Abstract
There are no studies discussing the significance of neutrophil-to-lymphocyte ratio (NLR), neutrophil-percentage-to-albumin ratio (NPAR), and systemic immune-inflammation index (SII) in predicting poor prognosis in patients with metabolic dysfunction associated steatotic liver disease (MASLD); this study aimed to investigate the relationship between these three inflammatory markers and all-cause mortality and cardiovascular disease (CVD) mortality in patients with MASLD. Survival data for 3970 participants were obtained from National Death Index (NDI) records associated with the National Health and Nutrition Examination Survey (NHANES) dataset, the associations of NPAR, NLR, and SII with all-cause and CVD mortality were analyzed using multivariate COX regression modeling, restricted cubic spline (RCS) was used to explore nonlinear relationships and to determine the inflection point, regrouping was done according to the nonlinear inflection point, using multivariate COX regression modeling, subgroup analysis, and the Kaplan-Meier survival curves to evaluate differences in risk of death between the two groups. Time-dependent receiver operating characteristic curve (ROC) analysis was conducted to assess the predictive efficacy of NPAR, NLR, and SII on survival outcomes. Multivariate COX regression and RCS analyses revealed a positive linear correlation between NLR and all-cause and CVD mortality, whereas a nonlinear relationship was found between NPAR and SII and all-cause and CVD mortality. Further reclassified into two groups according to the inflection point, multivariate COX regression analyses showed a significant difference in the risk of death between the two NPAR groups (HR 1.37, 95% CI = (1.01, 1.86) for all-cause mortality and HR 2.03, 95% CI = (1.24, 3.32) for CVD mortality ) and no difference in the risk of death between the two SII groups (HR 1.11, 95% CI = (0.87, 1.42) for all-cause mortality and HR 1.35, 95% CI = (0.86, 2.12) for CVD mortality), and Kaplan-Meier survival curves showed that both all-cause and CVD mortality rates were higher in patients with MASLD above the NPAR inflection point (log-rank P < 0.05). Subgroup analyses showed that the associations between high levels of NPAR and all-cause mortality were generally consistent across populations (P interaction > 0.05). Also, COPD subgroups had a significant effect on the correlation between high levels of NPAR and CVD mortality (P interaction < 0.05). Time-dependent ROC show the predictive value of NPAR, NLR, and SII for all-cause and CVD mortality in MASLD patients. The correlation between NPAR and mortality was nonlinear, and NLR was linearly and positively correlated with mortality, Measuring NPAR and NLR may be useful in assessing risk and predicting prognosis in populations of patients with MASLD.
Similar content being viewed by others
Background
Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disorder worldwide1, characterized by steatosis in more than 5% of hepatocytes without any other causes of liver damage, such as excessive alcohol consumption2. In recent years, the terminology of fatty liver disease has been controversial, with NAFLD noted to be difficult to summarize the relationship between liver steatosis and systemic metabolic disorders and suspected of stigmatizing obese patients3, Recently, a consensus group of the three major international societies of hepatology (AASLD, EASL, and ALEH) proposed a new definition of metabolic dysfunction associated steatotic liver disease (MASLD) to replace NAFLD4, the definition of MASLD was revised to include the presence of liver steatosis and the presence of at least one of five cardiometabolic risk factors, with the aim of better identifying metabolism-related risk factors in patients with fatty liver disease, increasing their clinical benefit, and eliminating potential stigmatizing connotations of the original definition5.
Previous studies have shown that patients with MASLD have a higher risk of death from liver-related complications, cardiovascular disease (CVD), and malignancy and that CVD is the leading cause of death in these patients6,7,8. However, prediction of adverse outcomes in MASLD remains difficult because the underlying mechanisms of its progression have not been identified9. It is believed that chronic inflammation contributes significantly to the development of MASLD10,11, which is characterized by lipotoxicity produced by excessive lipid deposition in the liver, which causes cellular damage and releases a large number of inflammatory factors, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), resulting in necroinflammation and progression of MASLD to metabolic dysfunction-associated steatohepatitis (MASH)12, and stimulation of liver stellate cells, leading to liver fibrosis13.
The neutrophil-to-lymphocyte ratio (NLR), calculated by absolute counts of neutrophils and lymphocytes, is a reliable biomarker reflecting the state of systemic inflammation14. Neutrophil-percentage-to-albumin ratio (NPAR), calculated by dividing the neutrophil percentage numerator serum albumin concentration, is another simple and easy-to-measure biomarker, and studies have confirmed that the prognosis of patients with acute kidney injury, cardiogenic shock, myocardial infarction, and cancer can be predicted with the help of NPAR15. Furthermore, the Systemic immune-inflammation index (SII, platelets count × neutrophil count/lymphocyte count ), a novel inflammatory marker that has been found to reflect the degree of local immune response and inflammation, can be used to assess the prognosis of liver cancer16 and is an independent predictor of prognosis in patients with CVD17.
Currently, none of the studies have evaluated the significance of NLR, NPAR, and SII in predicting the prognosis of adverse outcomes in patients with MASLD; therefore, this study aimed to investigate whether these three inflammatory markers have predictive value for all-cause and CVD mortality in patients with MASLD.
