Abstract
Lipopolysaccharide (LPS) exacerbates liver injury by activating various inflammatory pathways. Clusterin, a glycoprotein involved in lipid transport, and cytoprotection, is known to have inhibitory effects on liver steatosis and fibrosis. In this study, we investigated the role of clusterin in regulating LPS-induced liver injury and its effects on liver injury in C57BL/6 mice and clusterin knockout mice injected with LPS for 3 h. Primary Kupffer cells (KCs) and hepatocytes (HCs) were isolated from these mice and examined using immunohistochemistry, real-time RT-PCR, ELISA, and western blot analysis to assess the effects of clusterin. Clusterin deficiency significantly exacerbated LPS-induced liver injury, as evidenced by increased inflammatory cell infiltration, elevated serum alanine aminotransferase and aspartate aminotransferase levels, and upregulated expression of pro-inflammatory cytokines and components of the NLRP3 inflammasome. By contrast, overexpression of clusterin in primary Kupffer cells and hepatocytes significantly reduced these inflammatory markers. Furthermore, the protective mechanism of clusterin involved inhibition of the STAT3 signaling pathway. These findings suggest that clusterin is a useful therapeutic target to modulate cytokine production and key inflammatory signaling pathways in inflammatory liver diseases.
Similar content being viewed by others
Introduction
Inflammation resulting from acute liver injury is an important pathological mechanism in several liver diseases. Acute liver injury can be triggered by various factors, including infections, alcohol, toxins, and autoimmunity, and can lead to liver dysfunction and systemic complications1,2. Inflammatory responses play a central role in the progression of diseases such as hepatitis due to various causes, which can lead to severe liver damage, fibrosis, and ultimately liver failure. Understanding the pathways of the inflammatory response is essential for the development of effective therapies for the mitigation of liver disease progression3,4.
Lipopolysaccharide (LPS), an endotoxin found in the outer membrane of Gram-negative bacteria, plays an important role in inducing inflammation, especially in the liver5,6. When LPS enters the liver, it initiates an immune response that significantly increases inflammation. This process is predominantly mediated through the activation of Kupffer cells (KCs), the resident macrophages of the liver7, by LPS, resulting in the release of pro-inflammatory cytokines, such as interleukin (IL)−1, tumor necrosis factor (TNF)-α, and IL-6, which can exacerbate liver damage and contribute to various liver diseases such as steatohepatitis and cirrhosis8,9.
Among the intracellular inflammatory complexes activated during the hepatic inflammatory response, the NLRP3 (nod-like receptor (NLR) family pyrin domain-containing-3) inflammasome plays an important role10. It significantly contributes to the pathology of liver diseases by promoting release of IL-1β, a major pro-inflammatory cytokine involved in the pathogenesis of NAFLD, steatohepatitis, and fibrosis11,12,13.
Clusterin is a secreted glycoprotein involved in a variety of pathological conditions. It is involved in the regulation of key processes during the progression of liver diseases, such as NAFLD, cirrhosis, and hepatocellular carcinoma14. Recently, clusterin was reported to inhibit macrophag inflammation15,16and liver fibrosis17. Considering that inflammation plays a significant role in the development of fibrosis, in this study, we aimed to explore whether clusterin exerts anti-inflammatory effects in LPS-induced liver conditions and elucidate the underlying mechanisms.
Results
Loss of clusterin increases LPS-induced hepatic inflammation
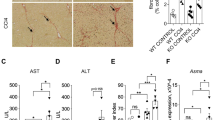
We first investigated the effect of clusterin on liver inflammation by examining the inflammatory response to acute liver injury following injection of LPS in clusterin-knockout (Clu-KO) mice. Clu-KO mice already have elevated serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels compared to wild-type controls, indicating increased liver vulnerability. In Clu-KO mice injected with LPS for 3 h, hematoxylin and eosin (H&E) staining revealed a trend toward increased inflammatory infiltrates, with significant further increases in ALT and AST levels. (Fig. 1A, B). Next, we investigated whether loss of clusterin affects liver inflammation. mRNA levels of inflammation-related cytokines (IL-1α, IL-6, and TNF-α) were increased in the liver of wild-type (WT) mice after LPS injection and were even higher in the liver of LPS-injected Clu-KO mice (Fig. 1C). These data suggest that loss of clusterin exacerbates LPS-induced acute liver injury in mice.
