Abstract
Low-density polyethylene (LDPE) is a widely used plastic that significantly contributes to environmental pollution, and its biodegradation remains challenging. This study investigates the dynamics of bacterial communities in consortia enriched with LDPE as the sole carbon source. The potential for microbial diversity to adapt to polluted environments underscores its role in bioremediation. Community analysis identified Actinobacteria and Proteobacteria as key contributors to LDPE degradation, with dominant genera including Mycobacterium, Cupriavidus, Gordonia, Ochrobactrum, Nocardia, Agromyces, Amycolatopsis, and Cellulosimicrobium. The biodegradation of untreated and pretreated LDPE films was also examined, revealing that UV pretreatment significantly enhances degradation, with weight losses of 2.22–5.17% after 120 days. In contrast, sunlight and thermal treatments resulted in lower weight losses of 1.67–4.56% and 1.42–3.22%, respectively, while untreated LDPE showed only 1.32–2.80% weight loss. These findings underscore the importance of UV pretreatment in facilitating plastic biodegradation. Furthermore, potential LDPE-degrading Actinobacteria and Proteobacteria were isolated, identified as key players in the communities and co-occurrence networks, suggesting promising candidates for developing sustainable plastic waste management solutions. Moreover, this study is the first to reveal the potential LDPE degradation abilities of several genera, including Mesorhizobium, Agromyces, Amycolatopsis, Olivibacter, Aquamicrobium, Pseudaminobacter, and others.
Similar content being viewed by others
Introduction
The demand for plastics is continuously increasing due to their diverse and attractive applications in households and industries. The high consumption of plastics has led to a global increase in plastic waste generation1. Low-density polyethylene (LDPE) is one of the most popular plastic materials due to its versatile nature and effectiveness and is widely used in the production of plastic bags. Annually, approximately 500 billion to 1 trillion plastic bags are used worldwide, with a short period of use before they are discarded2. Contaminated plastics can release harmful chemicals, such as phthalate plasticizers, organotins, and bisphenol A, into the surrounding soil or water sources. These chemicals may be released into the environment due to changes in temperature, oxygen levels, or pH, potentially causing adverse impacts on organisms and human health3,4.
The traditional methods for removing plastic waste include landfilling, incineration, and recycling. However, these methods have limitations; all of these methods are expensive and release toxic compounds that are considered potential carcinogens. The biodegradation of plastics by microorganisms has garnered increasing interest for plastic remediation due to its low cost and environmentally friendly nature5. Several bacterial genera, including Bacillus sp.6,7,8, Streptomyces sp.8,9, and Pseudomonas sp.10,11, have been reported to degrade LDPE. However, deep analysis of LDPE biodegradation pathways appears to be limited to a few bacterial phyla. Other phyla with the potential to degrade LDPE still need to be isolated and studied, particularly regarding their specific enzymes and metabolic pathways.
In addition, LDPE is a complex compound that requires the cooperative activity of different bacterial species. The use of bacterial consortia may increase the efficiency of plastic biodegradation due to their stability, functional robustness, and ability to perform complex tasks12. Investigating the dynamics and stability of bacterial composition in consortia is essential for ensuring the success, efficiency, and sustainability of bioremediation efforts aimed at mitigating environmental pollution, such as plastic waste. Moreover, following succession, which involves the gradual change in composition within a community until stability is reached, testing the community for degradation or other activities can lead to the prediction of key degraders and a deeper understanding of the involved mechanisms. This information will be valuable for optimizing biodegradation processes by providing conditions to promote the growth and activity of key degrading microorganisms; additionally, it can be used to develop guidelines for isolating key degrader strains for future applications.
The biodegradation of LDPE has been limited because of the hydrophobicity of the LDPE surface. Furthermore, the pretreatment of plastic is another way to improve biodegradation efficiency. Pretreatment can increase the surface roughness of the plastic surface or reduce the hydrophobicity of the polymer, increasing its susceptibility to microbial degradation13. Taghavi, et al.14 assessed the biodegradation of UV-pretreated and untreated PE by a consortium of four different strains: Penicillium raperi, Aspergillus flavus, Penicillium glaucoroseum, and Pseudomonas spp., over a period of 45 days. The results showed that UV pretreatment promoted roughness, hydrophilicity, and the loss of physical and molecular weight of PE. The weight loss of the UV-treated and untreated PE samples was approximately 7% and 3%, respectively. Kundungal, et al.15 studied the role of sunlight pretreatment in enhancing the biodegradation of LDPE by greater waxworm. The results showed that sunlight pretreatment of LDPE increased the surface roughness and enhanced the mineralization of LDPE. Arkatkar, et al.16 investigated the biodegradation of untreated and thermally pretreated polypropylene films by mixed soil culture and reported that after 12 months of incubation, weight losses of 10.7 and 0.43% were observed for the thermally pretreated films and untreated films, respectively. Although several studies on the pretreatment of plastics have been conducted, few studies have focused on the effect of different pretreatment methods on LDPE biodegradation17. Therefore, research in this area will contribute to the development of more effective strategies for managing LDPE waste through bioremediation. In this study, we analyzed the dynamics of the enriched culture composition during successive subcultures using high-throughput sequencing to identify the key degraders involved in LDPE biodegradation. Additionally, we aimed to investigate the potential of indigenous LDPE-degrading bacterial consortia for the biodegradation of LDPE and explore the effects of different pretreatment methods on LDPE biodegradation by indigenous bacterial consortia.