Materials and methods
Data source
For this study, data were extracted from the National Health and Nutrition Examination Survey (NHANES) database, which is subordinate to the National Center for Health Statistics (NCHS). The database was created to assess the health and nutritional status of the United States noninstitutionalized population using a complex stratified, multistage sampling statistic, with every two years being a survey cycle. The survey includes demographic and socioeconomic information, household diet and health status interviews, and physical and laboratory examinations. NHANES survey protocol was approved by the NCHS Ethics Review Committee, and all participants signed an informed consent form allowing secondary data analysis to be conducted without requiring additional ethical review18. The Declaration of Helsinki is followed by the survey methods employed in this research.
Definition of MASLD
Diagnosis of liver steatosis using the United States Multiracial Population Modified Fatty Liver Index (USFLI). MASLD was defined as the presence of liver steatosis (USFLI ≥ 30)19 and ≥ 1 of 5 cardiometabolic risk factors with no other identifiable cause of liver steatosis.
The USFLI is calculated using the following formula:
The five cardiometabolic risk factors are listed below20:
-
1.
Body mass index (BMI) ≥ 25 kg/m2 (23 Asia) OR waist circumference (WC) > 94 cm (males)/> 80 cm (females) OR ethnicity-adjusted;
-
2.
Fasting blood glucose (FBG) ≥ 5.6 mmol/L (100 mg/dL) OR 2-h post-load glucose levels ≥ 7.8 mmol/L (≥ 140 mg/dL) OR glycosylated hemoglobin A1c (HbA1c) ≥ 5.7% (≥ 39 mmol/L) OR type 2 diabetes mellitus (DM) OR treatment for type 2 DM;
-
3.
Blood pressure ≥ 130/85 mmHg OR specific antihypertensive drug treatment;
-
4.
Plasma triglycerides (TG) ≥ 1.70 mmol/L (≥ 150 mg/dL OR lipid-lowering treatment;
-
5.
Plasma high-density lipoprotein cholesterol (HDL-C) cholesterol ≤ 1.0 mmol/L (≤ 40 mg/dL) (male) and ≤ 1.3 mmol/L (≤ 50 mg/dL) (female) OR lipid-lowering treatment.
Cases selection
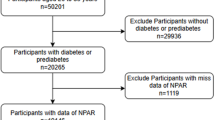
Data from six cycles of the NHANES database from 2005 to 2016 were collected for this study, and the included population was 36,287 adults aged ≥ 18 years. We excluded 31,311 individuals with missing USFLI components or USFLI < 30 and 51 pregnant individuals, the questionnaire-based 142 individuals with viral hepatitis (Positive serologic markers for hepatitis B or C virus and a doctor’s diagnosis of hepatitis B or C), 653 individuals with excessive alcohol consumption (Defined as > 1 alcoholic drink/day for female or > 2 alcoholic drinks/day for male), and 3 patients with liver cancer as well as 7 individuals with severe liver injury (aspartate aminotransferase (aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyl transpeptidase (GGT) > 500IU/L) and 4 individuals with end-stage renal disease (defined by an estimated glomerular filtration rate (eGFR) < 15), and 6 individuals without metabolic risk, 4110 participants were diagnosed with MASLD. Next, we excluded 20 participants with missing data on platelet counts, 6 with missing data on neutrophil percentages, neutrophil counts, and lymphocyte counts, 113 with missing disease status, and 1 with missing survival status. Finally, 3,970 participants were enrolled in the study (Fig. 1).
All-cause mortality and CVD mortality
We identified participant mortality using the death certificate records from the National Death Index (NDI) updated to the end of 2019, which includes information on nine potential cause-of-death variables coded using the International Classification of Diseases, Tenth Edition (ICD-10) coding scheme, with all-cause mortality defined as deaths from any cause, and CVD mortality defined separately by codes as Deaths due to CVD and cerebrovascular disease: UCOD_LEADING = 001 (“heart disease”) or 005 (“cerebrovascular disease”), were the primary study population for this investigation. More information on mortality rates can be found at: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm.
Measurement of the indicators NPAR, NLR, and SII
Data for hematology indices were obtained from the NHANES database complete blood count (CBC) profile, Coulter® DxH 800 analyzer was used for CBC, an automated dilution and mixing system was available for sample processing, hemoglobin was determined using a single-beam spectrophotometer, and blood count units are 103cells/µL. NLR for each participant was determined by dividing the absolute count of total neutrophils by the absolute count of total lymphocytes in the white blood cell (WBC). SII level was determined by multiplying the platelet count by the neutrophil count/lymphocyte count. Neutrophil percentage is the proportion of neutrophil counts to WBC counts and the formula for calculating NPAR is as follows: Neutrophil percentage %×100/Albumin(g/dL). Since SII has a right-skewed distribution, the SII values were natural log-transformed (lnSII), and NPAR, NLR, and lnSII were categorized into four subgroups based on quartiles (Q1-Q4).