Effect of clusterin deficiency on LPS-induced liver injury in mice. (A) Representative images of H&E staining of liver tissue sections from wild-type and Clu-KO mice injected with LPS for 3 h. (B) Serum levels of ALT and AST in WT and Clu-KO mice treated with and without LPS. (C) Representative real-time PCR analysis of pro-inflammatory cytokines in WT and Clu-KO mice treated with and without LPS. *p < 0.05, **p < 0.01, ***p < 0.001.
Loss of clusterin promotes activation of the NLRP3 inflammasome
Next, we determined whether the upregulation of pro-inflammatory cytokines upon LPS stimulation is associated with NLRP3 inflammasome activation. mRNA levels of IL-1β and NLRP3 and secretion of the inflammasome-specific cytokines IL-1β and IL-18 were higher in the liver of LPS-injected Clu-KO mice than in the liver of LPS-injected WT mice ((Fig. 2A, B). Immunohistochemical staining showed the expression of IL-1β was increased in the liver of LPS-injected Clu-KO mice (Fig. 2C). In addition NLRP3 and ASC proteins were further upregulated in LPS-injected Clu-KO mice (Fig. 2D). These findings indicate that clusterin deficiency enhances activation of the NLRP3 inflammasome after LPS injection.
Effect of clusterin deficiency on NLRP3 inflammasome activation in LPS-injected mice. (A) ELISA analysis of IL1-β and IL-18 levels in serum from wild-type and Clu-KO mice injected with LPS for 3 h. (B) Representative real-time PCR analysis of IL-1β and NLRP3 in WT and Clu-KO mice treated with and without LPS. (C) Immunohistochemical staining for IL-1β in liver sections of LPS-treated Clu-KO mice. (D) Representative western blot analysis of NLRP3 and ASC proteins in the liver of Clu-KO mice after LPS injection. *p < 0.05, **p < 0.01, ***p < 0.001.
Loss of clusterin promotes inflammatory responses in Kupffer cells and hepatocytes
Next, we isolated primary KCs and HCs from WT and Clu-KO mice to investigate liver inflammation and inflammasome activation in vitro. 24 h after LPS treatment, NLRP3, IL-1β, and various pro-inflammatory cytokines were upregulated in KCs and HCs, and these increases were exacerbated in cells derived from Clu-KO mice (Fig. 3A, B, Supplementary Fig. 1). In addition, LPS-induced secretion of IL-1β was increased in KCs and HCs isolated from Clu-KO mice, and LPS-induced protein expression of NLRP3 was increased in HCs isolated from Clu-KO mice (Fig. 3C, D). These data are consistent with the finding that loss of clusterin promotes LPS-induced liver inflammation and NLRP3 inflammasome activation in vivo.
Effects of clusterin deficiency on the inflammatory response in isolated primary Kupffer cells (KC) and hepatocytes (HC). (A, B) Representative real time PCR analysis of NLRP3, IL1-β, IL-6 and TNF-α in KC and HC cells isolated from WT and Clu-KO mice. Cells were treated with LPS (1 µg/ml) for 2 h and incubated for 24 h after media change. (C) ELISA of IL1-β secretion by KC and HC cells isolated from WT and Clu-KO mice and treated with and without LPS. (D) Representative western blot analysis of NLRP3 protein levels in HCs isolated from WT and Clu-KO mice and treated with and without LPS. *p < 0.05, **p < 0.01, ***p < 0.001.
Clusterin inhibits inflammation and the NLRP3 inflammasome in Kupffer cells and hepatocytes
To further evaluate its inhibitory effect on liver inflammation and the NLRP3 inflammasome, clusterin was overexpressed. Overexpression of clusterin using Ad-Clu decreased IL-1β and several inflammatory cytokines (Fig. 4A, B) and reduced protein expression of NLRP3, IL-1β and cleaved-caspase-1 (Fig. 4C, D) in HCs and KCs treated with LPS. These results suggest that clusterin inhibits liver inflammation and the NLRP3 inflammasome.
Effects of clusterin on inflammatory markers in LPS-treated Kupffer cells (KC) and hepatocytes (HC). (A, B) Real-time PCR analysis of the effects of clusterin on LPS-induced expression of IL-1β, IL-1α, iNOS, TNF-α, and IL-6 in KCs and HCs. Cells were treated with LPS (1 µg/ml) or infected with Ad-clusterin virus for 2 h, and incubated for 24 h after media change. (C, D) Western blot analysis of the effects of clusterin on LPS-induced expression of NLRP3, IL-1β, and cleaved caspase 1 in KCs and HCs. *p < 0.05, **p < 0.01, *** p < 0.001.
