Abstract
Purpose
Narrow resection margin hepatocellular carcinoma (NRM-HCC) has a high incidence of early recurrence. Our study was designed to identify prognostic factors in patients with NRM-HCC, establish and validate a nomogram model to predict early recurrence of NRM-HCC patients.
Methods
We retrospectively analyzed data from 2957 NRM-HCC patients who underwent radical hepatectomy at three medical centers between December 2009 and January 2015. Patients were randomly assigned to a training cohort (n = 2069) and a validation cohort (n = 888). Using univariate and multivariate COX regression to determine early relapse factors in NRM-HCC patients, and used these factors to construct a nomogram. The accuracy of the prediction was evaluated using the C-index, receiver operating characteristic (ROC) and calibration curve. Decision curve analysis (DCA) assessed the predictive value of the models. Finally, the recurrence-free survival of different risks was analyzed using Kaplan-Meier (K-M) method.
Results
The nomogram of NRM model contains alpha-fetoprotein (AFP), alkaline phosphatase (ALP), tumor size, tumor number, microvascular invasion (MVI), tumor capsular, and satellite nodules. The model shows good discrimination with C-indexes of 0.71 (95% CI: 0.69–0.72) and 0.72 (95% CI: 0.70–0.75) in the train cohort and test cohort respectively. Decision curve analysis demonstrated that the model is clinically useful and the calibration of our model was favorable. Our model stratified patients into two different risk groups, which exhibited significantly different early recurrence. The web-based tools are convenient for clinical practice.
Conclusions
NRM model demonstrated favorable performance in predicting early recurrence in NRM-HCC patients. This novel model will be helpful to guide postoperative follow-up and adjuvant therapy.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor worldwide, with approximately 910,000 new cases diagnosed annually. It is also the third leading cause of cancer-related deaths, accounting for around 830,000 deaths globally1. Hepatectomy remains one of the most effective and potentially curative treatment options for HCC2. However, the high rate of postoperative recurrence significantly undermines its therapeutic efficacy3. The surgical margin is a critical indicator of tumor resection completeness and is a key factor influencing postoperative recurrence. Despite advances such as preoperative three-dimensional liver reconstruction and intraoperative indocyanine green (ICG) fluorescence guidance, many patients still experience narrow or even positive margins due to factors such as deep tumor location, proximity to major blood vessels, and limited remnant liver volume4,5,6,7. Previous studies have shown that patients with narrow margins exhibit considerable variability in postoperative survival8,9,10,11,12,13. Therefore, further risk stratification is necessary to enhance postoperative recurrence monitoring and guide treatment.
HCC is a highly heterogeneous cancer, and traditional AJCC and BCLC staging systems are insufficient for accurately predicting postoperative recurrence, particularly early recurrence14. Based on the principles of precision medicine, developing predictive models tailored to specific HCC subtypes for monitoring early postoperative recurrence and guiding adjuvant therapy is an effective strategy for improving survival outcomes15.
Currently, there is no established risk stratification model specifically for early recurrence (ER) in patients with narrow margin HCC. This study aims to identify risk factors for early recurrence (≤ 2 years) in patients with narrow surgical margins and to develop a nomogram for clinical risk stratification and guidance for adjuvant treatment. Additionally, we have created an online calculator to facilitate the use of our model in clinical practice.
Method
Object and eligibility
HCC patients with narrow resection margin undergoing radical resection between January 2009 and December 2015 were extracted from a multicenter database (primary liver cancer big data, PLCBD) including Mengchao Hepatobiliary Hospital of Fujian Medical University, Eastern Hepatobiliary Surgery Hospital and the First Affiliated Hospital of Fujian Medical University. This study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Ethics Committee of Mengchao Hepatobiliary Hospital of Fujian Medical University (Approval No. [2021_089_01]). Given that the study involved de-identified data and posed minimal risk to participants, the Ethics Committee of Mengchao Hepatobiliary Hospital of Fujian Medical University waived the requirement for obtaining informed consent.
