Abstract
Metabolic-dysfunction-associated fatty liver disease (MAFLD) and serum uric acid are closely related to cardiovascular and cerebrovascular diseases. However, the causal association between MAFLD and serum uric acid remains unclear. A total of 3417 patients without hyperuricemia were included in the final analysis. MAFLD was defined as fatty liver index (FLI) ≥ 30. Multivariate Cox regression analysis was used to explore the association between FLI and new-onset hyperuricemia. Restricted cubic splines and threshold saturation effect analysis were used to detect nonlinear associations. The mean age was 62.8 ± 8.3 year, and 68.5% were women. A total of 738 (21.6%) hypertensive patients developed new-onset hyperuricemia, 388 (11.4%) new-onset hyperuricemia10 and 190 (5.6%) new-onset hyperuricemia20 during the 4-year midday follow-up period. In the fully adjusted model, compared with the Q1 (FLI ≤ 8.5) group, the risk of hyperuricemia increased by 56% (HR: 1.56; 95% CI: 1.02, 2.38) in the Q4 (FLI > 39.4) group, new-onset hyperuricemia10 increased by 108% (HR: 2.08; 95% CI: 1.15, 3.78), and new-onset hyperuricemia20 increased by 156% (HR: 2.56; 95% CI: 1.11, 5.94), respectively. Saturation effects showed a nonlinear association between FLI and new-onset hyperuricemia (p for log likelihood ratio test < 0.05). Subgroup analysis and stratified analysis showed that there had a significantly higher risk of new-onset hyperuricemia in the patients with normal body mass index (< 24 kg/m2) (p for interaction: 0.018) and non-central obesity (p for interaction: 0.024). MAFLD is an independent risk factor for hyperuricemia in hypertensive patients, especially in patients with normal body mass index and non-central obesity.
Similar content being viewed by others
Introduction
In recent years, the prevalence of hyperuricemia has been increasing year by year due to the improvement of the economic level and living standard of our country and abroad, especially in men1,2. A large number of studies have shown that elevated levels of uric acid can increase the risk of cardiovascular disease3,4,5,6,7, and even death8. Meanwhile, studies have shown that blood pressure may be a mediator between uric acid and cardiovascular disease9. Serum uric acid not only increases the risk of hypertension10, but also the risk of poor prognosis in patients with hypertension11,12. Therefore, it is also very important to prevent hyperuricemia while controlling blood pressure in hypertensive people.
According to the latest statistical data, metabolic-dysfunction-associated fatty liver disease (MAFLD) accounts for about 30% of the global population, and a considerable number of MAFLD are non-obese patients13,14,15. In low- and middle-income countries, this figure rises to a staggering 43%16. Fatty liver index (FLI), composed of gamma-glutamyl-transferase, body mass index (BMI), waist circumference and triglycerides, is the most valuable joint index for the diagnosis of fatty liver17. It has been confirmed that FLI has a strong predictive ability for fatty liver in both men and female in domestic and external databases, and a fatty liver index ≥ 30 can be considered to have MAFLD after exclusive heavy drinking19,20,21,22. Moreover, the FLI has been recommended by international clinical practice guidelines as a non-invasive alternative to diagnose fatty liver disease, mainly for use in large epidemiological studies22,23,24. Studies have shown that both elevated uric acid and blood pressure are independent risk factors for MAFLD25,26. However, However, there were cross-sectional and one longitudinal cohort study showing that FLI was significantly associated with hyperuricemia27,28. These studies suggest that there seems to be a close relationship between serum uric acid, blood pressure and MAFLD. The association between MAFLD and serum uric acid needs to be further verified. Although obesity is a major risk factor for MAFLD, non-obese MAFLD are gradually being recognized, especially in Asian populations29,30. Some studies indicate that non-obese patients with MAFLD exhibit an increased risk of certain diseases than obese MAFLD patients31,32. However, whether the association between MAFLD and hyperuricemia is also affected by obesity remains unclear.
It is important to identify the relationship between MAFLD and serum uric acid in hypertensive population for the prevention and treatment of diseases. This study investigated the association between FLI and new-onset hyperuricemia in a large non-alcoholic cohort of hypertensive patients. To explore whether there are frontal effect modifiers that alter the association between the two.