Clusterin inhibits the STAT3 signaling pathways upon LPS-induced liver injury
Next, we investigated the anti-inflammatory molecular mechanisms of clusterin by exploring nuclear translocation and phosphorylation of STAT3, which is part of an inflammatory signaling pathway activated by LPS. STAT3 accumulated in nuclei of liver tissues of Clu-KO mice injected with LPS (Fig. 5A). Additionally, the phosphorylation level of STAT3 was significantly increased in these mice (Fig. 5B). Furthermore, the absence of clusterin increased LPS-induced phosphorylation of STAT3 in primary HCs (Fig. 5C). Conversely, overexpression of clusterin inhibited LPS-induced STAT3 phosphorylation in KCs and HCs (Fig. 5D, E). These results indicate that the anti-inflammatory effect of clusterin is related to inhibition of STAT3.
Effects of clusterin on STAT3 signaling upon LPS-induced liver injury. (A) Immunohistochemical analysis of STAT3 nuclear translocation in liver tissues of WT and Clu-KO mice injected with LPS. **p < 0.01. (B) Representative western blot analysis of p-STAT3 in the liver of WT and Clu-KO mice injected with LPS. *p < 0.05. (C) Representative western blot analysis of p-STAT3 in HCs isolated from WT and Clu-KO mice and treated with LPS. (D, E) Western blot analysis of the effects of clusterin on LPS-induced p-STAT3 in KCs and HCs.
Clusterin inhibits the NLRP3 inflammasome in THP-1 cells
Phorbol 12-myristate 13-acetate-induced THP-1 macrophages release a variety of inflammatory mediators and serve as a cellular model of inflammatory diseases. These cells are activated by signals such as LPS and adenosine 5’-triphosphate (ATP), which promote expression of inflammasome-associated proteins. Therefore, we investigated the effect of clusterin on the NLRP3 inflammasome in THP-1 cells. Overexpression of clusterin using Ad-Clu decreased LPS/ATP-induced NLRP3 and IL-1β mRNA expression and IL-1β secretion (Fig. 6A, B). In addition, expression of NLRP3 inflammasome-associated proteins (NLPR3, IL-1β, and caspase 1) was increased upon treatment with LPS alone and LPS/ATP, and these effects were attenuated by co-treatment with Ad-Clu (Fig. 6C, D). Overexpression of clusterin also inhibited cytosolic release of IL-1β induced by LPS/ATP (Fig. 6D). In THP-1 cells, clusterin overexpression inhibited the increase of STAT3 phosphorylation induced by LPS (Fig. 6E). These data suggest that clusterin significantly inhibits the NLRP3 inflammasome in THP-1 cells.
Effects of clusterin on NLRP3 inflammasome activity in THP-1 macrophages. (A) Representative real time PCR analysis of NLRP3 and IL1-β in THP-1 cells. Cells were infected with Ad-Clusterin virus for 2 h, incubated for 18 h, and treated with LPS (1 µg/ml) or LPS/ATP (1 mM) for 6 h. *p < 0.05, **p < 0.01. (B) ELISA of IL1-β secretion by THP-1 cells treated with LPS or LPS/ATP. *p < 0.05, **p < 0.01. (C, D) Representative western blot analysis of NLRP3, IL1-β and caspase1 in THP-1 cells treated with LPS or LPS/ATP. (E) Representative western blot analysis of p-STAT3 in THP-1 cells.
Discussion
This study demonstrated that clusterin protects against liver inflammation. We found that the protective role of clusterin in LPS-induced liver injury is associated with inhibition of the NLRP3 inflammasome and STAT3 signaling pathways.