Covariates
General demographic variables included in this study were age, sex, race (categorized as Mexican American, non-Hispanic white, non-Hispanic black, or other race), education level (less than, equal to, and greater than a high school degree), and household income level (defined by the Poverty Impact Ratio (PIR) as low income (PIR < 1.3), moderate-income (PIR 1.3–3.5) and high income (PIR ≥ 3.5)). Smoking status was obtained from the NHANES Household Interviews, and smokers were categorized as past smokers (smoked more than 100 cigarettes in the past and are currently quit), current smokers (smoked more than 100 cigarettes in the past and are still smoking), and never smokers (smoked less than 100 cigarettes in the past). Physical activity was categorized as inactive, moderately active, and vigorously active according to whether or not the participant had engaged in moderately or vigorously intense fitness and recreational activities in the past 30 days. BMI and WC data were obtained from measurements by Mobile examination centers (MECs) professionals, calculating BMI by weight (in kilograms) divided by height (in meters squared). Diagnosis of diabetes mellitus (DM) was based on self-reported past medical history, current use of oral hypoglycemic medications or insulin injections, and FBG ≥ 7.0 mmol/L or HbA1c ≥ 6.5%21. Pre-DM was identified by a self-reported diagnosis of antecedent DM, FBG between 5.6 and 7.0 mmol/L, or HbA1c between 5.7 and 6.5%. Hypertension was diagnosed by doctor-diagnosed hypertension, taking antihypertensive medication, or having a mean of three consecutive measurements of systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg. Chronic kidney disease (CKD) was defined as an eGFR < 60 mL/ min/1.73 m2 based on the eGFR calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula22. CVD was defined as affirmative answers to the following questions: whether one had ever been told that one had a myocardial infarction, angina pectoris, coronary heart disease, congestive heart failure, or stroke. Chronic obstructive pulmonary disease (COPD) diagnosis was based on whether the participant had previously been diagnosed by a physician with chronic bronchitis, emphysema, and COPD23. Biochemical markers were obtained from NHANES laboratory data and included Albumin, HbA1c, FBG, fasting insulin (FINS), ALT, AST, GGT, TG, total cholesterol (TC), HDL-C, and low-density lipoprotein cholesterol (LDL-C).
Statistical analysis
The R software (version 4.3.1) was used to analyze the study, and complex weighting was applied to all data analyses. This was done while accounting for non-response to the survey, planned oversampling to create a representative sample, and the complexity of the design during the NHANES data collection process. Continuous variables were expressed as weighted means and standard error (SE); categorical variables were expressed as unweighted numbers and weighted proportions, according to the grouping of all-cause mortality and CVD mortality, and one-way ANOVA for between-group comparisons of continuous variables and chi-square test for between-group comparisons of categorical variables. Multivariate Cox regression was used to analyze the hazard ratio (HR) and 95% confidence interval (95%CI) for the associations between study variables and all-cause mortality and CVD mortality, three regression models were developed to adjust for confounders; model 1 was unadjusted for variables, model 2 was adjusted for age, sex, race, PIR, and education level, and model 3 was adjusted for physical activity, smoking status, BMI, WC, DM, Hypertension, CVD, CKD, COPD, TG, TC, HDL-C, LDL-C, ALT, AST, and GGT based on model 2. In addition, we performed a multicollinearity test for model 3 (Additional file: Table S1). We used multiple interpolations based on the random forest method to fill in the missing data, calculated Cronbach’s coefficients to reflect the internal reliability of the interpolated data, and selected the interpolated dataset with the largest Cronbach’s for the analysis. Using multivariate-adjusted restricted cubic spline (RCS) regression to further investigate the dose-response relationships between NPAR, NLR with SII, and mortality, the number of nodes was determined based on the minimum value of the Akaike Information Criterion (AIC). If a nonlinear relationship was detected, the groups were regrouped according to the nonlinearity inflection point, multivariate COX regression was performed to test the relationship between the newly grouped variables and mortality, and probabilities of survival outcomes between groups were calculated by the Kaplan-Meier method and compared using the log-rank test. In addition, we performed subgroup analyses and interaction analyses (based on age (< 60 or ≥ 60 years), sex, race, BMI (< 30 or ≥ 30), DM (no or yes), hypertension (no or yes), and COPD (no or yes)) to explore potential interaction effects of covariates. Finally, we performed time-dependent receiver operating characteristic curve (ROC) analyses to assess the accuracy of NPAR, NLR, and SII in predicting survival outcomes. A two-sided P-value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of participants
Table 1 describes the weighted baseline characteristics of the 3970 participants grouped according to all-cause and CVD mortality. The mean age of the participants was 51.48 years, and the majority were male (55.83%), non-Hispanic white (67.95%), never smokers (52.19%), those with a high school diploma or higher (52.28%), and moderate-income level (38.73%). The most common comorbidity was hypertension (55.04%). Mean NPAR, NLR, and SII were 14.19, 2.29, and 6.22, respectively. Compared with the surviving population, the death population had more patients with DM, hypertension, CVD, CKD, and COPD, higher BMI, and higher levels of ALT, FBG, HbA1c, TC, and LDL-C.
Relationship of NPAR, NLR, and SII to mortality
Table 2 shows the associations between NPAR, NLR, and SII and the risk of all-cause and CVD mortality. As continuous variables, increases in NPAR, NLR, and SII per unit were significantly and positively associated with increases in all-cause and CVD mortality in fully adjusted models (HR > 1 and P < 0.05). As an interquartile group, in the fully adjusted model, in group Q4 compared with Q1, NPAR, and NLR were significantly associated with increased all-cause mortality and there was no meaningful relationship between SII and all-cause mortality in group Q4 compared with group Q1 (P > 0.05), whereas NPAR, NLR, and SII were significantly associated with increased CVD mortality. In all three models, all-cause and CVD mortality increased with increasing NLR quartiles (P for trend < 0.05); whereas no significant linear increasing trend was observed for all-cause and CVD mortality with increasing NPAR versus SII quartiles (P for trend > 0.05).