Patients were eligible for inclusion in the study if they met the following criteria: (1) HCC (BCLC stage 0, A and part of B) diagnosis that was confirmed by postoperative pathology, (2) resection margin ≤ 1 cm, (3) Child–Pugh A or B liver function before surgery, (4) age between 18 and 80 years, and (5) hepatectomy as the initial treatment modality. Patients with the following criteria were excluded: (1) presence of major vascular invasion or distant metastasis, (2) perioperative mortality, (3) previous history of malignancy, (4) patients did not receive any antitumor treatments postoperatively (excluding antiviral therapy and TACE), and (5) incomplete clinical data or follow-up information. All patients were randomly divided into a training group and a test group in a 7:3 ratio. The flow chart of this study is shown in Fig. 1.
Clinicopathologic variables and follow-up
Baseline characteristics of the patients included age, sex, smoking history, alcohol consumption history, diabetes, hypertension and liver cirrhosis. Routine serological examination included Hepatitis B surface antigen (HBsAg), Alpha-fetoprotein (AFP), hepatitis B virus deoxyribonucleic acid (HBVDNA), white blood cell (WBC), platelet count (PLT), albumin (ALB), total bilirubin (TBIL), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP). Surgical factors included intraoperative blood transfusion, operative bleeding loss, resection margin, and extent of liver resection (major or minor). Major resection was defined as a resection extent ≥ 3 segments, while minor resection as a resection < 3 segments16. Tumor characteristics included tumor size, tumor number, microvascular invasion, Edmondson-Steiner grade, tumor capsular, satellite nodules and tumor staging. Postoperative adjuvant therapies included antiviral treatment (AVT) and transarterial chemoembolization (TACE).
Patients were followed up every three months for the first two years after hospital discharge and then every 3–6 months thereafter. The follow-up assessments included liver function tests, AFP measurements, and abdominal ultrasound. If there was a clinical suspicion of tumor recurrence, contrast-enhanced CT or MRI was conducted. As the primary end point, recurrence-free survival (RFS) was defined as the interval from hepatic resection to recurrence. Early recurrence (ER) of HCC was defined as a recurrence time ≤ 2 years after hepatic resection 17.
Definition of narrow margin
Previous studies have demonstrated that portal vein invasion and intrahepatic micrometastasis mainly occur within 1 cm of the main tumor, and rarely extend to more than 2 cm of the tumor. Therefore, narrow resection margin was defined as a resection margin ≤ 1 cm9.
Construction of model
Univariate and multivariate Cox proportional hazard regression was performed to select the independent factors of early recurrence. All factors with P < 0.05 in univariate Cox regression were selected for multivariate Cox regression. The narrow margin liver resection for early recurrence nomograms (NRM model) were built based on the results of multivariate Cox regression (p < 0.01) in the training cohort.
Assessment and comparison of model performance
We used various methods to evaluate different aspects of model performance, including discrimination, calibration, clinical utility, and overall effectiveness. For assessing early recurrence, we chose a 2-year period for dynamic time-dependent measures.
Model discrimination was assessed with Harrell’s c-index and time-dependent areas under the receiver operating characteristic curve (tdAUC). Calibration was measured using calibration plots. Recurrence probabilities for 2 years were estimated through bootstrapping with 600 resamples. Clinical utility was evaluated via decision curve analysis (DCA), and overall performance was gauged using the time-dependent Brier score. The NRM model was also compared with AJCC TNM and BCLC staging in each cohort.
Statistical analysis
All continuous variables were redefined continuous variables as number (%) and used Chi-square or Fisher exact tests for comparative analysis in our study. Survival curves were estimated using the Kaplan-Meier method and compared by log-rang test. COX regression was used for univariate and multivariate analysis, and the corresponding Hazard Ratio (HR) and 95% Confidence interval (CI) were analyzed. P < 0.05 was considered statistically significant. Our statistical analyses were performed with R version 4.3.3(http://www.r-project.org/).