Methods
Study design and participants
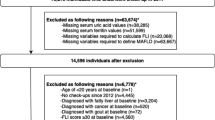
The data of this prospective cohort study were obtained from the Chinese H-type Hypertension registry database (registration number: ChiCTR1800017274), which has been reported in other studies33, and has been conducted since 2018. The inclusion and exclusion criteria for the baseline data population included the following: (1) All participants were > 18 years old and had hypertension. hypertension is defined as a systolic blood pressure of ≥ 140 mmHg, or/and diastolic blood pressure of ≥ 90 mmHg measured after 5 min of sitting still on three different days, or/and use of antihypertensive medication at baseline; (2) Who were unable to provide informed consent due to mental illness or impairment; (3) Long-term follow-up could not be conducted according to the study protocol due to relocation and other reasons; (4) Patients evaluated by physicians who were not suitable for enrollment or long-term follow-up (Such as patients who need to be hospitalized). Finally, a total of 14,243 hypertensive patients from Wuyuan, Jiangxi Province in China were recruited for the prospective study with a median follow-up duration of 4 years. Further data screening criteria are as follows: (1) Individuals who did not participate in field follow-up in 2022 (n = 4901); (2) patients with hyperuricemia at baseline (n = 5150) and missing baseline BMI data (n = 2); (3) individuals had a history of alcohol consumption (n = 732); (4) missing uric acid data at follow-up (n = 32). In the end, 3417 patients without hyperuricemia and no history of alcohol consumption were included in the final analysis (Fig. 1).
This study was approved by the Ethics Committee of the Institute of Biomedical Sciences, Anhui Medical University (Ethics NO.CH1059) and the Second Affiliated Hospital of Nanchang University (Ethics No. 2018019), and complied with the Declaration of Helsinki. All patients volunteered to participate and signed informed consent before participating in this study.
Data collection
The basic population information, measurement indicators, biochemical indicators, etc. of this study were collected by professional medical personnel and regular instruments, and have been verified for many times. Except for gender and age, lifestyle (current smoking and history of alcohol consumption), history of disease (diabetes and chronic kidney disease) and medication use (antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs and antiplatelet drugs) obtained by professional questionnaire. Current smoking was defined as smoking ≥ 1 cigarette per day from the date of follow-up for one year or more, or cumulative smoking ≥ 365 cigarettes per year. History of alcohol consumption defined as drinking in the last six months or having a previous history of alcohol consumption (≥ 210 g/week for men or ≥ 140 g/week for women). Diabetes was defined as fasting blood glucose ≥ 7.0 mmol/L, history of diabetes, or use of glucose-lowering drugs. Anthropometric measures included height (m), weight (kg), waist circumference (cm), hip circumference (cm). Weight is measured by a calibrated electronic scale and the result is retained to the tenth place. Waist circumference and hip circumference are measured as close to the skin as possible using a tape measure in accordance with international measurement standards. BMI was calculated as weight (kg) divided by the square of height (m). Overweight is defined as a BMI ≥ 24 kg/m234. Central obesity was defined as a waist-to-hip ratio (waist circumference/hip circumference) > 0.90 in men and > 0.85 in women.
All participants were informed one day in advance that fasting venous blood would be drawn the next morning and that they would be prohibited from eating for > 6 hours in advance. Venous blood samples from all study participants were frozen and transported to Shenzhen Biaojia Biotechnology Laboratory for testing. Plasma homocysteine (µmol/L), fasting blood glucose (mmol/L), alanine aminotransferase (U/L), Glutamyl transpeptidase (U/L), albumin (g/L), uric acid (µmol/L), total cholesterol (mmol/L), triglyceride (mmol/L), low-density lipoprotein cholesterol (LDL-C) (mmol/L) and high-density lipoprotein cholesterol (HDL-C) (mmol/L) were measured by Beckman Coulter automatic clinical analyzer. Laboratory staffs were blind to the study content. Estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) was estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, and parameters of the equation were measured by Beckman Coulter automatic clinical analyzer35.