The detection of nonlinear relationships
As shown in Fig. 2, we further assessed the dose-response relationship between NPAR, NLR, and SII with all-cause and CVD mortality using RCS analysis, which showed a nonlinear correlation (P for non-linear < 0.05) between NPAR and SII with all-cause and CVD mortality, and the RCS curves showed significant inflection point, while there was a linear correlation between NLR and all-cause and CVD mortality (P for non-linear > 0.05). In Table 3, NPAR and lnSII were further reclassified into two groups based on the inflection point, and multivariate COX regression analysis showed that the difference in risk of death between the NPAR groups was significant (P < 0.05), whereas no difference in risk of death was demonstrated between the lnSII groups (P > 0.05), and the Kaplan-Meier survival curves (Fig. 3) demonstrated that patients with MASLD above the inflection point for NPAR had higher all-cause (Fig. 3A) and CVD mortality (Fig. 3B) (log-rank P < 0.05). The risk of death (Fig. 3C and D) differed significantly between quartiles of NLR values (log-rank P < 0.05), but there was no significant difference in the risk of death (Fig. 3E and F) in patients with MASLD before and after the lnSII inflection point (log-rank P > 0.05).
Subgroup analysis
As depicted in Table 4, Subgroup analyses showed that the positive association between high NPAR levels (all-cause mortality ≥ 12.68, CVD mortality ≥ 12.39) and risk of all-cause mortality, compared with low NPAR levels (all-cause mortality < 12.68, CVD mortality < 12.39), was only present in patients with MASLD combined with COPD. It was found no significant interaction between high NPAR levels and various subgroups according to age, sex, race, BMI, DM, hypertension, and COPD (P interaction > 0.05). Positive associations between high NPAR levels and CVD mortality were found in older adults aged ≥ 65, women, non-Hispanic whites, non-Hispanic blacks, obese patients, non-DM populations, hypertensive patients, and COPD subgroups. The presence of COPD had a significant effect on the association between high NPAR levels and CVD mortality (P interaction < 0.05), and high NPAR levels in patients with MASLD combined with COPD corresponded to a higher risk of death.
Time-dependent ROC curves analyzed the predictive value of NPAR, NLR, and SII for all-cause and CVD mortality in patients with MASLD. Results of the analysis showed that the area under the curves (AUCs) for NPAR was 0.751 (95%CI 0.655–0.847), 0.677 (95% CI 0.631–0.722), 0.650 (95% CI 0.615–0.686), and 0.620 (95% CI 0.590–0.649) for 1-year, 3-year, 5-year and 10-year all-cause mortality, respectively (Fig. 4A). The AUCs of the NPAR were 0.706 (95% CI 0.538–0.873), 0.663 (95% CI 0.583–0.743), 0.667 (95% CI 0.605–0.729) and 0.628 (95% CI 0.575–0.680) for 1-year, 3-year, 5-year and 10-year CVD mortality, respectively (Fig. 4B). The AUCs of the NLR were 0.748 (95% CI 0.653–0.842), 0.658 (95% CI 0.610–0.706), 0.650 (95% CI 0.613–0.687) and 0.624 (95% CI 0.594–0.654) for 1-year, 3-year, 5-year and 10-year all-cause mortality, respectively (Fig. 4C). The AUCs of the NLR were 0.729 (95% CI 0.564–0.894), 0.671 (95% CI 0.593–0.749), 0.668 (95% CI 0.625–0.751) and 0.659 (95%CI 0.606–0.713) for 1-year, 3-year, 5-year and 10-year CVD mortality, respectively (Fig. 4D). The AUCs of the SII were 0.654 (95% CI 0.529–0.778), 0.572 (95% CI 0.519–0.624), 0.555 (95% CI 0.517–0.594) and 0.528 (95% CI 0.497–0.560) for 1-year, 3-year, 5-year and 10-year all-cause mortality, respectively (Fig. 4E). The AUCs of the NPAR were 0.609 (95% CI 0.411–0.807), 0.543 (95% CI 0.452–0.634), 0.593 (95% CI 0.526–0.659) and 0.559 (95% CI 0.503–0.614) for 1-year, 3-year, 5-year and 10-year CVD mortality, respectively (Fig. 4F). The above results demonstrate the predictive value of NPAR, NLR, and SII for all-cause and CVD mortality in patients with MASLD, and we also found that the predictive value of NPAR was more significant compared with NLR and SII, and that the predictive value of all three was better for short-term mortality than for long-term mortality.
Discussion
This was the first study to investigate the relationship between NPAR, NLR, and SII and survival outcomes in patients with MASLD based on a large sample population, and found that there was a nonlinear relationship between NPAR and all-cause and CVD mortality (Inflection point of 12.68 and 12.39, respectively), and a linear correlation between NLR and death outcomes, but after adjusting for confounders, the SII and mortality nonlinear relationship was no longer significant. Previous studies have found that CVD is the leading cause of death in patients with NAFLD7. New definitions of MASLD require the inclusion of at least one cardiometabolic risk factor based on liver steatosis, implying an increased risk of CVD in patients with MASLD24. Systemic inflammation plays an important role in the progression of MASLD and its interaction with CVD25.