Materials and methods
Materials and chemicals
Two types of LDPE (powder and film) were used in this study. LDPE powder was purchased from Sigma‒Aldrich, USA, and utilized as the carbon source to enrich LDPE-degrading consortia. LDPE films (35 μm thickness) were obtained from Thai P.E. Film Co., Ltd. (Thailand) and used for the biodegradation test. All the LDPE samples were sterilized by soaking in absolute ethanol for 2 h and drying in a laminar air flow. The medium used for the enrichment and biodegradation studies was minimal media (MM) containing (g/L of distilled water) the following: 0.25 MgSO4, 5.8 KH2PO4, 3.7 K2HPO4, 2.0 KNO3 and 0.1 yeast extract.
Sample collection and recovery of bacteria from plastic samples
Samples of plastic bag debris and surrounding soil were collected at the sanitary landfill located in Phitsanulok Province, Thailand (16°52’57.9"N, 100°15’50.1"E) on November 25, 2020 (Fig. S1). The samples were placed in sealed zip lock bags and stored at 4 °C until use. For recovery of bacteria from plastic samples, waste plastic bags were cut into squares of 10 × 10 cm. The plastic pieces were homogenized with 0.85% (w/v) NaCl solution using an ultrasonic bath machine for 5 min and then shaken for 10 min. This procedure was repeated three times. After extraction, the solution was centrifuged and resuspended in 0.85% (w/v) NaCl solution. An aliquot of the resuspension was then used for enrichment of LDPE-degrading consortia and for DNA extraction, as explained below.
Enrichment of LDPE-degrading consortia
Five milliliters of resuspension samples retrieved from the plastic surfaces and 5 g of soil sample were added to 45 mL of MM containing 1% LDPE powder and incubated with shaking at 150 rpm at 37 °C for 30 days. The pH of the MM was 6.6. Five successive subcultures were prepared by transferring 10% (v/v) of the culture to fresh medium containing 1% LDPE powder at 30-day intervals, resulting in a total treatment period of 150 days to obtain LDPE-degrading enriched consortia. To monitor the growth of the consortia, 0.1 mL of culture was spread onto tryptic soy agar (TSA) every 30 days, and the turbidity of the culture medium was observed. To analyze the bacterial succession during the enrichment procedure, cells from the consortium of each subculture were collected for DNA extraction.
DNA extraction, 16S rRNA gene amplicon sequencing and bioinformatics analysis
DNA was extracted from the initial plastic bag waste and soil samples, as well as from each of the enriched bacterial consortia, using DNeasy PowerSoil Pro Kits (Qiagen, Hilden, Germany). DNA concentrations were determined with a NanoDrop™ 2000 spectrophotometer (Thermo Scientific, USA). The 16S rRNA gene libraries were amplified using primers 341F and 805R and sequenced by paired-end sequencing on the Illumina MiSeq platform (CA, USA) at the Omics Sciences and Bioinformatics Center (Bangkok, Thailand). The raw data were processed using the QIIME2 (Quantitative Insight into Microbial Ecology) pipeline version 2023.9 (https://library.qiime2.org) as described by Ningthoujam, et al.18. Briefly, chimeric sequences and noisy sequences were filtered while picking amplicon sequence variants (ASVs) using the DADA2 module, and taxonomy classification was performed using the SILVA reference database. Alpha and beta diversity metrics were calculated using q2–diversity after subsampling without replacement on 44,516 reads. The bacterial co-occurrence network was calculated using Spearman’s rank correlation coefficient (cor ≥ 0.7, p < 0.05)19 and visualized using Cytoscape 3.8.2 software (https://cytoscape.org)20. The functional profiles of the bacterial communities were predicted by Tax4Fun2 based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (www.kegg.jp/kegg/kegg1.html)21.
LDPE film biodegradation assay
LDPE biodegradation by the enriched bacterial consortia was conducted in 125 mL Erlenmeyer flasks containing 45 mL of MM. Four pieces of sterilized LDPE film (2 × 2 cm) were aseptically added to each flask. The inocula were prepared in 4-fold-diluted Tryptic Soy Broth (TSB) medium and incubated at 37 °C overnight with shaking. The cells were harvested by centrifugation, washed twice, and suspended in a 0.85% (w/v) NaCl solution. Five milliliters of inoculum was added to each flask to obtain initial cell concentrations of 107 CFU mL− 1. The cultures were incubated at 37 °C with shaking at 150 rpm for 120 days. Fresh MM was supplied every 30 days to maintain sufficient nutrients for bacterial growth throughout the incubation. MM supplemented with LDPE film without the addition of bacterial inoculum was used as a control. The biodegradation of LDPE films was determined at the end of the 120th day using weight loss analysis, scanning electron microscopy (SEM), atomic force microscopy (AFM), and Fourier transform infrared (FTIR) spectrophotometry. The number of bacteria attached to LDPE films was determined by a viable plate count assessment on TSA.
Weight loss of LDPE films
The LDPE films were removed from the culture medium after 120 days of incubation to estimate the biodegradation efficiency. The LDPE films were washed with 2% (w/v) sodium dodecyl sulfate (SDS), followed by distilled water, and then dried overnight at 60 °C. The LDPE films were then weighed using a 4-digit balance (OHAUS, USA). The weight loss was calculated using the following formula:
% weight loss = (initial weight – final weight) / initial weight × 100.
Analysis of LDPE topographic changes
AFM analysis was conducted to investigate changes in surface morphology. At the end of incubation, the LDPE film was retrieved from the media, washed, and dried as described above. The surface topography of the LDPE films was observed using an Asylum Research-Model MFP-3D atomic force microscope (Bruker, USA). SEM analysis was conducted to investigate bacterial attachment and surface erosion. At the end of incubation, the LDPE film was retrieved from the media, fixed in 2.5% glutaraldehyde at 4 °C overnight and dehydrated with an ethanol gradient (25, 50, 70, 90, or 100%). The LDPE films were coated with gold and imaged by SEM (Model: JEOL JSM-IT500HR) (Jeol Ltd., Japan).