FLI and outcome
As an effective index for predicting fatty liver, FLI was proposed by Giorgio et al. and was calculated by glutamyl transpeptidase (U/L), BMI (kg/m2), waist circumference (cm) and triglycerides (mg/dL). The formula is as follows: \(FLI= \left(\frac{{e}^{0.953*\text{ln}\left(triglycerides\right)+0.139*BMI+0.718\text{ln}\left(glutamyl \; transpeptidase \right)+0.053*\left(\text{w}\text{a}\text{i}\text{s}\text{t}\; \text{c}\text{i}\text{r}\text{c}\text{u}\text{m}\text{f}\text{e}\text{r}\text{e}\text{n}\text{c}\text{e}\right)-15.745}}{1+{e}^{0.953*\text{ln}\left(triglycerides\right)+0.139*BMI+0.718\text{ln}\left(glutamyl\; transpeptidase \right)+0.053*\left(\text{w}\text{a}\text{i}\text{s}\text{t}\; \text{c}\text{i}\text{r}\text{c}\text{u}\text{m}\text{f}\text{e}\text{r}\text{e}\text{n}\text{c}\text{e}\right)-15.745}}\right)*100\)17. FLI was standardized transformed and divided into quartiles. Previous studies have shown that the likelihood of developing fatty liver disease increases with the increase of FLI, and FLI ≥30 can suspect fatty liver disease18,20,21. New-onset hyperuricemia was defined as serum uric acid levels >420µmol/L in males and >360µmol/L in females at follow-up in patients excluding a baseline diagnosis of hyperuricemia1. Although the patient’s blood samples were taken on an empty stomach, which maximizes the possibility of avoiding the influence of food on the results, a single sample collection may result in a misclassification of hyperuricemia. To avoid such errors, we performed sensitivity analyses on patients with hyperuricemia with serum uric acid increments greater than 10% (hyperuricemia10) and 20% (hyperuricemia20) on the basis of their diagnosis of hyperuricemia. Uric acid increments were calculated as follows: \({Uric\; acid\; increments}_{\left(female\right)}=\frac{{Uric\; acid}_{\left(follow\text{-}up\right)}-360}{360} \times 100\), \({Uric\; acid\; increments}_{\left(male\right)}=\frac{{Uric\; acid}_{\left(follow\text{-}up\right)}-420}{420}\times 100\).
Covariates
The covariates included gender, age, systolic blood pressure, diastolic blood pressure, diabetes, chronic kidney disease, current smoking, homocysteine, fasting blood glucose, total cholesterol, alanine aminotransferase, albumin, LDL-C, eGFR, antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs and antiplatelet drugs. In addition, collinearity analyses were performed for BMI, triglycerides, glutamyl transpeptidase, and waist-to-hip ratio, all of which had variance inflation factors < 5.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (mean ± SD), and categorical variables were expressed as frequencies and percentage (%). One-way ANOVA or χ2 test were used to test the differences of each variable among the FLI four groups, and t-test or χ2 test were used to test the differences between the two groups. Cox regression equation was used to explore the hazard ratio (HR) and 95% confidence interval (CI) of the association between FLI (continuous and categorical variable) and new-onset hyperuricemia after adjusting for known confounding factors. Tests for trend were performed by modeling the tertile classification of FLI as a continuous variable. At the same time, restricted cubic spline (RCS) model was used to estimate the potential nonlinear association between FLI and the relative risk of hyperuricemia. When a nonlinear relationship was found, Threshold saturation effect analysis and a recursive algorithm were further used to calculate inflection points.
In order to further evaluate the potential influence of different populations on FLI and the relative risk of new-onset hyperuricemia, subgroup analyses and interaction tests were performed to assess potential effect modifiers. The effects of gender, age (≥ 60 vs. < 60 year), BMI (≥ 24 vs. < 24 kg/m2), central obesity (Yes vs. No), current smoking (Yes vs. No), homocysteine (≥ 15 vs. < 15 µmol/L), LDL-C (≥ 2.6 vs. < 2.6 mmol/L), Triglyceride (≥ 1.7 vs. < 1.7mmol/L), diabetes (Yes vs. No), chronic kidney disease (Yes vs. No) were compared and performed subgroup analyses.
A two-sided p-value < 0.05 was considered to indicate statistical significance. All data were analyzed using the statistical packages R (R Foundation for Statistical Computing, http://www.r-project.org ) and Empower (R) (X&Y Solutions, Inc.; http://www.empowerstats.com ).
Results
Baseline characteristics of study participants
A total of 3417 patients without hyperuricemia and no history of alcohol consumption were included in the study final analysis (Fig. 1).
