Abstract
The European hoopoe (Upupa epops) conforms a paradigmatic example of animals cultivating bacteria in their uropygial gland that protect them against pathogenic infections. We here explore the hypothesis that enterococci are the responsible bacteria of such beneficial effect. We did so by comparing the antimicrobial activity against three indicator bacteria of colonies isolated from cultures of enterococci and mesophilic bacteria from the uropygial skin or secretion of nestlings, brooding or non-brooding females, and males of the subspecies longirostris in Hainan (China). In accordance with the hypothesis, enterococci isolated from nesting birds are more active than those from non-nesting birds. Moreover, enterococci from the uropygial secretion were more active than those isolated from the skin or than mesophilic bacteria isolates. These results therefore support the hypothesis that, during the nesting phase, hoopoe females and nestlings cultivate enterococci in their uropygial gland with relatively high antimicrobial activity.
Similar content being viewed by others
Introduction
Some animals cultivate antimicrobial-producing bacteria in particular locations of their body that help them preventing pathogenic infections. This is the case of fungus-growing ants1, beetles2, and other insects3 that cultivate antibiotic-producing bacteria in special cuticle locations. Chemical defence mediated by microorganisms has been described in a wide range of animal taxa with few examples in vertebrates4, in which the European hoopoe (Upupa epops) is the paradigmatic example as they cultivate antimicrobial-producing bacteria in their uropygial gland. While male and non-brooding female hoopoes produce a white and odourless uropygial gland secretion with only occasional presence of a few bacteria, secretions from breeding females and nestlings are brown, have a strong odour, and contain abundant Enterococcus faecalis strains5 that produce broad-spectrum antimicrobial substances6,7.
Particularities of the brown uropygial secretion of female and nestling hoopoes result from the presence of microbial symbionts in the gland8,9. When birds smear their secretion on the surface of feathers or on the eggshell, they also spread the cultivated bacteria on these surfaces10,11, the latter of which contain specialized structures to harbour the symbiont-containing secretion12. Secretion including antimicrobial substances (i.e., bacteriocines) and/or antimicrobial-producing bacteria, therefore, protects feathers from feather-degrading microorganisms13, and the embryos from trans-shell infection of pathogens12. Remarkably, enterococci isolated from the uropygial secretion of nesting hoopoes had similar effects on feather degradation in the lab13 and its abundance on the eggshell and in the secretion of females predicted hatching success of hoopoe eggs in natural conditions12. The background explained above, therefore, suggests that enterococci growing in the uropygial gland of hoopoes play an important role in the reproductive success and survival prospects of this species.
The mutualistic association between hoopoes and the enterococci that they cultivate in their uropygial gland predicts that traits facilitating the cultivation of bacterial strains with the highest antimicrobial properties should be selected in hoopoes. This scenario predicts that bacterial strains with higher abundance and prevalence in the uropygial secretion of hoopoes should be those with the highest antimicrobial properties. In accordance, previous work7 demonstrated that (i) all bacteria isolated from aerobic cultures of the uropygial secretion of European hoopoes belonged to the genera Enterococcus. Moreover, (ii) the genetic profile and the antimicrobial activity of isolates covaried with each other, and (iii) both genetic profile and antimicrobial activity varied among hoopoe nests suggesting that variation in the inhibitory capacity of symbionts is under selection. Moreover, the antimicrobial activity of isolates depended on the presence of bacteriocin genes, with the MR10 and AS-48 enterocins conferring the highest inhibition capacity and being the genes that appeared at the highest prevalence in bacterial strains isolated from hoopoe secretion14. Thus, it is likely that the benefits for hoopoes associated with the cultivation of the symbiont with the highest antimicrobial potential cause a higher frequency of strains carrying those genes. Interestingly, the selection of the most competitive bacterial symbionts is likely the result of a coevolutionary process, as some empirical and experimental evidence suggest that, although the microbiota of the uropygial secretion of hoopoes is mainly acquired horizontally from the environment, it is partially vertically transmitted from mothers to offspring15,16,17.