LPS plays a crucial role in liver injury, and LPS-associated liver damage is linked to activation of inflammation-related molecules18. In this study, loss of clusterin significantly exacerbated LPS-induced liver injury, with increased levels of ALT and AST, and upregulated expression of pro-inflammatory cytokines. Recent research indicated that LPS not only increases production of pro-inflammatory cytokines but also activates the NLRP3 inflammasome, which plays a key role in the pathology of LPS-induced acute liver injury19,20. This activation, along with upregulation of mature IL-1β in serum and liver, is a significant aspect of acute live disease21,22. In this study, we found that production of pro-inflammatory cytokines, including IL-1β, and the NLRP3 inflammasome was increased in Clu-KO mice injected with LPS. Previous studies have demonstrated that clusterin deficiency exacerbates inflammation, and clusterin expression is associated with decreased inflammationin osteoarthritis, and sepsis patients, suggesting that it has potential as a therapeutic agent23,24,25. Similarly, our findings show that clusterin deficiency increases liver inflammation and clusterin overexpression significantly decreases inflammatory marker levels, suggesting that clusterin could have potential as a therapeutic agent for the management of inflammatory liver disease.
IL-6 is a key mediator of inflammation in the liver and is stimulated by LPS to trigger STAT3 phosphorylation, leading to increased production of pro-inflammatory cytokines26. STAT3 activation is associated with liver fibrosis and disease progression, and its role in both acute and chronic liver diseases is being increasingly emphasized27,28. A recent study reported that inhibition of STAT3 elicits a protective effect by reducing inflammation and cell death in acute liver injury29. In this study, IL-6 mRNA and p-STAT3 protein were significantly upregulated in LPS-injected Clu-KO mice, and these changes were attenuated by overexpression of clusterin. Clusterin activate NRF2 and AMPK14, which regulates STAT3 expression30. However, in our study on the anti-inflammatory effects of clusterin, we observed little change in NRF2 and AMPK activation. Instead, clusterin mainly affected the regulation of STAT3 and NLRP3 inflammasomes. These results suggest that clusterin deficiency promotes LPS-induced inflammation by further upregulating the IL-6/STAT3 signaling pathway.
Although clusterin decreases macrophage inflammation15,16, the relationship between clusterin expression and inflammation may depend on cellular and environmental conditions. THP-1 cells, a human monocyte cell line used as a cellular model of inflammatory diseases, releases a variety of inflammatory mediators. Activation of THP-1 cells is associated with the release of pro-inflammatory cytokines, which contributes to the pathogenesis of non-alcoholic steatohepatitis31. Therefore, the interaction between THP-1 cells, inflammasomes, and liver inflammation is important in the pathophysiology of liver diseases. Our results show that clusterin inhibits the NLRP3 inflammasome not only in KCs and HCs, but also in THP-1 cells.
LPS triggers a signaling cascade that phosphorylates mitogen-activated protein kinases (ERK, p38, and JNK), which subsequently activate downstream transcription factors to increase production of various inflammatory factors32. Therefore, we examined mitogen-activated protein kinase phosphorylation in LPS-injected Clu-KO mice. p-ERK expression was markedly upregulated in LPS-injected Clu-KO mice, but p-p38 and p-JNK expression was not (Supplementary Fig. 2).
Because LPS-induced inflammation operates through multiple pathways, the overexpression of clusterin may result in residual effects even while it inhibits STAT3 and NLRP3 inflammasome. In a NAFLD model, overexpression of clusterin reduced steatosis through NRF2 activation but did not completely eliminate lipid droplets14. These consistent findings across various models underscore the potential of clusterin as a therapeutic target to reduce inflammation and alleviate associated pathologies. Although these findings highlight the protective role of clusterin against LPS-induced liver injury, further studies are needed to elucidate additional clusterin-independent mechanisms that contribute to liver pathology.
Conclusions
In conclusion, clusterin protects against liver inflammation by inhibiting the NLRP3 inflammasome and STAT3 signaling pathway in response to LPS-induced liver injury.
Methods
Chemicals
LPS was purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against clusterin (sc-6420) and ASC (sc-514414) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies against IL1β antibody (ab9722) were purchased from Abcam (Cambridge, UK). Antibodies against NLRP3 (CS1510), cleaved caspase 1 (CS89332), GAPDH (CS2118), tubulin (CS2146), caspase 1 (CS2225), p-STAT3 (CS9138), and STAT3 (CS4904), and rabbit (7074P2) and mouse (7076P2) secondary antibodies, were purchased from Cell Signaling Technology (Beverly, MA, USA).