The median follow-up was about 4 years, and the initial characteristics of the entire population and the subgroups based on FLI levels are depicted in Table 1.
In the whole population, the mean age was 62.8 years and the mean FLI was 19.5, which is significantly lower than the commonly used fatty liver threshold (30). Compared with group Q1, group Q4 had higher BMI, waist circumference, Triglyceride, Glutamyl transpeptidase, Alanine aminotransferase, and LDL-C levels (p < 0.001). In addition, the proportion of central obesity in four groups was 27.9%, 66.2%, 89.1% and 95.0%, respectively (p < 0.001). However, the mean age gradually decreased as the FLI increased (p <0.001). In addition, there were no significant differences in Systolic blood pressure and Antiplatelet drugs between FLI groups (p > 0.05). The characteristics of the populations grouped by new-onset hyperuricemia, new-onset hyperuricemia10, new-onset hyperuricemia20and BMI (≥ 24 vs. < 24 kg/m2) are shown in supplementary Tables 1, 2 and 3.
Nonlinear association between FLI and new-onset hyperuricemia
During a follow-up period of about 4 years, 738 (21.6%) patients developed hyperuricemia, 388 (11.4%) patients developed hyperuricemia10, and 190 (5.6%) patients developed hyperuricemia20. RCS were used to plot the association between FLI and the risk of new-onset hyperuricemia (Fig. 2 and Supplementary Fig. 1)
Association of FLI with the risk of new-onset hyperuricemia. Model was adjusted for sex, age, systolic blood pressure, diastolic blood pressure, diabetes, chronic kidney disease, current smoking, homocysteine, fasting blood glucose, total cholesterol, alanine aminotransferase, albumin, LDL-C, eGFR, antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs and antiplatelet drugs, body mass index, triglyceride, glutamyl transpeptidase and waist-hip ratio. HR hazard ratio, 95% CI 95% confidence interval, FLI fatty liver index, LDL-C low-density lipoprotein cholesterol, eGFR estimate glomerular filtration rate.
The risk of new-onset hyperuricemia increased significantly with the increase of FLI, and the risk of hyperuricemia increases most significantly when FLI reaches a certain value. Similar outcomes were observed for new-onset hyperuricemia, new-onset hyperuricemia10, and new-onset hyperuricemia20. In Cox proportional hazards regression analysis (Table 2), after adjusting for most of the common confounders in model II, the risk of hyperuricemia increased by 22% for each SD increase in FLI (HR: 1.22, 95% CI: 1.13, 1.32). In addition, the risk of new-onset hyperuricemia was increased by 89% in Q4 group compared with Q1 group (HR: 1.89, 95% CI: 1.49, 2.39). After excluding collinearity, the association between FLI and new-onset hyperuricemia remained stable after further adjustment for BMI, triglyceride, glutamyl transpeptidase and waist-hip ratio in model III. The p for trend test p < 0.05 in all model. Similar results were observed in new-onset hyperuricemia10 and new-onset hyperuricemia20. In the fully adjusted Model (Model III), we observed no statistical significance between the continuous variable of FLI and incident hyperuricemia, which may imply a nonlinear association between FLI and incident hyperuricemia.
Then, we used threshold saturation-effect models to further test the nonlinear association between FLI and new-onset hyperuricemia (Inflection point: 18.59; p for log likelihood ratio test: 0.004), new-onset hyperuricemia10(Inflection point: 13.55; p for log likelihood ratio test: 0.016) and new-onset hyperuricemia20 (Inflection point: 19.86; p for log likelihood ratio test: 0.039) (Table 3).
Subgroup analysis and stratified analysis
In subgroup analyses, interactions were seen in those with BMI (≥ 24 vs. <24 kg/m2) (p for interaction = 0.018) and central obesity (Yes vs. No) (p for interaction = 0.024) (Fig. 3). However, the relationship between FLI and new-onset hyperuricemia hazard ratios remained stable across other subgroups (gender, age, current smoking, diabetes, coronary heart disease, homocysteine, LDL-C and triglyceride) (All p for interaction > 0.05) (Supplementary Fig. 2). In the stratified analysis (Table 4), there was a significant positive correlation between FLI and new-onset hyperuricemia in patients with BMI < 24 kg/m2 and non-central obesity. However, this was not observed in patients with BMI ≥ 24 kg/m2 or central obesity.