Current knowledge of the mutualistic association between hoopoes and the bacterial communities of their uropygial gland therefore indicates that a high antimicrobial activity of symbionts is one of the target traits of the bacterial community of the uropygial secretion that hoopoes should select for7,14. Moreover, this selection is mainly exerted on the enterococci of the community, and the effect of this selection on enterococci in terms of antimicrobial activity of isolates should be detected mainly during the nesting phase when the secretion is full of bacteria5. Consequently, it can be predicted that (a) the antimicrobial potential of isolates from the uropygial secretion should, on average, be higher than that of isolates from the skin of the uropygial gland, simply because the latter can also be colonized by bacteria from sources other than secretion. Moreover, (b) the antimicrobial activity of enterococci isolates should, on average, be higher than that estimated for the entire community that may include non-enterococci strains14. Finally, (c) because antibiotic-producing bacteria are mainly cultivated by nesting individuals (breeding females and nestlings)5, the antimicrobial activity of isolates from those birds, should be higher than that of isolates from males and non-breeding females.
We here explore the above-mentioned three predictions from the current knowledge of the hypothesis that hoopoes maintain a mutualistic association with the enterococci inhabiting their uropygial gland, which as far as we know, have never been tested. We did so in a Chinese hoopoe population of the subspecies longirostris in the tropics (Hainan). There, we sampled the bacterial communities of the uropygial secretion and skin of nestlings, breeding and non-breeding females, and males. Moreover, samples were cultivated in a general medium for mesophilic bacteria and in a specific medium for Enterococcus spp. Finally, we quantified the antimicrobial activity of isolates from those two culture media against Bacillus licheniformis, Listeria innocua and Enterococcus faecalis. With this information, we explored and found support for the predicted effects of culture media, sample location, and individual reproductive stage on the antimicrobial activity of isolated bacterial colonies.
Results
Colonies isolated from the selective media for enterococci demonstrated higher antimicrobial activity than those of mesophilic bacteria when analysed against Enterococcus faecalis and Listeria innocua (Fig. 1). However, this effect did not reach statistical significance when confronting those colonies against Bacillus licheniformis (Table 1). Moreover, the antimicrobial activity of colonies isolated from the uropygial secretion of hoopoes was higher than that of colonies isolated from the uropygial skin when tested against Enterococcus faecalis, and, at a lower level (i.e., statistically non-significant), against Listeria innocua (Table 1). The antimicrobial activity of these two sample types against Bacillus licheniformis did not differ significantly (Table 1, Fig. 1). Interestingly, the interaction between culture media and sampled location did not explain variation in antimicrobial activity against any of the used indicator bacteria (F < 1.25, df = 1, > 21.9, P > 0.276).
Antimicrobial activity of colonies isolated from general (TSA for mesophilic bacteria) and selective (KF for enterococci bacteria) cultures of samples collected from the uropygial gland skin (Skin) and secretion (Secr) of nestling (N), brooding (BF) or non-brooding female (NF) and male (M) hoopoes. Pooled values of nesting (nestlings and brooding females) and non-nesting (non-brooding females and males) hoopoes are also shown. Showed values are means ± 95% confidence intervals.
Moreover, for the whole set of isolates and indicator strains, the antimicrobial activity of colonies isolated from hoopoes of different stages or sexes did not differ significantly (see effect associated to the factor “type of hoopoes” in ESM, Table S1). However, the interaction between the type of individual hoopoe sampled and the culture media from which colonies were isolated explained the antimicrobial activity against Listeria innocua and Enterococcus faecalis, but not that against Bacillus licheniformis (ESM, Table S1). In addition, when considering whether sampled individuals were (nesting) or were not (non-nesting) breeding, the interaction between the nesting state of sampled hoopoes and culture media explained antimicrobial activity of isolated colonies (Table 1, Fig. 1). Independently of the indicator bacterium used, colonies from nesting hoopoes (i.e., nestlings and brooding females) isolated from enterococci selective medium exhibited greater antimicrobial activity (Fig. 1).
Finally, in no case, the interaction between the type of hoopoe sampled (F < 0.56, df = 3, > 35.1, P > 0.647) or the nesting stage (F < 0.40, df = 1, > 54.5, P > 0.528) with the sampled location (uropygial gland skin or secretion) significantly explained antimicrobial activity of isolates.
Discussion
Our main results are that (i) independently of the indicator bacteria used, enterococci are more active than mesophilic bacteria isolated from hoopoes, (ii) isolates from the uropygial secretion are more active than bacterial colonies from the uropygial skin, and (iii) bacteria (mainly enterococci) collected from nesting individuals (nestlings and brooding females) demonstrated stronger antimicrobial activity than those from non-nesting individuals. Moreover, the effect of sampled location did not interact with other factors, but the interaction between nesting stage and culture media of isolates explained the antimicrobial activity of symbiotic bacteria of the hoopoe subspecies longirostris. Below we discuss the importance of these results supporting the main predictions of the hypothesis that, during the nesting phase, hoopoe females and nestlings cultivate enterococci in their uropygial gland with relatively high antimicrobial capacity.