Results
Baseline characteristics.
A total of 4258 HCC patients underwent hepatic resection at Mengchao Hepatobiliary Hospital of Fujian Medical University, Eastern Hepatobiliary Surgery Hospital and the First Affiliated Hospital of Fujian Medical University from January 2009 to December 2015 received careful reviews of their medical records, 2957 patients were eligible for the study, 1301 patients were excluded for resection margin > 1 cm (n = 797), portal invasion/extrahepatic spread (n = 484), perioperative death (n = 2), history of other malignancies (n = 4), incomplete clinical and follow-up data (n = 4), and age < 18 years or > 80 years (n = 10). 2069 eligible patients were enrolled as the train cohort and 888 eligible patients serve as the test cohort.
The clinicopathologic characteristics of patients in the train cohort and the test cohort are summarized in Table 1. Variables including age, gender, HBsAg, AFP, HBVDNA, BCLC staging system, AJCC 8th staging system, WBC, PLT, ALB, TBIL, GGT, ALP, ALBI grade, smoking and alcohol consumption history, hypertension, diabetes mellitus, liver cirrhosis, surgical method, blood transfusion, operative bleeding loss, tumor size, tumor number, microvascular invasion, edmondson-steiner grade, tumor capsule, satellite nodules, antiviral therapy, and postoperative TACE. In the overall cohort, the train cohort and the test cohort, respectively, 1372 patients (46.4%), 970 patients (46.9%), and 402 patients (45.3%) had early recurrence (≤ 2 years).
The comparison of clinicopathologic characteristics between patients with and without early recurrence is summarized in Table 2. There are differences in AFP, WBC, ALB, GGT, ALP, ALBI grade, surgical method, blood transfusion, operative bleeding loss, tumor size, tumor number, microvascular invasion, edmondson-steiner grade, tumor capsule, satellite nodules, tumor staging, antiviral therapy, and postoperative TACE.
Construction of the NRM model
The results of univariable cox regression analysis and multi-variable cox regression analysis of early recurrence are listed in Table 3. The independent risk factors of early recurrence including the HBsAg positive (HR: 1.33, 95% CI: 1.06–1.67, p = 0.013), AFP ≥ 400 µg/L (HR: 1.47, 95% CI: 1.28–1.68, p < 0.001), GGT ≥ 50 U/L (HR: 1.21, 95% CI: 1.04–1.41, p = 0.015), ALP ≥ 80 U/L (HR: 1.26, 95% CI: 1.09–1.46, p = 0.002), tumor size 5–10 cm (HR: 2.06, 95% CI: 1.75–2.41, p < 0.001), tumor size > 10 cm (HR: 2.26, 95% CI: 1.66–3.09, p < 0.001), multiple tumor (HR: 1.61, 95% CI: 1.35–1.92, p < 0.001), presence MVI (HR: 1.73, 95% CI: 1.51–1.98, p < 0.001), absence tumor capsular (HR: 1.35, 95% CI: 1.14–1.61, p < 0.001), presence satellite nodules (HR: 1.30, 95% CI: 1.11–1.52, p < 0.001).
7 variables (p < 0.010) were used to construct the NRM model. A nomogram integrating the independent risk factors described above was constructed to facilitate the use of our predicting model of the early recurrence Fig. 2.
Evaluation of the NRM model
Model discrimination was assessed and compared via the C-index and time-dependent AUC (2 years). The C-indexes of the NRM model were 0.71 (95% CI: 0.69–0.72) in the training cohort and 0.72 (95% CI: 0.70–0.75) in the test cohort. For the BCLC staging system, the C-indexes were 0.57 (95% CI: 0.56–0.59) and 0.58 (95% CI: 0.56–0.60), while for the AJCC 8th edition staging system, they were 0.64 (95% CI: 0.62–0.65) and 0.64 (95% CI: 0.62–0.67) in the respective cohorts. The C-index and time-dependent AUC (2 years) showed that NRM models were greater than the BCLC and AJCC 8th staging systems in the training and test cohorts (Figs. 3A and B and 4A and B).