FTIR spectroscopic analysis
The changes in the chemical composition of the LDPE films were analyzed by using the ATR-FTIR spectrum (Perkin Elmer, USA). At the end of incubation, the LDPE film was retrieved from the media, washed, and dried as described above. Then, the LDPE films were scanned at 600–4000 cm− 1 at 1 cm− 1 resolution. The percentage of transmittances versus wavenumbers in cm− 1 are plotted in the graph.
Viability of bacteria attached to the surface of LDPE
The viability of bacterial cells in biofilms on the LDPE film surface was measured at the end of incubation through serial dilution. The LDPE films were removed from the culture medium and washed with distilled water. The LDPE films were suspended in phosphate urea magnesium sulfate buffer22 and subjected to ultrasonication in a bath to remove the bacterial biofilm. An aliquot of this solution was serially diluted, plated on TSA agar plates and incubated at 37 °C. The results were obtained in the form of colony forming units.
Effect of different pretreatment methods on LDPE biodegradation by bacterial consortia
To enhance LDPE biodegradation, the LDPE films were pretreated using various methods. For photooxidation, one set of LDPE films was exposed to artificial UV-C irradiation (15 W, 50 Hz) in an ultraviolet chamber for 40 h. Another set was subjected to natural sunlight exposure from 9:00 AM to 5:00 PM for 5 days, totaling 40 h of sunlight. Additionally, thermal oxidation was performed by pretreating the LDPE films at 60 °C in a hot air oven for 40 h. The cultures were prepared as described above, and biodegradation was carried out using each pretreated LDPE film as a substrate. The cultures were incubated at 37 °C with shaking at 150 rpm for 120 days. MM with pretreated LDPE film without the addition of bacterial inoculum was used as controls. The biodegradation of LDPE films was determined at the end of the 120th day using weight loss analysis, SEM, AFM, and FTIR, as described above. The number of bacteria attached to the LDPE films was determined by a viable plate count assessment as described above.
Isolation of pure cultures from LDPE-degrading consortia and assessment of their LDPE biodegradation potential
Serial dilutions of all bacterial consortia were prepared and spread on TSA agar plates. Colonies with different morphologies were selected and purified. The purified isolated strains were then cultured in MM supplemented with 1% LDPE powder. The strains that grew were selected for screening LDPE degradation using 2,3,5-triphenyl tetrazolium chloride (TTC) salt23, and identification was conducted based on the analysis of their 16S rRNA gene sequences. For screening, 1% bacterial culture was added to 5 mL of MM without yeast extract, containing 1% LDPE powder and 1% TTC solution as an indicator. The cultures were incubated at 37 °C with shaking at 180 rpm for 7 days. The change in color of the medium indicated the utilization of carbon from LDPE for bacterial growth24.
Statistical analysis
All the experiments were carried out in triplicate. The data are expressed as the mean ± standard error. One-way ANOVA was employed to confirm the significance level of the experiments. Statistical analysis was performed using SPSS Software 28 (https://www.ibm.com/spss), and p ≤ 0.05 indicated statistical significance.
Results and discussion
Bacterial succession during the enrichment procedure
In the enrichment conditions, the bacterial consortia were cultivated in MM containing LDPE powder at 37 °C with shaking at 150 rpm and a pH of 6.6. The enrichment period lasted for 150 days. Five enriched stages of the consortia were collected for analysis of bacterial succession using Illumina high-throughput sequencing. The original plastic bag waste (PB1_O, PB2_O, and PB3_O) and soil (LS_O) samples exhibited higher alpha diversity indices of bacterial communities compared to the enrichment culture samples (Table S1). After enrichment with LDPE, the alpha diversity of the mixed cultures decreased. With each subsequent enrichment process, the alpha diversity indices of the mixed cultures further decreased (Table S1). Exposure to LDPE has the potential to eliminate microorganisms that cannot utilize LDPE for growth and to enhance the presence of LDPE-consuming bacteria, leading to a reduction in bacterial diversity. Wang, et al.25 also reported that the richness and diversity of the bacterial community in enrichment culture with PE mulching film were lower than those in agricultural soil. Principal coordinate analysis (PCoA) based on distances of beta diversity was conducted to determine the dissimilarity among samples. The results revealed that after exposure to LDPE, the communities in the enrichment culture differed from the communities in the original environmental sample (Fig. 1). The bacterial communities achieved stability after several subcultures (4th − 5th subcultures). Furthermore, the communities of LDPE-enriched cultures (PB1, PB2, and PB3) isolated from plastic bag wastes clustered separately from the LDPE-enriched cultures (LS) isolated from the soil.
The bacterial community structures of the original plastic bag waste samples and the original soil and enrichment culture samples at different time points were analyzed at the phylum, class, and genus levels (Fig. 2a–c). Proteobacteria (61–82%) predominated in the original plastic bag waste samples, followed by Firmicutes (14–38%). In the original soil sample, Actinobacteriota (55%) and Firmicutes (43%) were the major phyla (Fig. 2a). Gammaproteobacteria was the dominant class among the Proteobacteria during the initiation of enrichment but decreased after long-term enrichment. Conversely, the abundance of Alphaproteobacteria and Actinobacteria increased during the final 3rd − 5th subcultures (Fig. 2b). This observation suggests that both bacterial groups can adapt to the presence of LDPE. Previous studies have reported that Alphaproteobacteria and Actinobacteria are enriched when exposed to polymers. Shi, et al.26 reported that the relative abundance of Alphaproteobacteria increased with increasing concentrations of PE residue in the soil. Huang, et al.27 also evaluated the impacts of LDPE microplastics on microbial communities in soil and reported that Actinobacteria were significantly enriched on the microplastic. Additionally, it was noted that the increase in the abundance of Alphaproteobacteria and Actinobacteria followed the same trend, suggesting potential synergistic interactions. Actinobacteria are well known for their role in breaking down complex hydrocarbons, including LDPE. These bacteria produce extracellular enzymes that degrade long-chain polymers, making them primary degraders directly involved in LDPE breakdown28. Their increased abundance reflects their participation in the degradation process. In contrast, Alphaproteobacteria may play a supporting role in the microbial community. Their increased abundance in the presence of LDPE may be linked to the utilization of byproducts generated from the initial breakdown of LDPE by Actinobacteria.