The present study showed that NPAR has favorable predictive properties for survival outcomes in patients with MASLD, especially for short-term survival outcomes (1-year all-cause mortality and 1-year CVD mortality AUCs of 0.751 and 0.706, respectively), with a significant difference in the risk of death between the two groups of patients classified according to the inflection point. Neutrophil percentage and albumin concentration are simple and easy-to-measure clinical indicators. Neutrophils are the main component of human WBC and play a crucial role in mediating inflammatory responses. Albumin acts as an antioxidant and anti-inflammatory, and its concentration is affected by inflammation26. Hypoalbuminemia is often an indicator of poor prognosis in patients due to nutritional deficiencies and inflammation27,28. Albumin is specifically synthesized by the liver and decreases significantly with increasing severity of liver disease; patients with cirrhosis not only have reduced albumin synthesis but also structural and functional damage29,30. High NPAR levels increase the short- and long-term risk of death in patients with septic shock, coronary artery disease, and cardiogenic shock31,32,33. A retrospective cohort study revealed that the predictive efficacy of NPAR for the risk of death in patients with cirrhosis was comparable to that of the model of end-stage liver disease score34. Inflammatory progression leads to opposite trends in neutrophils and albumin, so the ratio of the two better reflects the inflammatory state of the body. In addition, we found that the presence of COPD significantly affects the association between MASLD patients with high NPAR levels and CVD mortality and that MASLD patients with high NPAR levels have an increased risk of death in combination with COPD. A key factor in the development of COPD is the inflammatory response, and the main indicator of chronic inflammation in COPD is the infiltration of inflammatory cells including neutrophils, macrophages, and lymphocytes into the small airways. According to research from the NHANES database, NPAR was better at predicting 5-year all-cause mortality than other inflammatory blood biomarkers including NLR and eosinophil-to-lymphocyte ratio (ELR)35. The reason for this is that MASLD in combination with COPD is more indicative of a significant systemic inflammatory state, which amplifies the mortality predictive advantage of NPAR.
Lymphocyte count is an essential marker of immune function, elevated NLR represents an elevated neutrophil or lowered lymphocyte count, which is indicative of an inflammatory state or lack of immune function of the body, and may result in decreased disease resistance of the body36. Elevated NLR is associated with the risk of MASH and liver fibrosis in patients with NAFLD37, and has also demonstrated positive efficacy in the prediction of risk for liver cancer and coronary artery disease38,39. A study from Brazil showed that NLR was an independent predictor of all-cause and CVD mortality in patients with type 2 DM40. In the present study, we observed a linear positive association between NLR and the risk of death in patients with MASLD, which remained significant after adjusting for other influencing factors and performed well in the prediction of short-term all-cause and CVD mortality ((1-year all-cause mortality and CVD mortality AUCs of 0.748 and 0.729, respectively).
SII is a comprehensive indicator of platelet count, neutrophil count, and lymphocyte count, Neutrophils, through mechanisms like neutrophil extracellular traps (NETs), as crucial players in the systemic immune-inflammatory response to metabolic disorders41. NETs are reticular structures made of DNA, histones, and antimicrobial proteins. They capture and eliminate external infections, but they can also cause tissue harm by inciting autoimmune42. While animal models have demonstrated that NETs exacerbate hypertensive conditions and harm organs including the heart and kidneys43,44, elevated plasma levels of NETs have been reported to promote the faster production of arterial lipid plaques and thrombi45,46. In addition to their role in inflammation, T-lymphocytes have been demonstrated to mediate the intestinal flora’s endotoxin toxicity, contributing to the development and progression of MASLD47. Platelets are mainly produced by the liver, which is closely associated with inflammatory response48. Reduced platelet count is a characteristic of chronic liver disease, especially cirrhosis, and impaired function and splenomegaly due to structural liver injury are the main causes of reduced platelet count. Some animal experiments have found that accumulation of platelet-derived platelet factor 4 (PF4) in experimental mice with liver fibrosis stimulates the proliferation and activation of hematopoietic stem cell (HSC) proliferation and activation to induce liver fibrosis. In contrast, deletion of the PF4 gene significantly reduced the risk of liver fibrosis, although the exact mechanism remains unclear49. Findings in a mouse model of biliary fibrosis are consistent with the above studies, and in this experimental setting, antiplatelet therapy with low-dose aspirin slowed the progression of fibrosis50. SII has been shown in many studies to be representative of the body’s integrated immune level and inflammatory state and is linearly associated with the prognosis of many diseases, especially CVD51,52,53. High levels of SII were linked to a higher risk of death in nearly all atherosclerotic CVD, including peripheral artery disease, ischemic stroke, and acute myocardial infarction, according to a comprehensive meta-analysis54. Additionally, it was discovered that patients with intrahepatic cholangiocarcinoma who had elevated SII levels following liver transplantation had lower survival rates55. MASLD is characterized by low-grade systemic inflammation, and in this study, we found that elevated levels of SII were positively associated with an increased risk of all-cause and CVD mortality in patients with MASLD, and subsequent RCS analyses revealed a nonlinear relationship between SII and the two and yielded a nonlinear inflection point, i.e., the value of SII at which the risk of death was lowest, but furthermore, there was no significant difference in the risk of death among populations with different SII levels grouped according to inflection point. One possible explanation is that SII is a composite of platelets, neutrophils, and lymphocytes, and the functional differences and interactions among the three lead to differences in risk of death among populations with different SII levels, complicating their prediction of adverse outcomes56.