The subgroup analysis between FLI with the risk of new-onset hyperuricemia. Model was adjusted, if not stratified, for sex, age, systolic blood pressure, diastolic blood pressure, diabetes, chronic kidney disease, current smoking, homocysteine, fasting blood glucose, total cholesterol, alanine aminotransferase, albumin, LDL-C, eGFR, antihypertensive drugs, glucose-lowering drugs, lipid-lowering drugs and antiplatelet drugs, body mass index, triglyceride, glutamyl transpeptidase and waist-hip ratio. HR hazard ratio, 95% CI 95% confidence interval, FLI fatty liver index, LDL-C low-density lipoprotein cholesterol, eGFR estimate glomerular filtration rate.
Discussion
In the present study, we found an independent, nonlinear positive association between FLI and new-onset hyperuricemia, especially in individuals with BMI < 24 kg/m2 and non-central obesity. Similar results were also observed when uric acid levels were adjusted to higher values.
Similar to serum uric acid, previous studies have shown that MAFLD is closely related to early atherosclerosis36,37, hypertension26, atrial fibrillation31 and other Cardiovascular and metabolic diseases38,39. However, the association between MAFLD and hyperuricemia remains inconclusive, especially in the nonobese population. Similar to our results, So‑Ryoung et al. showed a significant association between FLI and new-onset atrial fibrillation in people with a lower BMI in a follow-up study of 8,048,055 Korean participants with no apparent liver disease, and the association disappeared as BMI increased31. In addition, a study of 13,640 U.S. general population patients showed that non-obese MAFLD patients had a higher 10-year risk of death compared with obese MAFLD(BMI > 30 kg/m2) people32. KARLZ et al. pointed out that in addition to BMI, the classification of obesity and non-obesity should be optimized in conjunction with other human body indices (waist circumference, waist-to-hip ratio, etc.)40. Goh Eun et al.’s 8.3-year follow-up study of 10,585,844 Korean participants also showed that high FLI had the highest impact on all-cause mortality in subjects with lower BMI (< 18.5 kg/m2) and non-central obesity (p for interaction < 0.001), compared with other BMI and central obesity groups39. In our population, subgroup and stratified analyses showed that patients with non-central obesity had a significantly higher risk of uric acid than those with central obesity.
Obese and nonobese forms of MAFLD appear to have different pathophysiological mechanisms, possibly for the following reasons. First, the genetic susceptibility of obese and non-obese MAFLD patients is different41. For non-obese MAFLD patients, there is a higher rate of fatty liver related gene mutation, such as PNPLA3 gene42. For obese MAFLD patients, fatty liver may be the result of obesity, rather than the cause of obesity. Genetically related MAFLD patients show decreased peripheral fat and increased visceral fat, and may not manifest as overall obesity or abdominal obesity43. Thus, obesity may mask or attenuate the effects of MAFLD on other diseases. Second, obese and non-obese people have different tolerance and response to fatty liver, and non-obese MAFLD patients may show more severe liver damage44. Salvatore Petta et al. evaluated liver histology in 946 Italian patients with nonalcoholic steatohepatitis and found that IFNL-4 genetic variants were significantly associated with severe liver fibrosis and necrotizing inflammation in nonobese, but not in obese subjects45. A cohort study of 646 patients with MAFLD confirmed by liver biopsy over a period of approximately 19.9 years showed that nonobese MAFLD (< 25 kg/m2) patients had an increased risk for severe liver disease compared with overweight patients46. In addition, study have shown that sarcopenia is significantly associated with the development of non-alcoholic steatohepatitis and liver fibrosis in subjects with NAFLD, which is independent of age, gender, BMI, insulin resistance, hypertension, and diabetes47. In our study, the mean age was 62 years, and the mean age was significantly older in the BMI < 24 kg/m2 group than in the BMI ≥ 24 kg/m2 group (p < 0.001), which means that nonobese persons are more likely to have steatohepatitis and liver fibrosis. The mechanism by which MAFLD increases the risk of hyperuricemia is mainly mediated by the increased activity and expression of xanthine oxidoreductase (XOR) in patients with fatty liver48. Studies have shown that the significant increase in plasma XOR activity in nonalcoholic fatty liver disease/steatohepatitis (NAFLD/NASH) mouse model is significantly correlated with the increase in serum liver transaminases caused by liver injury49, but not with BMI50. This suggests that increased BMI is not the main cause of increased XOR activity and expression. Non-obese MAFLD patients show more significant hepatic lobular inflammation, which in turn promotes increased XOR activity, may be the main mechanism of this study. However, more studies are needed for further confirmation. In conclusion, for non-obese patients with MAFLD, they are often overlooked because they are not clearly characterized. The accurate identification and prevention of non-obese MAFLD patients are the main problems to be solved in future research. However, whether obese or non-obese MAFLD, appropriate exercise, especially resistance exercise, can reduce the occurrence of hepatic steatosis and steatohepatitis51,52.