At the genus level, the dominant groups of each enriched consortium were different (Fig. 2c). At the end of the enrichment process, consortium PB1 exhibited dominance, with Mycobacterium (27%) and Cupriavidus (22%) as the dominant genera, while consortium PB2 was characterized by the prevalence of Gordonia (23%), Ochrobactrum (21%), and Cupriavidus (18%) in its bacterial communities. In contrast, consortium PB3 was dominated by Ochrobactrum (24%) and Nocardia (20%). In consortium LS, the dominant genera were Hyphomicrobium (21%) and Gordonia (15%) (Table 1). Rong, et al.29 reported that the genera Mycobacterium and Hyphomicrobium were significantly enriched in soil amended with LDPE microplastics. Huerta Lwanga, et al.30 reported that a bacterial consortium, which included Microbacterium awajiense, Rhodococcus jostii, Mycobacterium vanbaalenii, Streptomyces fulvissimus and Bacillus spp., isolated from the gut of earthworms was able to degrade LDPE microplastics within 4 weeks. Montazer, et al.31 reported that the LDPE powder showed a maximum weight loss of up to 33.7% within 21 days due to Cupriavidus necator H16. Reports have shown that Gordonia and Nocardia can degrade PE films32,33. Gao and Sun34 reported that a consortium that included Ochrobactrum sp. demonstrated efficient degradation of PET and PE films.
Co-occurrence network analysis of bacterial communities
Given that LDPE has an impact on bacterial communities, co-occurrence networks were constructed for each consortium during the enrichment process to predict bacterial interactions and the core bacterial taxa. According to Fig. 3, the PB1, PB2, PB3, and LS consortia incorporated 25, 18, 22, and 23 nodes, respectively, connecting 56, 25, 41, and 66 edges, respectively. The nodes represent bacterial genera, whereas the edges depict notable positive correlations (green) and negative correlations (red). Most of the nodes in the network of each consortium belonged to the Proteobacteria and Actinobacteria phyla, which implies that exposure to PE targets specific bacterial groups. Agromyces could be considered the hub of the consortium PB1 network because it had the greatest number of edges, as shown in Fig. 3a. In the case of consortium PB2, Cupriavidus, Isoptericola, and Gordonia could be considered hubs of the network (Fig. 3b). Similarly, Achromobacter, Amycolatopsis, Cellulosimicrobium, and Isoptericola could be considered hubs of the consortium PB3 network (Fig. 3c). Finally, for consortium LS, Edaphobaculum, Bacillus, and Mycobacterium could be considered hubs of the network (Fig. 3d). These results suggest that these bacteria might play an important role in the degradation of LDPE. Some studies have also shown that LDPE can be degraded by the genera Cupriavidus31, Gordonia32, Achromobacter35, and Bacillus36.
Predicted bacterial community functions
To analyze the functional changes in the bacterial community during subculturing, Tax4Fun2 based on 16S rRNA gene sequencing data was used to predict metabolic potential. Six functional groups were identified, namely, metabolism, cellular processes, environmental information processing, genetic information processing, organismal systems, and human diseases (Fig. 4a). Metabolism (68.8–80.4%) was the most abundant KEGG level 1 pathway of all consortia. Environmental information processing (7.7–13.1%) and cellular processes (4.6–9.6%) were the second and third most abundant KEGG level 1 pathways, respectively.
In terms of xenobiotic degradation at KEGG level 2, benzoate degradation was the most abundant function and increased during the enrichment process (Fig. 4b). Benzoate is known to be a byproduct of the degradation of polymers, such as PE37. Moreover, the relative abundances of other subgroups involved in the degradation of bisphenol, furfural, dioxins, xylene, toluene, polycyclic aromatic hydrocarbons, naphthalene, aminobenzoate, atrazine, caprolactam, and steroids all increased during the enrichment process.