In addition, it is worth noting that in the ROC analysis, we found that the predictive ability of NPAR, NLR, and SII for long-term mortality in patients with MASLD was lower than that of short-term mortality, and the AUCs values for predicting long-term mortality were relatively moderate, all less than 0.70. The reason for this result may be that inflammation in patients with MASLD tends to be chronic and persistent during the long-term course of the MASLD and that changes in risk factors and the emergence of comorbidities over time make the role of the inflammatory response no longer significant, which should be taken into account in clinical practice.
The strengths of this study are the large sample size and the long follow-up period, which makes the findings broadly representative, and the fact that all data collected from the NHANES database ensures the reliability of the data source. Of course, there are some limitations of our study: firstly, this is a single-center observational study, which does not allow for the determination of causality; secondly, the diagnosis of MASLD was derived from a non-invasive score rather than liver biopsy, which has some error in patient inclusion. Finally, some variables were determined based on questionnaires, and recall bias was inevitable. Therefore, the results of this study have yet to be confirmed by large-scale prospective studies.
Conclusion
This study revealed the correlation of NPAR, NLR, and SII with all-cause and CVD mortality in patients with MASLD, and we found that the relationship between NPAR and SII and mortality was nonlinear and that the relationship between NLR and mortality was linear and positive, but after adjusting for the relevant variables, the nonlinear relationship between SII and mortality was no longer significant.
Data availability
All of the data used in this study is publicly available and can be accessed at https://wwwn.cdc.gov/nchs/nhanes.
Abbreviations
- AIC:
-
Akaike Information Criterion
- AUCs:
-
Area under the curves
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CBC:
-
complete blood count
- CVD:
-
Cardiovascular disease
- CKD:
-
Chronic kidney disease
- COPD:
-
Chronic obstructive pulmonary disease
- DM:
-
Diabetes mellitus
- FBG:
-
Fasting blood glucose
- FINS:
-
Fasting insulin
- GGT:
-
γ-glutamyl transpeptadase
- HbA1c:
-
Glycosylated hemoglobin A1c
- HDL-C:
-
High-density lipoprotein cholesterol
- HR:
-
Hazard ratio
- IL-6:
-
Interleukin-6
- LDL-C:
-
Low-density lipoprotein cholesterol
- MASLD:
-
Metabolic dysfunction associated with steatotic liver disease
- MASH:
-
Metabolic dysfunction-associated steatohepatitis
- MECs:
-
Mobile examination centers
- NPAR:
-
Neutrophil-percentage-to-albumin ratio
- NLR:
-
Neutrophil-to-lymphocyte ratio
- NCHS:
-
National Center for Health Statistics
- NDI:
-
National Death Index
- NHANES:
-
National Health and Nutrition Examination Survey
- NASH:
-
Non-alcoholic steatohepatitis
- RCS:
-
Restricted cubic spline
- ROC:
-
Receiver operating characteristic curve
- SE:
-
Standard error
- SII:
-
Systemic immune-inflammation index
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- TNF-α:
-
Tumor necrosis factor-α
- USFLI:
-
United States Multiracial Population Modified Fatty Liver Index
- WC:
-
Waist circumference
- WBC:
-
White blood cell
References
Riazi, K. et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 7(9), 851–861 (2022).
Devarbhavi, H. et al. Global burden of liver disease: 2023 update. J. Hepatol. 79(2), 516–537 (2023).
Eslam, M., Sanyal, A. J. & George, J. MAFLD: A Consensus-Driven proposed nomenclature for metabolic Associated fatty liver disease. Gastroenterology 158(7), 1999–2014 (2020). e1991.
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Ann. Hepatol. 29(1), 101133 (2023).
Chan, W. K. et al. Metabolic dysfunction-Associated Steatotic Liver Disease (MASLD): a state-of-the-art review. J. Obes. Metabolic Syndrome 32(3), 197–213 (2023).
Tan, D. J. H., Ng, C. H., Muthiah, M. D., Loomba, R. & Huang, D. Q. Clinical features and outcomes of NAFLD-related hepatocellular carcinoma - authors’ reply. Lancet Oncol. 23(6), e244 (2022).
Targher, G., Byrne, C. D. & Tilg, H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 69(9), 1691–1705 (2020).
Bilson, J. & Byrne, C. D. Fatty liver disease and risk of all cause and cause-specific mortality outcomes in the older population. Hepatobiliary Surg. Nutr. 12(6), 949–952 (2023).
Cheung, A., Figueredo, C. & Rinella, M. E. Nonalcoholic fatty liver disease: identification and management of high-risk patients. Am. J. Gastroenterol. 114(4), 579–590 (2019).
Velliou, R. I., Legaki, A. I., Nikolakopoulou, P., Vlachogiannis, N. I. & Chatzigeorgiou, A. Liver endothelial cells in NAFLD and transition to NASH and HCC. Cell. Mol. Life Sci. 80(11), 314 (2023).
Motta, B. M., Masarone, M., Torre, P. & Persico, M. From Non-alcoholic Steatohepatitis (NASH) to Hepatocellular Carcinoma (HCC): epidemiology, incidence, predictions, risk factors, and Prevention. Cancers 15(22). (2023).
Lekakis, V. & Papatheodoridis, G. V. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. (2023).
Imamori, M. et al. Thrombospondin-1 promotes liver fibrosis by enhancing TGF-β action in hepatic stellate cells. Biochem. Biophys. Res. Commun. 693, 149369 (2023).
