. Adu. Investigation: Justice Asante, Godswill Hygeinus, Vincent A. Opoku. Supervision: Michael O. Adu, Paul A. Asare, Mathias N. Andersen. Data curation: Justice Asante, Vincent A. Opoku. Formal analysis: Vincent A. Opoku. Writing—original draft: Vincent A. Opoku. Writing—review & editing: Michael O. Adu, Paul A. Asare, Mathias N. Andersen. Validation: Michael O. Adu, Paul A. Asare, Mathias N. Andersen. Funding acquisition: Michael O. Adu, Paul A. Asare, Mathias N. Andersen. Project administration: Michael O. Adu, Paul A. Asare, Mathias N. Andersen. Resources: Michael O. Adu, Paul A. Asare, Mathias N. Andersen.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Compliance and consents
The experiment complied with relevant institutional, national, and international guidelines and legislation. The Council of Scientific and Industrial Research—Crop Research Institute (CSIR-CRI) of Ghana and the World Vegetable Center Genebank supplied the okra germplasm. They granted permission to use the genetic materials in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Asante, J., Opoku, V.A., Hygienus, G. et al. Photosynthetic efficiency and water retention in okra (Abelmoschus esculentus) contribute to tolerance to single and combined effects of drought and heat stress. Sci Rep 14, 28090 (2024). https://doi.org/10.1038/s41598-024-79178-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79178-5