Animals experiments
In vivo experiments were conducted using 7- to 8-week-old male C57BL/6 mice (Central Lab Animal, Seoul, Korea). To generate clusterin KO mice on the C57BL/6 genetic background, clusterin-deficient mice that were originally generated using a Swiss black genetic background were backcrossed onto the C57BL/6 strain for at least seven generations. Mice were injected with LPS (1 mg/kg) and anesthetized with 100 mg/kg pentobarbital via intravenous injection 3 h after treatment and sacrificed (n = 7 per group), and liver tissue samples were collected. Blood samples were collected to determine the activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using specific assay kits from Abcam, Cambridge, UK. The experiments were performed in compliance with the Keimyung University Guidelines for the Use of Laboratory Animals. All experiments were carried out in accordance with the approved guidelines. Mice were euthanized with carbon dioxide (CO2). All efforts were made to minimize animal suffering.
Ethics statement
All experiments were approved by the Institutional Animal Care and Use Committee of Keimyung University (KM-2023-32). All animal procedures were performed in accordance with the ARRIVE guidelines for animal research.
Isolation of primary KCs and hepatocytes
Male 6 week-old C57BL/6 mice were purchased from Central Lab Animal (Seoul, Korea) and housed in a facility under a 12 h light/dark cycle. Mouse Kupffer cells and hepatocytes were isolated by perfusion of the liver with EGTA solution and collagenase solution (collagenase type I, Worthington Biochemical Corp, Lakewood, NJ, USA) through the portal vein. After incubation at 37 °C for 20 min and filtration through a 70 μm nylon mesh, the liver mixture was centrifuged at 500 rpm for 5 min to separate the hepatocyte pellet from the supernatant containing KCs. Hepatocytes were then resuspended in Williams’ medium E (Sigma-Aldrich) and cultured on type I collagen-coated dishes (IWAKI Scitech Kiv, Tokyo, Japan) for 1–2 h before being transferred to medium 199 (Sigma-Aldrich). The hepatocyte pellet and supernatant were centrifuged at 1600 rpm for 10 min; the pellet was further purified using OptiPrep density-gradient centrifugation (Sigma-Aldrich) to isolate KCs. Isolated KCs were cultured in RPMI 1640 medium (Gibco-BRL, Grand Island, NY, USA) with 10% fetal bovine serum, incubated for 30 min, and then the medium was replaced to purify the KCs. Cells were used for experiments the next day after isolation.
Isolation of primary HSCs
Hepatic stellate cells (HSCs) were isolated from both wild type and clusterin knockout (KO) C57BL/6 mice by perfusing the liver via the inferior vena cava. Initially, the liver was perfused with EGTA buffer (comprising 136.89 mmol/L NaCl, 5.37 mmol/L KCl, 0.64 mmol/L NaH2PO4.H2O, 0.85 mmol/L Na2HPO4, 9.99 mmol/L HEPES, 4.17 mmol/L NaHCO3, 0.5 mmol/L EGTA, and 5 mmol/L glucose, pH 7.35–7.4) at a rate of 5 mL/min for 2 min. This was followed by an Enzyme buffer containing 0.4 mg/mL pronase (Roche Diagnostics, Indianapolis, IN, USA) and 0.193 U/mg collagenase (Roche Diagnostics), perfused at the same rate for 5 and 7 min respectively. Post-perfusion, the liver was agitated for 25 min at 37 °C, filtered through a 70 μm nylon mesh, and centrifuged at 580 × g for 10 min at 4 °C to pellet the HSCs. The pelleted HSCs were resuspended in Gey’s Balanced Salt Solution (GBSS) (Sigma-Aldrich), layered onto a Cell-OptiPrep™ (Sigma-Aldrich) gradient prepared in GBSS, and centrifuged at 1380 × g for 17 min at 4 °C. The HSCs were collected from a thin white layer at the interface, washed in Hank’s Balanced Salt Solution, and cultured in DMEM (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS). The culture medium was refreshed every two days.
Generation of recombinant adenovirus
The cDNA for rat clusterin was cloned into the pAd-Track-CMV shuttle vector and electroporated into BJ5138 cells containing the AdEasy adenoviral vector to generate recombinant adenoviral plasmids. These were transfected into HEK-293 cells, and adenoviruses expressing clusterin were amplified and purified using CsCl density centrifugation (Sigma-Aldrich). Finally, the viruses were desalted and their titers were assessed using the Adeno-X Rapid Titer Kit (BD Bioscience, San Jose, CA, USA).