All evidence of a mutualistic association between hoopoes and enterococci growing in their uropygial gland comes from studies with the European hoopoe. Culture-independent methods have revealed that the microbial community of the uropygial secretion of hoopoes is quite complex and varies between sexes and among annual seasons18. However, the use of traditional culture methods almost invariably shows that Enterococcus is the only genus that grows in aerobic culture for mesophilic bacteria5,7. Moreover, in the European hoopoes, the uropygial secretion of breeding females, but not that of males, demonstrated antimicrobial activity and harboured bacteria that grew in mesophilic culture media, linking the antimicrobial properties of the uropygial secretion of hoopoes to the presence of enterococci. Here, for the first time, we have compared the antimicrobial capacity of bacterial strains isolated from general and from enterococci-specific cultures of hoopoe samples. Although isolates from a general medium would also include enterococci, the proportion of these bacteria should necessarily be higher in plates with specific medium for enterococci, especially in samples taken from the gland surface. Thus, since we found that the antimicrobial activity of isolates from the specific medium is higher than that of isolates from a general medium, our results further suggest that cultivating enterococci would imply some advantages for hoopoes. Enterococci are a group of bacteria known to produce a wide range of potent antimicrobial chemicals, i.e., bacteriocins19,20, with positive effects on avian growth21 and applicability on food safety and poultry22,23,24. Thus, it is not surprising that the antimicrobial activity of enterococci isolates was higher than that of isolates that included some other groups of bacteria.
The hypothesis of mutualistic association between hoopoes and antibiotic-producing bacteria also posits that hoopoes cultivate those bacteria in their uropygial gland. Thus, even if bacteria from the gland colonize other locations10,11, isolates from secretion samples should produce wider inhibition halos than isolates from the skin of the uropygial gland. Our results also supported that prediction for two of the three indicator bacteria used. Bacterial isolates from both sampled locations, the skin and the secretion, showed similar antagonistic capacities against Bacillus licheniformis. However, it is the indicator bacteria against which isolates produced the biggest inhibition halos (Fig. 1). Therefore, it suggests that not only symbionts living inside the uropygial gland, but also those from the gland skin are highly active against this feather-degrading bacteria. We know that, not only bacteriocins from symbiotic enterococci of hoopoes, but also the bacteria from the uropygial secretion that reach hoopoe feathers, protect them from degradation by B. licheniformis13. Moreover, we also know that the antimicrobial capabilities of bacteria isolated from the uropygial skin of different bird species associate positively with species-specific risk of infection (i.e., nesting habits)25. Thus, because the bacterial symbionts that are active against feather degrading bacteria would benefit hoopoes when reaching the feathers, it is possible that, facilitated by the preening activity/behaviour10, hoopoes also harbour these bacteria on the skin of the uropygial gland (for a similar argument, see Martínez-Renau et al.25). Sequencing and taxonomically identifying isolates from the skin of the uropygial gland and its secretion is necessary to test this possibility.
The mutualistic association between hoopoes and antibiotic-producing bacteria mainly occurs during reproduction in incubating or brooding females and nestlings5 and, thus, the antimicrobial capacity of isolates from nesting hoopoes should be higher than that of non-nesting hoopoes (males and non-breeding females). Again, our results supported the prediction, except when confronting isolates with B. licheniformis. The rationality of that prediction is that hoopoes rarely show nest sanitation behaviours, with nestlings’ faeces and debris from reproductive activities accumulating within the nest cavities26,27, which should result in a high risk of pathogenic infection that antimicrobial symbionts would counteract5.
Even though we found support for the expected effects of all considered factors explaining the antimicrobial activity of bacterial symbionts of hoopoes, only nesting activity and culture media interacted with each other. Since the expected effect of enterococci should appear more pronounced in samples from the uropygial secretion of nestlings or brooding females5, these two factors should interact explaining variance in antimicrobial activity of isolates. Moreover, the hypothesis also predicts that enterococci from the secretion should be the isolates with the highest antimicrobial activity and, thus, there should be a significant interaction between these two factors. Although the absence of statistical evidence supporting these interactions might be interpreted as opposed to the hypothesis tested, it may be due to statistical and/or biological reasons. It might for instance be explained by the relatively small sample size inherent to ecological studies with wild animals that many times prevent detecting statistical significance of relatively small size effects28.