Decision curve analysis (DCA) was used to compare clinical usefulness. As shown in Fig. 5, DCA revealed that the NRM model had better net benefits in both the training and test cohorts than other models.
Calibration analysis also displayed a favorable agreement between the prediction and actual outcome of ER in both cohorts (Fig. 6A, B).
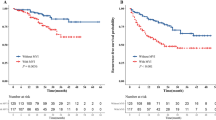
Using a 50th centile of the ER risk score, 0.28, in overall cohort, train cohort, and test cohort, respectively, we stratified the patients into two risk groups with significant recurrence outcome (P < 0.001) (Fig. 7A-C).
Discussion
Although the optimal surgical margin for hepatectomy remains a subject of debate, the prevailing view suggests that a narrow margin may increase the risk of microscopic residual disease, potentially leading to early recurrence of hepatocellular carcinoma post-surgery12. Previous studies have demonstrated that portal vein invasion and intrahepatic micrometastasis mainly occur within 1 cm of the main tumor, and rarely extend to more than 2 cm of the tumor18,19. Therefore, narrow resection margin was defined as a resection margin ≤ 1 cm. Our study aimed to identify prognostic factors for patients with narrow resection margin hepatocellular carcinoma (NRM-HCC) and to develop a nomogram for predicting early recurrence. We analyzed data from 2957 NRM-HCC patients who underwent radical hepatectomy. Key factors influencing early recurrence, including alpha-fetoprotein (AFP), alkaline phosphatase (ALP), tumor size, tumor number, microvascular invasion (MVI), tumor capsule integrity, and satellite nodules, were incorporated into the nomogram. This model demonstrated robust predictive performance with C-indexes of 0.71 in the training cohort and 0.72 in the validation cohort. The novelty of this study is based on: (1) a large multicenter study comprising 2957 HCC patients, (2) the first to report nomogram specifically for NRM-HCC candidates, (3) providing individualized and stratified survival estimates with favorable performance.
MVI is a significant prognostic indicator, associated with increased recurrence risk due to its role in tumor invasiveness and the likelihood of residual cancer cells. Previous studies have demonstrated that the width of the surgical margin is associated with postoperative recurrence and survival in patients with HCC combined with MVI. A study included 2,508 HCC patients (929 patients with MVI) showed that Compared with a wide resection margin, a narrow margin was associated with worse recurrence and overall survival in patients with microvascular invasion (hazard ratio: 1.50 and 1.75) and a wide margin resection also had a lesser incidence of early recurrence developed within the first postoperative 24 months (58.1% versus 72.7%; P < 0.001)20. Another multicenter study demonstrated that concomitant having narrow RM and positive MVI increases the risks of postoperative death and recurrence by about 2-fold in patients with solitary HCC10. It is worth mentioned that even for solitary small hepatocellular carcinoma (≤ 2 cm), wide-margin liver resection can still improve disease-free survival time in patients with MVI21. In the future, it is worth further exploring whether different grades of MVI have an effect on the recurrence of HCC with narrow margins.
Similar with MVI, tumor capsule integrity and satellite nodules also impact resection margins. A breached tumor capsule and the presence of satellite nodules suggest multifocal tumor growth, raising the likelihood of microscopic residual disease beyond the margin. Other models for predicting HCC recurrence have also found that the inclusion of tumor capsule and satellite nodules as model variables can improve the accuracy of prediction22.