Evaluation of the biodegradation of LDPE films with different pretreatment methods
Weight loss analysis
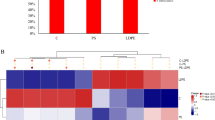
For the biodegradation assays, the bacterial consortia were cultivated in MM containing LDPE films at 37 °C with shaking at 150 rpm and a pH of 6.6. The assays were conducted over a period of 120 days. Initially, the biodegradation of LDPE films was confirmed through weight loss analysis. There was no weight reduction in the control LDPE. The untreated LDPE films inoculated with all four consortia showed 1.3–2.8% weight loss in 120 days (Fig. 5). However, to enhance the biodegradation process, three different pretreatment methods were employed for the present study. The LDPE films were subjected to thermal, sunlight, and artificial UV pretreatments for 40 h. The results showed that the degradation of pretreated LDPE films was better than that of untreated films. The LDPE films exposed to artificial UV-C irradiation demonstrated greater degradation potential compared to other pretreatment methods, with weight losses ranging from 2.2 to 5.27%. In contrast, natural sunlight and thermal treatments resulted in weight losses of 1.7–4.6% and 1.4–3.2%, respectively. The UV-treated LDPE films, when incubated with all consortia, significantly lost weight compared to the control, except for those treated with consortium PB1 (Fig. 5). Taghavi, el al14 investigated the effect of UV pretreatment on PE, PS and PET and reported that UV pretreatment improved the biodegradation efficiency of PE and PS. UV pretreatment of plastics results in increased hydrophilicity of the plastic structure, bacterial colonization, and improved biodegradation efficiency38. Artificial UV-C light, especially in controlled environments, tends to have more concentrated and consistent wavelength exposure, enhancing degradation more effectively than natural sunlight. Sunlight contains a broader spectrum of UV light (including UVA, UVB, and UVC), but due to atmospheric filtering and intensity variability, its effect is often weaker than artificial UV-C in controlled settings39. However, pretreating LDPE with sunlight has the advantage of incurring no external costs, making it a more economical and environmentally friendly option. To assess bacterial growth after biodegradation, the numbers of bacteria attached to the LDPE films were determined. The results showed that bacteria from the PB2, PB3, and LS consortia adhered to the LDPE surface, while the bacterial growth of consortium PB1 on the LDPE surface was lower than that of the other consortia (Table S2). When comparing bacterial growth with biodegradation efficiency, LDPE was degraded by the PB1 consortium at a low efficiency, and the number of bacteria associated with PB1 on the LDPE surface was low. This result is consistent with previous reports indicating the attachment of bacterial cells, which is an important step in the biodeterioration of LDPE. Gupta and Devi40 studied the biodegradation of LDPE by Bacillus spp. ISJ36, ISJ38, and ISJ40. Strain ISJ40 showed the maximum cell density on the LDPE surface and resulted in a greater weight reduction in the LDPE films than did the other two strains. Although the weight reduction of LDPE in this study was lower than in other studies, such as the one by Esmaeili, et al.41, which investigated the effect of UV pretreatment on LDPE, the results showed that a mixed culture of Lysinibacillus xylanilyticus and Aspergillus niger degraded UV-treated LDPE film by 29.5% and untreated film by 15.8% within 126 days. The duration of pretreatment was 25 days. Factors such as the grade of LDPE, impurities, pretreatment duration, and the capabilities of the bacteria contributed to this difference in efficiency35.
FTIR analysis
FTIR analysis was conducted on different pretreated LDPE films over a period of 120 days to validate the chemical modification of the basic structure of the polymer. In this study, LDPE films inoculated with PB3 and LS consortia were selected as representatives to observe changes in the chemical structure of LDPE. Figure 6 (a–d) shows the FTIR spectra of the control and experimental groups. The corresponding functional groups for all wavenumbers characteristic of LDPE are presented in Table S3. The FTIR spectra of LDPE treated with bacterial consortia were similar to the identified peaks in the control films, but the peaks were generally sharper. Sharper peaks (indicating lower transmittance) were interpreted as indicating a greater presence of the corresponding functional group in that part of the spectra. A similar spectrum was previously reported by several researchers42,43. All the spectra showed the presence of functional group peaks at 2910 cm− 1, 2846 cm− 1, 1462 cm− 1, and 718 cm− 1, corresponding to the CH2 stretching, C–H stretching, C-H scissoring and C-H rocking deformation, respectively35. The peaks attributed to CH2 stretching and C–H stretching at 2910 cm− 1 and 2846 cm− 1 were sharper for LDPE treated with the bacterial consortia. Moreover, several peaks between 650 and 712 cm− 1 were observed for the LDPE inoculated with both consortia, which might have resulted from CH2- deformation43. These modifications of the functional group peaks represent the interaction of bacteria with the LDPE films.
AFM analysis
The surface deterioration of LDPE films by the PB3 and LS consortia was examined using AFM technique. This technique has been employed to observe surface roughness7,44. Compared with those of the controls, the AFM images revealed changes in surface topography after 120 days of bacterial treatment (Fig. 7). The topographic images of LDPE films inoculated with bacterial consortia showed the formation of ridges, pits, and grooves, confirming disruption of the LDPE surface morphology. Several previous studies have suggested similar morphological changes in polymer surfaces due to the formation of bacterial biofilms and enzymatic action on the surface of LDPE23,45. Furthermore, the topographic images of pretreated LDPE films inoculated with bacterial consortia exhibited more surface erosion and roughness than did those of the untreated LDPE films. Kundungal, et al.15 reported that pretreatment of LDPE increased its surface roughness, increasing its susceptibility to microbial degradation.
SEM analysis
SEM analysis has demonstrated its effectiveness in investigating the degradation of plastics in many studies6,46. In this study, UV-treated LDPE films inoculated with all consortia were selected as representatives to observe structural changes on LDPE films (Fig. 8). The degradation potential of the consortia was characterized by comparing the images of control LDPE films. SEM images revealed biofilm formation, cracks, pits, grooves, and surface erosion on LDPE surfaces inoculated with all consortia after 120 days of incubation (Fig. 8d–o). In contrast, no evident structural changes occurred in the control films (Fig. 8a–c). The bacteria that penetrated into the polymer matrix confirmed the susceptibility of the polymer surface to microbial degradation and the ability of bacteria to break down the polymers35.
SEM micrographs of LDPE films without bacteria (control) at 2000× (a), 7000× (b), and 10,000× magnification (c) and UV-treated LDPE films incubated with consortium PB1 at 2500× (d), 7000× (e), and 12,000× magnification (f), consortium PB2 at 2000× (g), 7000× (h), and 12,000× magnification (i), consortium PB3 at 2500× (j), 7000× (k), and 12,000× magnification (l), and consortium LS at 2500× (m), 7000× (n), and 12,000× magnification (o) after 120 days of incubation. Red arrows indicate holes on the surface of the LDPE film produced from the bacterial consortia.