Another non-exclusive explanation of non-detected interactions is that characteristics of the mutualistic association between hoopoes and antimicrobial-producing symbionts differ for different hoopoes subspecies. Here we worked with the subspecies longirostris in the tropics, where selection pressures from pathogenic disease should be higher than in the semiarid climate of south-eastern Spain, where most work on this hypothesis has been carried out. One apparent difference between these two populations is that, in Spain, bacteria from the uropygial secretion of males or non-breeding females did not grow in general media5, whereas some colonies grew when cultivating samples from the uropygial secretion of Chinese hoopoes (see Table 2). Interestingly, because bacteria from the secretions of males and non-breeding females did not grow in specific media for enterococci, it is likely that, contrary to what has been detected for the Spanish hoopoes, non-enterococci bacteria grew in cultures from the secretion of the subspecies longirostris. Genetic identification of isolated colonies from this subspecies is necessary to confirm this possibility. The non-significant interactions between culture media and sampled location on the one hand, and between sample location and hoopoe breeding stage on the other, also suggest that bacterial symbionts, others than enteroccoci, might be important for Chinese hoopoes breeding in the tropics. Future studies should focus on the genetic identification of bacteria that grew in cultures of different samples and individuals of the subspecies longirostris.
Summarising, our results confirm most of the general assumptions of the mutualistic association between hoopoes and enterococci of the uropygial secretion and suggest that it may vary among subspecies of hoopoes under different selection pressures.
Material and methods
Study area and fieldwork
The study was conducted in Wenchang countryside (19° 40′ N, 110° 45′ E), in Hainan Island, southern China. The area has a tropical monsoon maritime climate, with a mild weather, abundant heat and rainfall, and an average annual temperature of 24.2 °C29. Tree holes are scarce in the area and, although nest boxes are available, they are mainly occupied by Crested Mynas (Acridotheres cristatellus), Common Mynas (Acridotheres tristis), Oriental Magpie Robins (Copsychus saularis), and White-shouldered Starlings (Sturnus sinensis), while hoopoes (Upupa epops longirostris) prefer to nest in the holes of walls of buildings in local farms.
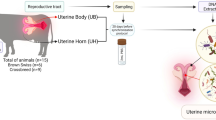
The fieldwork was carried out from the beginning of May to the end of June of 2023 and consisted of an intense search for hoopoe nests in the area. We also asked farmers and local people for locations where they had seen hoopoes carrying prey in the beak. Once a hoopoe nest was found, with an endoscope, we looked inside and estimated the reproduction stage (eggs, or nestlings of different ages). When the female was inside and the older nestlings were less than 8 days old, we carefully removed pieces of the wall and captured the female. After sampling the female, we pasted the removed material to the wall, and the female was then released into the nest through the entrance hole, which we kept closed for 5 min with a cotton bag to calm the female and reduce the risk of nest abandonment. Nestlings were sampled when the older one was around 18 days old. None of the sampled hoopoe nests were abandoned after sampling.
Sampling bacterial communities in the field
Females and nestlings were sampled following the same protocol. We wore new latex gloves cleaned with ethanol during the whole sampling process. First, we sampled the bacterial community of the skin by rubbing a sterile cotton swab wetted in sterile phosphate buffered saline (PBS) over the surface of the uropygial gland, the surroundings of the papilla opening and the tuft. The swab with the bacterial sample was included in a sterile microfuge vial containing 1 ml of PBS. Afterwards, we softly washed the skin and feathers around the gland with ethanol (70%) to avoid contamination of the secretion with external alive bacteria. Once the alcohol had evaporated, and following the sampling protocol described in Martín-Vivaldi et al.8, we gently introduced a sterilized micropipette tip (1–10 µl) inside the uropygial-gland papilla, and collected all the uropygial secretion that was directly included in an empty and sterilized microfuge vial. The used vials were kept at approximately 4 °C in a portable icebox until their use in the lab within the next 48 h.