Tumor size and number are critical factors influencing resection margin adequacy11. Larger tumors and multiple complicate complete resection, leading to higher recurrence rates23. Multiple hepatocellular carcinoma is divided into two categories. One is the result of intrahepatic metastasis of isolated nodules. The other is multicentric origin, which is a second primary tumor24. In either case, surgical clearance of multiple lesions is significantly more difficult, especially for narrow-margin HCC. The optimal tumor size for predict prognosis of HCC is still unclear. The cutoff values of tumor size of BCLC staging system were 2 cm and 3 cm, while those of AJCC 8th staging were 2 cm and 5 cm. A tumor larger than 5 cm in diameter is considered a large liver cancer, and a tumor larger than 10 cm in diameter is considered a giant liver cancer. Therefore, we used 5 cm and 10 cm as cutoff values in our NRM model is suitable. Study from Yao Li et al. also revealed that solitary HCC with a tumor diameter greater than 5 cm has a high probability of narrow margins25. And optimal tumor size cut-offs require more research in the future.
Our NRM model also incorporates two important serum markers, AFP and ALP. As we known, AFP and ALP levels are linked with increased HCC invasiveness and poorer prognosis in HCC patients. These biomarkers are especially pertinent in cases with narrow resection margins, as they may indicate residual tumor cells or a higher risk of recurrence. Although they were not included in BCLC and AJCC, many predictive models used it as a variable to predict HCC recurrence and survival. The predictive value of AFP in narrow marginal HCC has also been reported11.
Given that HCC is a comprehensive disease, conventional staging systems (BCLC and AJCC 8th TNM staging system) are not able to assess favorable predictive performance in NRM-HCC patients. The factors identified in this study, including AFP and ALP levels and various tumor characteristics, corroborate their prognostic value in narrow margin scenarios. MVI, tumor capsule status, and satellite nodules contribute to the challenges of achieving complete resection thereby increasing early recurrence risk. The integration of AFP, ALP, and other prognostic factors into our nomogram model provides a valuable tool for personalized risk assessment in patients with narrow resection margins. Additionally, we have created an online calculator to facilitate the use of our model in clinical practice. (https://zjy-msdatastudio.shinyapps.io/NRM-HCC-ER/)
Despite the large sample size and multicenter data, this study has limitations. Firstly, the retrospective nature of the study may introduce selection bias. Secondly, the data from three centers might affect generalizability. Thirdly, other factors such as anatomical resection, tumor location and liver function were not included, which could impact recurrence. Lastly, due to differences in baseline characteristics and sample sizes between the training and test groups, the slight difference in C-index between the two groups is acceptable under the principle of random allocation. Future research should address these limitations through prospective studies and validation in diverse populations.
Inconclusion, the NRM model demonstrates favorable performance in predicting early recurrence in NRM-HCC patients, providing significant clinical value. This model aids in postoperative follow-up and the formulation of individualized adjuvant therapy strategies. Continued research should focus on refining the model and evaluating its applicability in real-world settings.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3), 209–249 (2021).
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L: EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69(1), 182–236 (2018).
Portolani, N. et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 243(2), 229–235 (2006).
Tangsirapat, V. et al. Surgical margin status outcome of intraoperative indocyanine green fluorescence-guided laparoscopic hepatectomy in liver malignancy: a systematic review and meta-analysis. BMC Surg 24(1), 181 (2024).
She, W. H. et al. Correlation of pathological examination with indocyanine green (ICG) intensity gradients: a prospective study in patients with liver tumor. Surg Endosc 38(6), 3441–3447 (2024).
Ramalhinho, J. et al. The value of Augmented Reality in surgery - A usability study on laparoscopic liver surgery. Med Image Anal 90, 102943 (2023).
Adballah, M. et al. Augmented reality in laparoscopic liver resection evaluated on an ex-vivo animal model with pseudo-tumours. Surg Endosc 36(1), 833–843 (2022).
Ke, Q. et al. Resection Margin Width Does Not Influence the Prognosis of Solitary Hepatocellular Carcinoma After Anatomic Resection: A Real-World Study from China. J Hepatocell Carcinoma 10, 1353–1365 (2023).