Screening and identification of LDPE-degrading bacteria
A total of 26 cultivable strains were isolated from the LDPE-degrading consortia that exhibited a color change resulting from the reduction of TTC to triphenyl formazan (red color) (Fig. S2). The reduction of TTC occurred due to the bacterial electron transport system, which utilizes carbon from LDPE as an energy source in the biodegradation process for bacterial growth23. All 26 bacterial isolates were identified by 16S rRNA gene sequencing analysis and classified into 16 different genera, namely, Mesorhizobium, Agromyces, Paracoccus, Microbacterium, Bordetella, Cellulosimicrobium, Gordonia, Olivibacter, Stenotrophomonas, Mycobacterium, Aquamicrobium, Pseudaminobacter, Mycolicibacterium, Amycolatopsis, Corynebacterium, and Nocardia (Table 2). Interestingly, most of the bacterial isolates were Actinobacteria and Proteobacteria, which were shown to be the major bacterial groups in the LDPE-degrading communities. Additionally, several bacterial isolates were found to be key bacteria in the co-occurrence networks. Various bacterial genera have been previously reported to be involved in the biodegradation of LDPE, such as Microbacterium47, Paracoccus10, Cellulosimicrobium48, Gordonia32, Stenotrophomonas49, and Nocardia50. However, studies on LDPE degradation by other genera, including Mesorhizobium, Agromyces, Bordetella, Olivibacter, Mycobacterium, Aquamicrobium, Pseudaminobacter, Mycolicibacterium, Amycolatopsis, and Corynebacterium, are still rare. These results contribute to expanding our understanding of bacterial taxa capable of utilizing LDPE as a carbon source. Furthermore, although some bacteria, such as Ochrobactrum and Cupriavidus, showed high abundance in the bacterial community, they were not successfully isolated in this study. We suggest that this may be due to the difficulty of cultivating certain bacteria, which may have specific growth requirements that were not met during our isolation attempts.
Conclusions
This study investigated the succession patterns of bacterial communities in enriched consortia in response to LDPE. We identified Actinobacteria and Proteobacteria as potential key players in LDPE biodegradation, with dominant genera including Mycobacterium, Cupriavidus, Gordonia, Ochrobactrum, Nocardia, Agromyces, Amycolatopsis, and Cellulosimicrobium. Additionally, we explored the potential of bacterial consortia for degrading both untreated and pretreated LDPE films. The degradation of the pretreated LDPE films was superior to that of the untreated films. UV-treated LDPE films incubated with all four consortia showed maximum degradation within 120 days. AFM, SEM, and FTIR analyses confirmed surface changes and chemical modifications in LDPE films treated with all consortia. Therefore, UV pretreatment can be effectively used as a strategy before LDPE is subjected to biodegradation. Furthermore, the isolation of potential LDPE-degrading Actinobacteria and Proteobacteria from the enriched consortia further supports their importance in plastic degradation. These findings provide valuable insights for the development of sustainable applications in plastic waste management, highlighting the potential of bacterial consortia, individual strains, and pretreatment methods for efficient plastic biodegradation. For a more complete understanding of the LDPE biodegradation process, further research should include an analysis of the intermediates formed during LDPE biodegradation.
Data availability
The 16S rRNA gene amplicon sequences were deposited in the NCBI Sequence Read Archive (SRA) under BioProject accession no. PRJNA1096991. The sequences of isolated strains have been deposited in the GenBank database under accession numbers PP579354 to PP579379.
References
Tong, Y. et al. The occurrence, speciation, and ecological effect of plastic pollution in the bay ecosystems. Sci. Total Environ. 857, 159601. https://doi.org/10.1016/j.scitotenv.2022.159601 (2023).
Suleman, R. et al. Impact of plastic bags usage in food commodities: an irreversible loss to environment. Environ. Sci. Pollut Res. 29, 49483–49489. https://doi.org/10.1007/s11356-022-21091-3 (2022).
Dey, A. et al. Challenges and possible solutions to mitigate the problems of single-use plastics used for packaging food items: a review. J. Food Sci. Technol. 58, 3251–3269. https://doi.org/10.1007/s13197-020-04885-6 (2021).
Li, Y. et al. Leaching of chemicals from microplastics: a review of chemical types, leaching mechanisms and influencing factors. Sci. Total Environ. 906, 167666. https://doi.org/10.1016/j.scitotenv.2023.167666 (2024).
Thew, C. X. E. et al. Recent advances and challenges in sustainable management of plastic waste using biodegradation approach. Bioresour Technol. 374, 128772. https://doi.org/10.1016/j.biortech.2023.128772 (2023).
Jayan, N., Skariyachan, S. & Sebastian, D. The escalated potential of the novel isolate Bacillus cereus NJD1 for effective biodegradation of LDPE films without pre-treatment. J. Hazard. Mater. 455, 131623. https://doi.org/10.1016/j.jhazmat.2023.131623 (2023).
Ji, S. H. et al. Biodegradation of low-density polyethylene by plasma-activated Bacillus strain. Chemosphere 349, 140763. https://doi.org/10.1016/j.chemosphere.2023.140763 (2024).
Rajapandi, J. D. & Rajamanickam, U. Low–density polyethylene management by using selective bacterial strains from garbage soil. Biologia 79, 985–1001. https://doi.org/10.1007/s11756-023-01595-0 (2024).
Kong, D. et al. Enhanced biodegradation activity toward polyethylene by fusion protein of anchor peptide and Streptomyces sp. strain K30 latex clearing protein. Int. J. Biol. Macromol. 264, 130378. https://doi.org/10.1016/j.ijbiomac.2024.130378 (2024).