Cell culture
The human monocytic cell line THP-1 (Korean Cell Line Bank, Seoul, Korea) was a kind gift from Dr. Shin Kim (Keimyung university school of medicine). Cells was cultured in DMEM (Gibco-BRL), supplemented with 10% FBS (Hyclone, Logan, UT, USA) and antibiotics, and maintained in a 5% CO2/95% air environment at 37 °C. The cells were serum-starved in a medium containing 0.5% FBS and then infected with viruses (Ad-Clu)15. After 2 h the medium was changed and cells were treated with LPS (1 µg/ml) or LPS/ATP (1 mM).
Quantitative real-time RT-PCR
Total RNA was isolated from cells using TRIzol reagent (Life Technologies, Grand Island, NY, USA). Reverse transcription was performed using the Maxima First Strand cDNA Synthesis Kit (Thermo Scientific, Rockford, IL, USA). Real-time RT-PCR was performed using a SYBR Green PCR master mix kit (Roche Diagnostics, Indianapolis, IN, USA) and a CFX Connect real-time PCR system (Bio-Rad, Richmond, CA, USA). Primer sequences were as follows: NLRP3 (forward) 5`-ATTACCCGCCCGAGAAAGG-3` and (reverse) 5`-CATGAGTGTGGCTAGATCCAAG-3`; iNOS (forward) 5`-CATGCTACTGGAGGTGGGTG-3` and (reverse) 5`-CATTGATCTCCGTGACAGCC-3`; IL1α (forward) 5`-CAACGTCAAGCAACGGGAAG-3` and (reverse) 5`-AAGGTGCTGATCTGGCTTGG-3`; IL1β (forward) 5`-CTTTCCCGTGGACCTTCCAG-3` and (reverse) 5`-AATGGGAACGTCACACACCA-3`; IL6 (forward) 5`-TTGCCTTCTTGGGACTGATG-3` and (reverse) 5`-CTCATTTCCACGATTTCCCA-3`; TNFα (forward) 5`-ACCGTCAGCCGATTTGCTAT-3` and (reverse) 5`-CCGGACTCCGCAAAGTCTAA-3`; GAPDH (forward) 5`-ACGACCCCTTCATTGACCTC-3` and (reverse) 5`-ATGATGACCCTTTTGGCTCC-3`. Expression of GAPDH mRNA was used as an internal control.
Western blotting
Liver tissue and cells were lysed with RIPA lysis buffer (Thermo Fisher Scientific) containing proteinase inhibitors and dithiothreitol. Proteins were resolved by SDS-PAGE and then transferred electrophoretically to a polyvinyl difluoride membrane (Millipore, Burlington, MA, USA). The membranes were sequentially incubated in primary antibodies, and then with appropriate horseradish peroxidase-conjugated secondary antibodies. Signals were visualized using the Clarity™ Western ECL Substrate Kit (Bio-Rad, Richmond, CA, USA). Protein expression was detected using the Fusion Solo ChemiDoc system (VILBER LOURMAT, Germany). Protein band intensities were measured using ImageJ software version 1.52a (NIH, Bethesda, MD, USA).
Immunohistochemical analysis
Livers were isolated from mice, fixed in 4% formaldehyde, and then paraffin-embedded. Histochemical staining was performed using hematoxylin and eosin. Immunohistochemical staining was performed by incubation with primary antibodies against STAT3 (1:200) and IL1β (1:200), followed by incubation with horseradish peroxidase-conjugated anti-rabbit (Dako, Glostrup, Denmark) IgG secondary antibodies. All data were normalized against equivalent data in the control.
Measurement of cytokine levels
Mouse serum and cell culture medium were stored at − 80˚C. Levels of IL1β (R&D Systems, Abingdon, UK) were measured using enzyme-linked immunosorbent assay (ELISA) kits, following the manufacturers’ instructions.
Statistical analysis
Experimental results were statistically analyzed by a one-way analysis of variance with the Bonferroni correction or the two-tailed student’s t test (GraphPad, Prism version 9.5.1). Data are presented as mean ± standard error of the mean (SEM). All experiments were performed at least three times.
Data availability
Data availabilityAll data associated with this study are presented in the manuscript and supplementary information files.
References
Wu, Z., Han, M., Chen, T., Yan, W. & Ning, Q. Acute liver failure: mechanisms of immune-mediated liver injury. Liver Int. 30, 782–794. https://doi.org/10.1111/j.1478-3231.2010.02262.x (2010).
Stravitz, R. T. & Lee, W. M. Acute liver failure. Lancet 394, 869–881. https://doi.org/10.1016/s0140-6736(19)31894-x (2019).