Ethic declaration
The experiments comply with the current laws of China. All applicable guidelines for the care and use of animals were followed. Experimental procedures for hoopoe manipulation were approved by the Animal Research Ethics Committee of Hainan Provincial Education Centre for Ecology and Environment, Hainan Normal University (no. HNECEE-2023-007). Sample sizes were relatively small and restricted to nests with hoopoe nestlings older than 16 days and to adults caught at the right breeding stage (breeding and non-breeding females and males). Thus, in accordance with ARRIVE guidelines, nest visits and the number of sampled individuals were reduced to those allowing reliable statistical tests.
Estimating antimicrobial activity of bacterial colonies from hoopoe samples
Samples collected from the uropygial gland skin and secretion were first cultivated in a general medium for mesophilic bacteria and in a selective medium for enterococci. Briefly, 5 µl of secretion were diluted in 45 µl of sterile PBS and serial dilutions (by mixing 30 µl of the initial dilution in 270 µl of sterile PBS) were prepared until 10–4. For bacterial samples from swabs kept in PBS, we also prepared serial dilutions with 10 µl of original dilution per 990 µl of sterile PBS until 10–4. We used 100 µl of each buffer dilution for the cultivation of aliquots on two different solid media (See Electronic Supplementary Material (ESM), Fig S1): Tryptic Soy Agar (TSA), a broadly used general medium for growing aerobic mesophilic bacteria (Scharlau Chemie S.A., Barcelona), and a specific medium for enterococci (Kenner fecal agar (KFA) Scharlau Chemie S.A., Barcelona). The KF medium also included TTC (2,3,5-Triphenyl tetrazolium chloride solution) (1%), which colour the enterococci colonies red. Petri dishes were incubated at 37 °C for 24 h and 72 h, respectively.
From each cultivated sample, a maximum of five colonies, preferably of different morphology (i.e. size, shape, and colour) were isolated and inoculated individually into petri dishes with TSA, by streaking with a sterile loop, and incubated at 37 °C for 24 h. This step was repeated twice consecutively to minimize the possibility of contamination of each isolate (see ESM, Fig S1). For antimicrobial assays, each colony of the isolated strains was replicated by spotting from the source colony onto three TSA plates (35 colonies per plate), which were then incubated for 24 h at 37 °C. As a control strain, we spotted a colony of an Enterococcus faecalis strain (MRR 10-3) that was originally isolated from the uropygial secretion of the European hoopoe. This strain is known for producing a special bacteriocin (MRR10) with a wide range of antimicrobial activity6. After growth, the plates were covered with 6 ml of soft agar (Brain Heart Infusion added 0.8% agar, Scharlau Chemie S.A., Barcelona), previously heated until liquefied and tempered to 50 °C. Once liquified, the soft agar was inoculated with 100 μl of an overnight culture of the indicator strain at 37 °C. Finally, covered plates were incubated for 16 h at 37 °C. The antimicrobial activity of each isolated colony was revealed by the presence of growth inhibition halos around the spot of isolates. The intensity of antimicrobial activity was measured as the width of the halo ring around the isolated colony, measured with a ruler to the nearest 0.5 mm (for more details see ESM, Fig S1 and Ruiz-Castellano et al.30, Ruiz-Rodríguez et al.7 and Martínez-Renau et al.25).
Tests of antimicrobial activity were performed against three typified bacterial strains from the Spanish Type Culture Collection (CECT) and from our laboratory collection of taxonomic groups that could be potentially pathogenic for birds31,32,33. We used Bacillus licheniformis D13, Enterococcus faecalis JH2-2, and Listeria innocua CECT340. The first one is a feather degrading bacteria, the second is a commensal bacteria that might cause some animal diseases, and the third one is innocuous but phylogenetically close related to pathogenic ones31,32,33.
Sample sizes and statistical analyses
We were able to estimate the antagonistic capacity of 677 bacterial colonies against Bacillus licheniformis, Listeria innocua and/or Enterococcus faecalis. 377 and 300 of the tested strains were isolated from samples of the skin and secretion of the uropygial gland of nestlings (N = 23 and 24, respectively), adult males (N = 5), and brooding (N = 3) and non-brooding females (N = 2), that were cultivated in a general medium for mesophilic bacteria (TSA, N = 378), and in specific media for enterococci (KF, N = 299). No bacterial colony grew in cultures of the secretion of males and non-brooding females in specific media, while some few bacteria isolated from the general media grew in waves occupying the entire plates, which impeded the estimations of their antagonistic capacities. As a result, the number of evaluated bacterial strains differed depending on the culture media and the sampled location (see sample sizes in Table 2).