Strengths of the study
The association between MAFLD and hyperuricemia was further verified in a cohort study, which provided some insights for future research. In addition, a differential population of non-obese MAFLD was found. With the deepening of research, it is found that this population is not rare and deserves people’s attention. Limitations of this study: (1) The degree of hepatic steatosis was not assessed using abdominal color ultrasound, or transient elastography of the liver, or liver biopsy, but this is not applicable in large studies. However, several large sample studies showed that FLI showed obvious advantages in the diagnosis of nonalcoholic fatty liver disease. (2) Despite adjustment for a variety of covariates, potential confounders such as diet, lifestyle such as exercise, and pre-existing viral, drug-induced, or immune liver disease remain. (3) This study is only for Chinese patients with hypertension, and whether it can be extended to other populations needs further research. (4) No multiple follow-up data were used to calculate the association between dynamic changes in FLI and hyperuricemia.
Conclusion
The results of this study indicate a significant nonlinear positive association between FLI and new hyperuricemia in hypertensive populations. Patients with normal BMI (< 24 kg/m2) and non-central obesity had a higher risk of high uric acid. The screening of fatty liver and uric acid should also be strengthened for non-obese people with hypertension in China, which is conducive to the risk stratification of hypertension and improve the prognosis of patients.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due the sensitivity of the questions asked in this study, but are available from the corresponding author on reasonable request.
References
Zhang, M. et al. Prevalence of Hyperuricemia among Chinese adults: findings from two nationally Representative cross-sectional surveys in 2015–16 and 2018–19. Front. Immunol. 12, 791983 (2021).
McCormick, N. et al. Racial and sex disparities in gout prevalence among US adults. JAMA Netw. Open 5(8), e2226804 (2022).
Feig, D. I., Kang, D. H. & Johnson, R. J. Uric acid and cardiovascular risk. N. Engl. J. Med. 359(17), 1811–1821 (2008).
Wu, A. H., Gladden, J. D., Ahmed, M., Ahmed, A. & Filippatos, G. Relation of serum uric acid to cardiovascular disease. Int. J. Cardiol. 213, 4–7 (2016).
Richette, P. et al. Improving cardiovascular and renal outcomes in gout: what should we target? Nat. Rev. Rheumatol. 10(11), 654–661 (2014).
Messerli, F. H. & Burnier, M. Cardiovascular disease and uric acid: is the not-so-innocent bystander becoming a true culprit and does the US black box warning for febuxostat indicate that not all uric acid lowering is beneficial? Eur. Heart J. 40(22), 1787–1789 (2019).
Wen, S., Arakawa, H. & Tamai, I. Uric acid in health and disease: from physiological functions to pathogenic mechanisms. Pharmacol. Ther. 256, 108615 (2024).
Stack, A. G. et al. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM Int. J. Med. 106(7), 647–658 (2013).
Gill, D. et al. Urate, blood pressure, and cardiovascular disease: evidence from mendelian randomization and meta-analysis of clinical trials. Hypertens. Dallas Tex. 1979 77(2), 383–392 (2021).
Kuwabara, M. et al. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-year Japanese Cohort Study. Hypertens. Dallas Tex. 1979 71(1), 78–86 (2018).
Hu, F. et al. Prospective study of serum uric acid levels and first stroke events in Chinese adults with hypertension. Front. Physiol. 12, 807420 (2021).
Liu, F. et al. The prevalence, distribution, and extent of subclinical atherosclerosis and its relation with serum uric acid in hypertension population. Front. Cardiovasc. Med. 8, 638992 (2021).
Younossi, Z. et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15(1), 11–20 (2018).