Chen, T. T. et al. Prediction value of neutrophil and eosinophil count at risk of COPD exacerbation. Ann. Med. 55(2), 2285924 (2023).
Dai, K. et al. Neutrophil percentage-to-albumin ratio and monocyte-to-lymphocyte ratio as predictors of free-wall rupture in patients with acute myocardial infarction. J. Clin. Lab. Anal. 36(1), e24136 (2022).
Hu, B. et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. cancer Research: Official J. Am. Association Cancer Res. 20(23), 6212–6222 (2014).
Ö ırakoğlu, Ç. & AS Yılmaz, F. Systemic immune-inflammation index is associated with increased carotid intima-media thickness in hypertensive patients. Clin. Experimental Hypertens. (New York NY: 1993). 43(6), 565–571 (2021).
Curtin, L. R. et al. National Health and Nutrition Examination Survey: sample design, 2007–2010. Vital Health Stat. Ser. 2 Data Evaluation Methods Res. 2013(160):1–23 .
Ruhl, C. E. & Everhart, J. E. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther. 41(1), 65–76 (2015).
Song, S. J., Lai, J. C., Wong, G. L., Wong, V. W. & Yip, T. C. Can we use old NAFLD data under the new MASLD definition? J. Hepatol. (2023).
Zou, X., Zhou, X., Zhu, Z. & Ji, L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol. 7(1), 9–11 (2019).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612 (2009).
Ibrahimou, B., Azim, S. I. & Sun, N. Interaction between blood lead level and chronic obstructive pulmonary disease (COPD) on risk of heart attack or stroke: USA NHANES, 2013–2014. Pulm. Pharmacol. Ther. 58, 101805 (2019).
Mellemkjær, A., Kjær, M. B., Haldrup, D., Grønbæk, H. & Thomsen, K. L. Management of cardiovascular risk in patients with metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. (2023).
Lechner, K. et al. High-risk atherosclerosis and metabolic phenotype: the roles of ectopic adiposity, Atherogenic Dyslipidemia, and inflammation. Metab. Syndr. Relat. Disord. 18(4), 176–185 (2020).
Eckart, A. et al. Relationship of nutritional status, inflammation, and serum albumin levels during Acute illness: a prospective study. Am. J. Med. 133(6), 713–722e717 (2020).
Huang, H. et al. Independent and joint effects of high-sensitivity c-reactive protein and hypoalbuminemia on long-term all-cause mortality among coronary artery disease: a prospective and multicenter cohort study. BMC Cardiovasc. Disord. 21(1), 613 (2021).
Jäntti, T. et al. Hypoalbuminemia is a frequent marker of increased mortality in cardiogenic shock. PloS One 14(5), e0217006 (2019).
Spinella, R., Sawhney, R. & Jalan, R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hep. Intl. 10(1), 124–132 (2016).
Domenicali, M. et al. Posttranscriptional changes of serum albumin: clinical and prognostic significance in hospitalized patients with cirrhosis. Hepatol. (Baltimore Md) 60(6), 1851–1860 (2014).
Gong, Y., Li, D., Cheng, B., Ying, B. & Wang, B. Increased neutrophil percentage-to-albumin ratio is associated with all-cause mortality in patients with severe sepsis or septic shock. Epidemiol. Infect. 148, e87 (2020).
Sun, T. et al. Association between Neutrophil Percentage-to-Albumin Ratio and All-Cause Mortality in Critically Ill Patients with Coronary Artery Disease. BioMed research international 2020:8137576. (2020).
Yu, Y. et al. The Neutrophil Percentage-to-Albumin Ratio as a New Predictor of All-Cause Mortality in Patients with Cardiogenic Shock. BioMed research international 2020:7458451. (2020).
Du, X. et al. Higher levels of neutrophil percentage-to-albumin ratio predict increased mortality risk in patients with liver cirrhosis: a retrospective cohort study. Eur. J. Gastroenterol. Hepatol. 35(2), 198–203 (2023).
Lan, C. C., Su, W. L., Yang, M. C., Chen, S. Y. & Wu, Y. K. Predictive role of neutrophil-percentage-to-albumin, neutrophil-to-lymphocyte and eosinophil-to-lymphocyte ratios for mortality in patients with COPD: evidence from NHANES 2011–2018. Respirol. (Carlton Vic) 28(12), 1136–1146 (2023).
Bhat, T. et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev. Cardiovasc. Ther. 11(1), 55–59 (2013).
Abdel-Razik, A. et al. A novel model using mean platelet volume and neutrophil to lymphocyte ratio as a marker of nonalcoholic steatohepatitis in NAFLD patients: multicentric study. Eur. J. Gastroenterol. Hepatol. 28(1), e1–9 (2016).
Thomas, C. E. et al. Neutrophil-lymphocyte ratio in relation to risk of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Cancer Med. 12(3), 3589–3600 (2023).
Verdoia, M. et al. Impact of diabetes on neutrophil-to-lymphocyte ratio and its relationship to coronary artery disease. Diabetes Metab. 41(4), 304–311 (2015).
Cardoso, C. R. L., Leite, N. C. & Salles, G. F. Importance of hematological parameters for micro- and macrovascular outcomes in patients with type 2 diabetes: the Rio De Janeiro type 2 diabetes cohort study. Cardiovasc. Diabetol. 20(1), 133 (2021).