Pathak, V. M. & Navneet Exploitation of bacterial strains for microplastics (LDPE) biodegradation. Chemosphere 316, 137845. https://doi.org/10.1016/j.chemosphere.2023.137845 (2023).
Shilpa, Basak, N. & Meena, S. S. Biodegradation of low-density polythene (LDPE) by a novel strain of Pseudomonas aeruginosa WD4 isolated from plastic dumpsite. Biodegradation 35, 641–655. https://doi.org/10.1007/s10532-023-10061-2 (2024).
Han, Y. N. et al. Greater biofilm formation and increased biodegradation of polyethylene film by a microbial consortium of Arthrobacter sp. and Streptomyces sp. Microorganisms 8, 1979. https://doi.org/10.3390/microorganisms8121979 (2020).
Ciuffi, B., Fratini, E. & Rosi, L. Plastic pretreatment: the key for efficient enzymatic and biodegradation processes. Polym. Degrad. Stab. 222, 110698. https://doi.org/10.1016/j.polymdegradstab.2024.110698 (2024).
Taghavi, N., Zhuang, W. Q. & Baroutian, S. Enhanced biodegradation of non-biodegradable plastics by UV radiation: part 1. J. Environ. Chem. Eng. 9, 106464. https://doi.org/10.1016/j.jece.2021.106464 (2021).
Kundungal, H. et al. Role of pretreatment and evidence for the enhanced biodegradation and mineralization of low-density polyethylene films by greater waxworm. Environ. Technol. 42, 717–730. https://doi.org/10.1080/09593330.2019.1643925 (2021).
Arkatkar, A. et al. Degradation of unpretreated and thermally pretreated polypropylene by soil consortia. Int. Biodeterior. Biodegradation. 63, 106–111. https://doi.org/10.1016/j.ibiod.2008.06.005 (2009).
Rani, R. et al. Isolation, characterization and optimization of bacterial isolate SARR1 for biodegradation of pretreated low density polyethylene. J. Appl. Nat. Sci. 13, 561–570. https://doi.org/10.31018/jans.v13i2.2663 (2021).
Ningthoujam, R. et al. Bacterial community shifts in a di-(2-ethylhexyl) phthalate-degrading enriched consortium and the isolation and characterization of degraders predicted through network analyses. Chemosphere 310, 136730. https://doi.org/10.1016/j.chemosphere.2022.136730 (2023).
Schwager, E. et al. CCREPE: compositionality corrected by permutation and renormalization. (2020). https://bioconductor.org/packages/devel/bioc/vignettes/ccrepe/inst/doc/ccrepe.pdf
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Wemheuer, F. et al. Tax4Fun2: prediction of habitat-specific functional profiles and functional redundancy based on 16S rRNA gene sequences. Environ. Microbiome. 15, 1–12 (2020).
Balasubramanian, V. et al. High-density polyethylene (HDPE)-degrading potential bacteria from marine ecosystem of Gulf of Mannar, India. Lett. Appl. Microbiol. 51, 205–211. https://doi.org/10.1111/j.1472-765X.2010.02883.x (2010).
Khandare, S. D. et al. Marine bacterial based enzymatic degradation of low-density polyethylene (LDPE) plastic. J. Environ. Chem. Eng. 10, 107437. https://doi.org/10.1016/j.jece.2022.107437 (2022).
Khandare, S. D., Chaudhary, D. R. & Jha, B. Bioremediation of polyvinyl chloride (PVC) films by marine bacteria. Mar. Pollut Bull. 169, 112566. https://doi.org/10.1016/j.marpolbul.2021.112566 (2021).
Wang, P. et al. Does bacterial community succession within the polyethylene mulching film plastisphere drive biodegradation? Sci. Total Environ. 824, 153884. https://doi.org/10.1016/j.scitotenv.2022.153884 (2022).
Shi, Z. et al. Alteration of bacterial communities and co-occurrence networks as a legacy effect upon exposure to polyethylene residues under field environment. J. Hazard. Mater. 426, 128126. https://doi.org/10.1016/j.jhazmat.2021.128126 (2022).
Huang, Y. et al. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 254, 112983. https://doi.org/10.1016/j.envpol.2019.112983 (2019).
Skariyachan, S. et al. Recent advances in plastic degradation – from microbial consortia-based methods to data sciences and computational biology driven approaches. J. Hazard. Mater. 426, 128086. https://doi.org/10.1016/j.jhazmat.2021.128086 (2022).
Rong, L. et al. LDPE microplastics affect soil microbial communities and nitrogen cycling. Sci. Total Environ. 773, 145640. https://doi.org/10.1016/j.scitotenv.2021.145640 (2021).
Huerta Lwanga, E. et al. Decay of low-density polyethylene by bacteria extracted from earthworm’s guts: a potential for soil restoration. Sci. Total Environ. 624, 753–757. https://doi.org/10.1016/j.scitotenv.2017.12.144 (2018).
Montazer, Z., Habibi Najafi, M. B. & Levin, D. B. Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can. J. Microbiol. 65, 224–234. https://doi.org/10.1139/cjm-2018-0335 (2018).
Wang, H. et al. Biofilm formation promoted biodegradation of polyethylene in Gordonia polyisoprenivorans B251 isolated from bacterial enrichment acclimated by hexadecane for two years. Chemosphere 344, 140383. https://doi.org/10.1016/j.chemosphere.2023.140383 (2023).
Soleimani, Z. et al. A survey of intact low-density polyethylene film biodegradation by terrestrial Actinobacterial species. Int. Microbiol. 24, 65–73. https://doi.org/10.1007/s10123-020-00142-0 (2021).