Deng, J. et al. The prevalence, popular trends, and associated and predictive factors of non-obese fatty liver disease. Front. Endocrinol. 12, 744710 (2021).
Chen, J., Zhou, H. & Liu, K. Normal weight and central obesity as predictors of increased all-cause mortality in metabolic dysfunction associated steatotic liver disease. J. Hepatol. 80 (4), e143–e145 (2024).
Danpanichkul, P. et al. Disparities in metabolic dysfunction-associated steatotic liver disease and cardiometabolic conditions in low and lower middle-income countries: a systematic analysis from the global burden of disease study 2019. Metabolism 158, 155958 (2024).
Bedogni, G. et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33 (2006).
Ruhl, C. E. & Everhart, J. E. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment. Pharmacol. Ther. 41(1), 65–76 (2015).
Xu, Z. et al. Blood biomarkers for the diagnosis of hepatic steatosis in metabolic dysfunction-associated fatty liver disease. J. Hepatol. 73(5), 1264–1265 (2020).
Yang, B. L. et al. External validation of fatty liver index for identifying ultrasonographic fatty liver in a large-scale cross-sectional study in Taiwan. PLoS One 10(3), e0120443 (2015).
Huang, X. et al. Validation of the fatty liver index for nonalcoholic fatty liver disease in middle-aged and elderly Chinese. Medicine (Baltim) 94(40), e1682 (2015).
European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes. Facts 9(2), 65–90 (2016).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 73 (1), 202–209 (2020).
Lee, H. H. et al. Metabolic dysfunction-associated steatotic liver disease and risk of cardiovascular disease. Gut 73(3), 533–540 (2024).
Wijarnpreecha, K. et al. Hyperuricaemia and risk of nonalcoholic fatty liver disease: a meta-analysis. Liver Int. Off J. Int. Assoc. Study Liver. 37(6), 906–918 (2017).
Yuan, M. et al. Hypertension and NAFLD risk: insights from the NHANES 2017–2018 and Mendelian randomization analyses. Chin. Med. J. (Engl) 137(4), 457–464 (2024).
Qu, J. et al. Fatty liver index for hyperuricemia diagnosis: a community-based cohort study. BMC Endocr. Disord. 22(1), 114 (2022).
Yu, C. et al. Positive correlation between fatty liver index and hyperuricemia in hypertensive Chinese adults: a H-type hypertension registry study. Front. Endocrinol. 14, 1183666 (2023).
Duseja, A., De, A. & Wong, V. Special population: lean nonalcoholic fatty liver disease. Clin. Liver Dis. 27(2), 451–469 (2023).
Fan, J. G., Kim, S. U. & Wong, V. W. S. New trends on obesity and NAFLD in Asia. J. Hepatol. 67(4), 862–873 (2017).
Lee, S. R., Han, K. D., Choi, E. K., Oh, S. & Lip, G. Y. H. Nonalcoholic fatty liver disease and the risk of atrial fibrillation stratified by body mass index: a nationwide population-based study. Sci. Rep. 11(1), 3737 (2021).
Dao, A. D., Nguyen, V. H., Ito, T., Cheung, R. & Nguyen, M. H. Prevalence, characteristics, and mortality outcomes of obese and nonobese MAFLD in the United States. Hepatol. Int. 17(1), 225–236 (2023).
Ding, C. et al. Associations of total homocysteine and kidney function with all-cause and cause-specific mortality in hypertensive patients: a mediation and joint analysis. Hypertens. Res. Off J. Jpn Soc. Hypertens. 47(6), 1500–1511 (2024).
Zhou, B. F. & Cooperative Meta-Analysis Group of the Working Group on Obesity in China. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. BES 15(1), 83–96 (2002).
Meeusen, J. W., Kasozi, R. N., Larson, T. S. & Lieske, J. C. Clinical impact of the refit CKD-EPI 2021 creatinine-based eGFR equation. Clin. Chem. 68(4), 534–539 (2022).
Pais, R. et al. Fatty liver is an independent predictor of early carotid atherosclerosis. J. Hepatol. 65(1), 95–102 (2016).
Lee, S. B. et al. Association between non-alcoholic fatty liver disease and subclinical coronary atherosclerosis: an observational cohort study. J. Hepatol. 68(5), 1018–1024 (2018).