Zhang, X. P. et al. Significance of anatomical resection and resection margin status in patients with HBV-related hepatocellular carcinoma and microvascular invasion: a multicenter propensity score-matched study. Int J Surg 109(4), 679–688 (2023).
Han, J. et al. The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford) 21(8), 962–971 (2019).
Endo, Y. et al. Impact of Surgical Margin Width on Prognosis Following Resection of Hepatocellular Carcinoma Varies on the Basis of Preoperative Alpha-Feto Protein and Tumor Burden Score. Ann Surg Oncol 30(11), 6581–6589 (2023).
Lafaro, K., Grandhi, M. S., Herman, J. M. & Pawlik, T. M. The importance of surgical margins in primary malignancies of the liver. J Surg Oncol 113(3), 296–303 (2016).
Spolverato, G. et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 22(12), 4020–4028 (2015).
Zeng, J. et al. Development of a machine learning model to predict early recurrence for hepatocellular carcinoma after curative resection. Hepatobiliary Surg Nutr 11(2), 176–187 (2022).
Chan, A. W. H. et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 69(6), 1284–1293 (2018).
Belghiti, J. et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 191(1), 38–46 (2000).
Poon, R. T. Differentiating early and late recurrences after resection of HCC in cirrhotic patients: implications on surveillance, prevention, and treatment strategies. Ann Surg Oncol 16(4), 792–794 (2009).
Nakashima, Y. et al. Portal vein invasion and intrahepatic micrometastasis in small hepatocellular carcinoma by gross type. Hepatol Res 26(2), 142–147 (2003).
Shi, M., Zhang, C. Q., Zhang, Y. Q., Liang, X. M. & Li, J. Q. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg 28(4), 376–381 (2004).
Yang, P. et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery 165(4), 721–730 (2019).
Lin, W. D. et al. Wide surgical margins improve prognosis for HCC with microvascular invasion. Eur Rev Med Pharmacol Sci 27(5), 2052–2059 (2023).
Costentin, C. et al. ERS: A simple scoring system to predict early recurrence after surgical resection for hepatocellular carcinoma. Liver Int 43(11), 2538–2547 (2023).
Sasaki, K. et al. Minimum resection margin should be based on tumor size in hepatectomy for hepatocellular carcinoma in hepatoviral infection patients. Hepatol Res 43(12), 1295–1303 (2013).
Yamamoto, S. et al. Spatial and temporal expansion of intrahepatic metastasis by molecularly-defined clonality in multiple liver cancers. Cancer Sci 111(2), 601–609 (2020).
Li, Y. et al. One- versus two-stage partial hepatectomy for large resectable solitary hepatocellular carcinomas determined preoperatively to have a narrow resection margin: a propensity score matching analysis. Hepatobiliary. Surg. Nutr. 11(5), 662–674 (2022).
Acknowledgements
The authors thank the staff of the primary liver cancer big data (PLCBD).
Funding
This study was supported by the Startup Fund for scientific research, Fujian Medical University (2021QH1159) and the Scientific Foundation of Fuzhou Municipal Health commission(2021-S-wp1).
Author information
Authors and Affiliations
Contributions
Jinyu Zhang, Jianxing Zeng and Jingfeng Liu contributed to the study conception and design. Material preparation and data collection was performed by Jinyu Zhang, Jianxing Zeng, and Qionglan Wu. Analysis and interpretation of the data were done by Jinyu Zhang and Zhiping Wang. The first draft of the manuscript was written by Jinyu Zhang, Zhiping Wang and Jianxing Zeng. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Wang, Z., Wu, Q. et al. Nomogram for predicting early recurrence of hepatocellular carcinoma with narrow resection margin. Sci Rep 14, 28103 (2024). https://doi.org/10.1038/s41598-024-79760-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-79760-x