Ghanim, H. et al. Increase in plasma endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care. 32, 2281–2287. https://doi.org/10.2337/dc09-0979 (2009).
Szabo, G. & Bala, S. Alcoholic liver disease and the gut-liver axis. World J. Gastroenterol. 16, 1321–1329. https://doi.org/10.3748/wjg.v16.i11.1321 (2010).
Yan, J., Li, S. & Li, S. The role of the liver in sepsis. Int. Rev. Immunol. 33, 498–510. https://doi.org/10.3109/08830185.2014.889129 (2014).
Kubes, P. & Mehal, W. Z. Sterile inflammation in the liver. Gastroenterology 143, 1158–1172. https://doi.org/10.1053/j.gastro.2012.09.008 (2012).
Dixon, L. J., Barnes, M., Tang, H., Pritchard, M. T. & Nagy, L. E. Kupffer cells in the liver. Compr. Physiol. 3, 785–797. https://doi.org/10.1002/cphy.c120026 (2013).
Del Campo, J. A., Gallego, P. & Grande, L. Role of inflammatory response in liver diseases: therapeutic strategies. World J. Hepatol. 10, 1–7. https://doi.org/10.4254/wjh.v10.i1.1 (2018).
Tukov, F. F. et al. Modeling inflammation–drug interactions in vitro: a rat Kupffer cell-hepatocyte coculture system. Toxicol. In Vitro. 20, 1488–1499. https://doi.org/10.1016/j.tiv.2006.04.005 (2006).
Wang, L. & Hauenstein, A. V. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol. Aspects Med. 76, 100889. https://doi.org/10.1016/j.mam.2020.100889 (2020).
de Carvalho Ribeiro, M. & Szabo, G. Role of the Inflammasome in Liver Disease. Annu. Rev. Pathol. 17, 345–365. https://doi.org/10.1146/annurev-pathmechdis-032521-102529 (2022).
Szabo, G. & Csak, T. Inflammasomes in liver diseases. J. Hepatol. 57, 642–654. https://doi.org/10.1016/j.jhep.2012.03.035 (2012).
Koyama, Y. & Brenner, D. A. Liver inflammation and fibrosis. J. Clin. Investig. 127, 55–64. https://doi.org/10.1172/jci88881 (2017).
Park, J. S. et al. Clusterin overexpression protects against western diet-induced obesity and NAFLD. Sci. Rep. 10, 17484. https://doi.org/10.1038/s41598-020-73927-y (2020).
Wang, Y. et al. Clusterin is closely associated with adipose tissue insulin resistance. Diab./Metab. Res. Rev. 39, e3688. https://doi.org/10.1002/dmrr.3688 (2023).
Ungsudechachai, T., Honsawek, S., Jittikoon, J. & Udomsinprasert, W. Clusterin exacerbates interleukin-1β-induced inflammation via suppressing PI3K/Akt pathway in human fibroblast-like synoviocytes of knee osteoarthritis. Sci. Rep. 12, 9963. https://doi.org/10.1038/s41598-022-14295-7 (2022).
Seo, H. Y. et al. Clusterin attenuates hepatic fibrosis by inhibiting hepatic stellate cell activation and downregulating the Smad3 Signaling Pathway. Cells 8 https://doi.org/10.3390/cells8111442 (2019).
Hamesch, K., Borkham-Kamphorst, E., Strnad, P. & Weiskirchen, R. Lipopolysaccharide-induced inflammatory liver injury in mice. Lab. Anim. 49, 37–46. https://doi.org/10.1177/0023677215570087 (2015).
Wang, X. et al. Luteolin ameliorates LPS-induced acute liver injury by inhibiting TXNIP-NLRP3 inflammasome in mice. Phytomedicine 87, 153586. https://doi.org/10.1016/j.phymed.2021.153586 (2021).
Seo, H. Y. et al. Lobeglitazone inhibits LPS-induced NLRP3 inflammasome activation and inflammation in the liver. PloS One. 18, e0290532. https://doi.org/10.1371/journal.pone.0290532 (2023).
Nath, B. & Szabo, G. Alcohol-induced modulation of signaling pathways in liver parenchymal and nonparenchymal cells: implications for immunity. Semin. Liver Dis. 29, 166–177. https://doi.org/10.1055/s-0029-1214372 (2009).