Malamud, M. et al. Recognition and control of neutrophil extracellular trap formation by MICL. Nature 633(8029), 442–450 (2024).
Euler, M. & Hoffmann, M. H. The double-edged role of neutrophil extracellular traps in inflammation. Biochem. Soc. Trans. 47(6), 1921–1930 (2019).
Fabbiano, S. et al. Immunosuppression-Independent Role of Regulatory T Cells against hypertension-driven renal dysfunctions. Mol. Cell. Biol. 35(20), 3528–3546 (2015).
Siminiak, T., Flores, N. A. & Sheridan, D. J. Neutrophil interactions with endothelium and platelets: possible role in the development of cardiovascular injury. Eur. Heart J. 16(2), 160–170 (1995).
Li, J. et al. Inflammatory cytokines induce neutrophil extracellular traps interaction with activated platelets and endothelial cells exacerbate coagulation in moderate and severe essential hypertension. J. Hypertens. 40(11), 2219–2229 (2022).
Chrysanthopoulou, A. et al. Angiotensin II triggers release of neutrophil extracellular traps, linking thromboinflammation with essential hypertension. JCI insight 6(18). (2021).
Yaghmaei, H. et al. The Role of Gut Microbiota Modification in Nonalcoholic Fatty Liver Disease Treatment Strategies. International journal of hepatology 2024:4183880. (2024).
Pavlovic, N., Rani, B., Gerwins, P. & Heindryckx, F. Platelets as key factors in Hepatocellular Carcinoma. Cancers 11(7). (2019).
Zaldivar, M. M. et al. CXC chemokine ligand 4 (Cxcl4) is a platelet-derived mediator of experimental liver fibrosis. Hepatol. (Baltimore Md) 51(4), 1345–1353 (2010).
Yoshida, S. et al. Extrahepatic platelet-derived growth factor-β, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology 147(6), 1378–1392 (2014).
Liu, Y. Y. et al. Systemic inflammation with Sarcopenia predicts survival in patients with gastric cancer. J. Cancer Res. Clin. Oncol. 149(3), 1249–1259 (2023).
He, K. et al. Preoperative systemic Immune-inflammation index (SII) as a Superior Predictor of Long-Term Survival Outcome in patients with stage I-II gastric Cancer after radical surgery. Front. Oncol. 12, 829689 (2022).
Orhan, A. L. et al. Evaluating the systemic immune-inflammation index for in-hospital and long-term mortality in elderly non-ST-elevation myocardial infarction patients. Aging Clin. Exp. Res. 34(7), 1687–1695 (2022).
Ye, Z. et al. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis. Front. Cardiovasc. Med. 9, 933913 (2022).
Ren, A. et al. Systemic Immune-Inflammation Index Is a Prognostic Predictor in Patients with Intrahepatic Cholangiocarcinoma Undergoing Liver Transplantation. Mediators of inflammation 2021:6656996. (2021).
Xie, R. et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front. Immunol. 13, 925690 (2022).
Acknowledgements
Thanks to the staff of the National Center for Health Statistics of the Centers for Disease Control for collecting and organizing the NHANES data.
Funding
This work is supported by The Joint Scientific Research Fund of Gansu Province (Grant number: 23JRRA1487) The Health Industry Research Project of Gansu Province (Grant number: GSWSQN2023-02) The Foundation of The First Hospital of Lanzhou University (Grant number: ldyyyn2023-8).
Author information
Authors and Affiliations
Contributions
Authors’ contributions The study design was conceived by K.-X.D., Y.Z., Y.-P.W., and Q.-H.G.. K.-X.D. organized the data, conducted the analyses, and wrote and edited the manuscript. Y.Z., Y.-P.W., and Q.-H.G. contributed to the interpretation of the results, revision, and finalization of the manuscript. All authors have reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Institutional review board statement
The NHANES survey protocol was approved by the NCHS Ethics Review Committee, and all respondents signed an informed consent form so that secondary analyses of the data were conducted without the need for another ethical review. The approval of the NCHS Research Ethics Review Board can be accessed on the NHANES website (https://www.cdc.gov/nchs/nhanes/irba98.htm) (accessed on June 4, 2024).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, K., Zheng, Y., Wang, Y. et al. Predictive role of neutrophil percentage-to-albumin ratio, neutrophil-to-lymphocyte ratio, and systemic immune-inflammation index for mortality in patients with MASLD. Sci Rep 14, 30403 (2024). https://doi.org/10.1038/s41598-024-80801-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80801-8
Keywords
This article is cited by
-
Prognostic impact of systemic inflammation indicators on all-cause and CVD mortality in adults with MASLD
BMC Gastroenterology (2025)
-
Neutrophil percentage-to-albumin ratio as predictor for mortality in patients undergoing endoscopic intervention for variceal hemorrhage
European Journal of Medical Research (2025)
-
Sex-specific associations between Neutrophil-Percentage-to-Albumin Ratio(NPAR) and Neutrophil-to-Lymphocyte Ratio (NLR) with positive and negative symptoms in schizophrenia
BMC Psychiatry (2025)
-
Association between saturated and polyunsaturated fatty acid proportions in total fat intake and mortality risk: mediation by the neutrophil percentage-to-albumin ratio
Lipids in Health and Disease (2025)
-
Pan-immune-inflammation value and mortality in the US adult MASLD: a nonlinear NHANES analysis
BMC Gastroenterology (2025)