Fresneda, S. et al. Sex and age differences in the association of fatty liver index-defined non-alcoholic fatty liver disease with cardiometabolic risk factors: a cross-sectional study. Biol. Sex. Differ. 13(1), 64 (2022).
Chung, G. E. et al. Association of fatty liver index with all-cause and disease-specific mortality: a nationwide cohort study. Metabolism 133, 155222 (2022).
Nadolsky, K. Z. Use of the term lean for nonalcoholic fatty liver disease in individuals with low body mass index. Gastroenterology 164(3), 502–503 (2023).
Eslam, M. et al. Metabolic (dysfunction)-associated fatty liver disease in individuals of normal weight. Nat. Rev. Gastroenterol. Hepatol. 19(10), 638–651 (2022).
Feldman, A. et al. Clinical and metabolic characterization of lean caucasian subjects with non-alcoholic fatty liver. Am. J. Gastroenterol. 112(1), 102–110 (2017).
Liu, Z. et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J. Hepatol. 73(2), 263–276 (2020).
Cruz, A. C. D. et al. 379 characteristics and long-term prognosis of lean patients with nonalcoholic fatty liver disease. Gastroenterology 146(5), S909 (2014).
Petta, S. et al. Interferon lambda 4 rs368234815 TT > δG variant is associated with liver damage in patients with nonalcoholic fatty liver disease. Hepatol. Baltim. Md. 66(6), 1885–1893 (2017).
Hagström, H. et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long-term follow-up study. Hepatol. Commun. 2(1), 48–57 (2018).
Koo, B. K. et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 66(1), 123–131 (2017).
Furuhashi, M. et al. Independent links between plasma xanthine oxidoreductase activity and levels of adipokines. J. Diabetes Investig. 10(4), 1059–1067 (2019).
Parisinos, C. A. et al. Genome-wide and mendelian randomisation studies of liver MRI yield insights into the pathogenesis of steatohepatitis. J. Hepatol. 73(2), 241–251 (2020).
Kawachi, Y. et al. Increased plasma XOR activity induced by NAFLD/NASH and its possible involvement in vascular neointimal proliferation. JCI Insight 6(17), e144762 (2021).
Hallsworth, K. et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 60(9), 1278–1283 (2011).
Zelber-Sagi, S. et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J. Gastroenterol. 20(15), 4382–4392 (2014).
Funding
This work was supported by: (1) The Cultivation of backup projects for National Science and Technology Awards (Grant number: 20223AEI91007). (2) Jiangxi Science and Technology Innovation Base Plan-Jiangxi Clinical Medical Research Center (Grant number: 20223BCG74012). (3) Key Research and Development Program of Jiangxi (Grant number: 20243BBI91021). (4) Jiangxi Provincial Natural Science Foundation (Grant number: 20224BAB206090, 20232BAB206140). (5) Fund project of the Second Affiliated Hospital of Nanchang University (Grant number: 2021efyA01, 2023efyA05). (6) The National Natural Science Foundation of China (Grant number: 82460670). (7) Jiangxi Provincial Drug Administration Science and Technology Project (Grant number: 2022JS41,2023JS26). (8) Jiangxi Provincial Health Commission Science and Technology Project (Grant number: 202310528).
Author information
Authors and Affiliations
Contributions
C.L.Y. is the main contributors of the article. C.L.Y. completed the literature search, data analysis, and data interpretation and the writing of the article, and with the help of C.C.D. and C.Y., he further improved the article before it was presented. X.S.C, H.H.B, C.Y. and C.C.D. conceived the study and participated in its design, coordination and participated in the field data collection. H.H.B. is the corresponding authors of this paper and participated in the study design and provided critical revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Clinical trial registration
All data in this study were obtained from the Chinese H-type Hypertension Registry (ChiCTR1800017274), which is a prospective multicenter cohort study.
Ethical approval and patient consent statement
This study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Institute of Biomedical Sciences, Anhui Medical University (Ethics NO.CH1059) and the Second Affiliated Hospital of Nanchang University (Ethics NO.2018019). All patients signed informed consent before participating in this study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, C., Ding, C., Yu, C. et al. Decoding the fatty liver-hyperuricemia link in the obese and nonobese hypertensive patients: insights from a cohort study. Sci Rep 14, 29525 (2024). https://doi.org/10.1038/s41598-024-80895-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-80895-0