Kaufmann, B., Kim, A. D. & Feldstein, A. E. in Inflammasome Biology (ed Pablo Pelegrin) 355–368Academic Press, (2023).
Ungsudechachai, T., Jittikoon, J., Honsawek, S. & Udomsinprasert, W. Protective effect of clusterin against interleukin-1β-induced apoptosis and inflammation in human knee osteoarthritis chondrocytes. Clin. Transl Sci. 17, e13881. https://doi.org/10.1111/cts.13881 (2024).
Jun, Y. K. et al. Regulation of psoriasis, colitis, and the intestinal microbiota by clusterin. Sci. Rep. 13, 15405. https://doi.org/10.1038/s41598-023-42019-y (2023).
Augusto, J. F. et al. Clusterin neutralizes the inflammatory and cytotoxic properties of Extracellular histones in Sepsis. Am. J. Respir. Crit Care Med. 208, 176–187. https://doi.org/10.1164/rccm.202207-1253OC (2023).
Klein, C. et al. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J. Clin. Investig. 115, 860–869. https://doi.org/10.1172/jci23640 (2005).
Ploeger, C. et al. STAT1 and STAT3 exhibit a crosstalk and are Associated with increased inflammation in Hepatocellular Carcinoma. Cancers (Basel). 14. https://doi.org/10.3390/cancers14051154 (2022).
Liu, Y. et al. HERC2 promotes inflammation-driven cancer stemness and immune evasion in hepatocellular carcinoma by activating STAT3 pathway. J. Experimental Clin. Cancer Res. 42 https://doi.org/10.1186/s13046-023-02609-0 (2023).
Jiao, J. et al. Spatial molecular and cellular determinants of STAT3 activation in liver fibrosis progression in non-alcoholic fatty liver disease. JHEP Reports: Innov. Hepatol. 5, 100628. https://doi.org/10.1016/j.jhepr.2022.100628 (2023).
Gong, H. et al. Nrf2-SHP Cascade-mediated STAT3 inactivation contributes to AMPK-Driven Protection against endotoxic inflammation. Front. Immunol. 11, 414. https://doi.org/10.3389/fimmu.2020.00414 (2020).
Fu, L., Zhou, F., Wang, X. & Lu, F. [Effect of free fatty acid on NALP3 inflammasome signaling pathway in THP-1 macrophages]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 39, 811–817. https://doi.org/10.3969/j.issn.1672-7347.2014.08.010 (2014).
Jang, S. I., Kim, H. J., Kim, Y. J., Jeong, S. I. & You, Y. O. Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW 264.7 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur. J. Pharmacol. 542, 1–7. https://doi.org/10.1016/j.ejphar.2006.04.044 (2006).
Acknowledgements
We submit our paper to Scientific Reports at the suggestion of our editorial submission advisor at the Springer Nature Transfer Desk. Thank you.Western imaging files, anesthesia methods, availability, and ethical approval are attached or included in the text.Thank you.We have made further revisions to reflect the reviewers’ valuable comments and hope that the current version of the manuscript will be accepted for publication in Scientific Reports.We also revised the Technical Check section below:1. Included the caption for Fig. 6 E in the manuscript file.2. Removed the supplementary figure caption from the main manuscript and attached it to the supplementary file.3. Included multiple exposure images for some images (western blots image).- Fixed high contrast (overexposed) areas of the western blot, and included a multi-exposure image in the additional information file (western blots image)0.2024.11.151 We checked the title.2. We made a change in the methods section: (line 223)3 Corrected the information for THP1 cells. (Line 267)
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A3046593, NRF‐ 2021R1I1A3059150), and was supported by a NRF grant funded by the Korea government (MIST) (NRF‐2022R1A2C1006416).
Author information
Authors and Affiliations
Contributions
Hye-Young Seo, Mi Kyung Kim, and Byoung Kuk Jang designed the study and obtained funding. Hye-Young Seo, Ji Yeon Park, So-Hee Lee, and Seong Hwan Cho performed the experiments. So-Hee Lee and Ji Yeon Park acquired the data. Eugene Han and Jae Seok Hwang analyzed the data. Hye-Young Seo wrote the manuscript. Mi Kyung Kim and Byoung Kuk Jang reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Seo, HY., Park, J.Y., Lee, SH. et al. Clusterin inhibits lipopolysaccharide induced liver injury. Sci Rep 15, 5975 (2025). https://doi.org/10.1038/s41598-024-80903-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-80903-3