Gao, R. & Sun, C. A marine bacterial community capable of degrading poly(ethylene terephthalate) and polyethylene. J. Hazard. Mater. 416, 125928. https://doi.org/10.1016/j.jhazmat.2021.125928 (2021).
Maleki Rad, M., Moghimi, H. & Azin, E. Biodegradation of thermo-oxidative pretreated low-density polyethylene (LDPE) and polyvinyl chloride (PVC) microplastics by Achromobacter denitrificans Ebl13. Mar. Pollut Bull. 181, 113830. https://doi.org/10.1016/j.marpolbul.2022.113830 (2022).
Liu, X. et al. Rapid colonization and biodegradation of untreated commercial polyethylene wrap by a new strain of Bacillus velezensis C5. J. Environ. Manage. 301, 113848. https://doi.org/10.1016/j.jenvman.2021.113848 (2022).
Amobonye, A. et al. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 759, 143536. https://doi.org/10.1016/j.scitotenv.2020.143536 (2021).
Erdmann, M. et al. Photo-oxidation of PE-HD affecting polymer/fuel interaction and bacterial attachment. Npj Mater. Degrad. 4, 18. https://doi.org/10.1038/s41529-020-0122-1 (2020).
Doğan, M. Ultraviolet light accelerates the degradation of polyethylene plastics. Microsc Res. Tech. 84, 2774–2783. https://doi.org/10.1002/jemt.23838 (2021).
Gupta, K. K. & Devi, D. Biofilm mediated degradation of commercially available LDPE films by bacterial strains isolated from partially degraded plastic. Rem. J. 30, 39–47. https://doi.org/10.1002/rem.21660 (2020).
Esmaeili, A. et al. Biodegradation of low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus Niger in soil. PLoS One. 8, e71720. https://doi.org/10.1371/journal.pone.0071720 (2013).
Taghavi, N. et al. Degradation of plastic waste using stimulated and naturally occurring microbial strains. Chemosphere 263, 127975. https://doi.org/10.1016/j.chemosphere.2020.127975 (2021).
Gowthami, A. et al. Biodegradation efficacy of selected marine microalgae against low-density polyethylene (LDPE): an environment friendly green approach. Mar. Pollut Bull. 190, 114889. https://doi.org/10.1016/j.marpolbul.2023.114889 (2023).
Rong, Z. et al. Degradation of low-density polyethylene by the bacterium Rhodococcus sp. C-2 isolated from seawater. Sci. Total Environ. 907, 167993. https://doi.org/10.1016/j.scitotenv.2023.167993 (2024).
Jebashalomi, V., Emmanuel Charles, P. & Rajaram, R. Microbial degradation of low-density polyethylene (LDPE) and polystyrene using Bacillus cereus (OR268710) isolated from plastic-polluted tropical coastal environment. Sci. Total Environ. 924, 171580. https://doi.org/10.1016/j.scitotenv.2024.171580 (2024).
Dimassi, S. N. et al. Investigation on the effect of several parameters involved in the biodegradation of polyethylene (PE) and low-density polyethylene (LDPE) under various seawater environments. Sci. Total Environ. 912, 168870. https://doi.org/10.1016/j.scitotenv.2023.168870 (2024).
Montazer, Z., Habibi Najafi, M. B. & Levin, D. B. In vitro degradation of low-density polyethylene by new bacteria from larvae of the greater wax moth, Galleria mellonella. Can. J. Microbiol. 67, 249–258. https://doi.org/10.1139/cjm-2020-0208 (2021).
Muhonja, C. N. et al. Biodegradability of polyethylene by bacteria and fungi from Dandora Dumpsite Nairobi-Kenya. PLoS One. 13, e0198446. https://doi.org/10.1371/journal.pone.0198446 (2018).
Dey, A. S. et al. Biodegradation of unpretreated low-density polyethylene (LDPE) by Stenotrophomonas sp. and Achromobacter sp., isolated from waste dumpsite and drilling fluid. Front. Microbiol. 11 https://doi.org/10.3389/fmicb.2020.603210 (2020).
Koutny, M. et al. Acquired biodegradability of polyethylenes containing pro-oxidant additives. Polym. Degrad. Stab. 91, 1495–1503. https://doi.org/10.1016/j.polymdegradstab.2005.10.007 (2006).
Acknowledgements
This research project is supported by the Second Century Fund (C2F), Chulalongkorn University, the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), and Thailand Science Research and Innovation Fund Chulalongkorn University (DIS66230009).
Author information
Authors and Affiliations
Contributions
C.M. conceived the original idea, performed the experiments, analyzed data, wrote the original manuscript and edited the manuscript. O.P. conceived the original idea, supervised the project, provided resources and wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Muangchinda, C., Pinyakong, O. Enrichment of LDPE-degrading bacterial consortia: Community succession and enhanced degradation efficiency through various pretreatment methods. Sci Rep 14, 28795 (2024). https://doi.org/10.1038/s41598-024-80306-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80306-4
Keywords
This article is cited by
-
Enhanced gaseous chlorobenzene removal and its microbial mechanism through innovative modified packings coupled with magnetic field in a biotrickling filter
Frontiers of Environmental Science & Engineering (2025)
-
A comprehensive review on microbial degradation of polyethylene plastics: ecotoxicity, deterioration process, recurrent intermediates and key enzymes involved
Archives of Microbiology (2025)
-
Microbial diversity analysis of municipal solid waste landfills soils of Delhi (NCR) and plastic dump sites of Uttar Pradesh region of India and their function prediction for plastic degrading enzymes
World Journal of Microbiology and Biotechnology (2025)