To explore the variation in antimicrobial activity of isolated bacterial strains in relation to the type of individual (nestlings, males, brooding and non-brooding females), sampling location (skin or secretion of the uropygial gland) and bacterial type (enterococci or mesophilic bacteria), we included these variables as fixed factors in General Linear Mixed Models (GLMM). These models also included the identity of sampled individuals (nested within the type of individual hoopoes) as the first random factor, and the interactions with sampling location and bacterial type as two additional random factors. These three random factors accounted for the non-independence of data from colonies isolated from the same individual as well as for the repeated-measures nature of information from samples of different locations and bacterial types from the same individual. The effects of the interactions between fixed factors ((i) type of bacteria (mesophilic vs. enterococci) and location of samples, (ii) type of bacteria and type of individual, and (iii) location of samples and type of individuals) on the antimicrobial activities were explored in separate models that also included main effects and the second order interaction between individual identity (nested within the type of individual hoopoes), location and bacterial types as additional random effects. The effects of second order interactions between fixed factors could not be estimated because enterococci did not grow in samples from non-nesting individuals. Non-significant interactions were sequentially removed with final models only including that between type of bacteria and type of individual hoopoes. Moreover, since the antimicrobial activity of nesting (i.e., nestlings and brooding females) and non-nesting hoopoes (i.e., males and non-brooding females) showed similar patterns (see results), we repeated all these statistical models by including information on whether sampled hoopoes were or were not in the nests (i.e., nesting). For simplicity, we presented the results of these last models in the Results section, while models including information of the “type of hoopoes samples” were reported in the Electronic Supplementary Material (ESM).
Values of antimicrobial activity were Log10 transformed before the analyses to approach a normal distribution, and residuals of GLMMs were plotted against expected normal values to detect outliers (extreme and isolated points that separated from the expected values and from other points). When detected, the model was run again without considering detected outliers. Although statistical inferences (i.e., statistically significant factors) did not depend on considering outliers, we show results that excluded those points.
All statistical analyses were performed in Statistica 13.034.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files). For further information contact to JJS.
References
Currie, C. R., Scott, J. A., Summerbell, R. C. & Malloch, D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398, 701–704 (1999).
Scott, J. J. et al. Bacterial protection of beetle-fungus mutualism. Science 322, 63–63 (2008).
Brownlie, J. C. & Johnson, K. N. Symbiont-mediated protection in insect hosts. Trends Microbiol. 17, 348–354 (2009).
Flórez, L. V., Biedermann, P. H. W., Engl, T. & Kaltenpoth, M. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat. Prod. Rep. 32, 904–936. https://doi.org/10.1039/C5NP00010F (2015).
Soler, J. J. et al. Symbiotic association between hoopoes and antibiotic-producing bacteria that live in their uropygial gland. Funct. Ecol. 22, 864–871 (2008).
Martín-Platero, A. M. et al. Characterization of antimicrobial substances produced by Enterococcus faecalis MRR 10–3, isolated from the uropygial gland of the hoopoe Upupa epops. Appl. Environ. Microbiol. 72, 4245–4249 (2006).
Ruiz-Rodríguez, M. et al. Antimicrobial activity and genetic profile of enteroccoci isolated from hoopoes uropygial gland. PLoS ONE 7, e41843. https://doi.org/10.1371/journal.pone.0041843 (2012).
Martín-Vivaldi, M. et al. Seasonal, sexual and developmental differences in hoopoe Upupa epops preen gland morphology and secretions: evidence for a role of bacteria. J. Avian Biol. 40, 191–205 (2009).
Martín-Vivaldi, M. et al. Antimicrobial chemicals in hoopoe preen secretions are produced by symbiotic bacteria. Proc. R Soc. Lond. B Biol. Sci. 277, 123–130. https://doi.org/10.1098/rspb.2009.1377 (2010).
Martínez-García, A. et al. Preening as a vehicle for key bacteria in hoopoes. Microb. Ecol. 70, 1024–1033. https://doi.org/10.1007/s00248-015-0636-1 (2015).
Soler, J. J. et al. Nestedness of hoopoes’ bacterial communities: symbionts from the uropygial gland to the eggshell. Biol. J. Linn. Soc. 18, 763–773. https://doi.org/10.1111/bij.12772 (2016).
Martín-Vivaldi, M. et al. Special structures of hoopoe eggshells enhance the adhesion of symbiont-carrying uropygial secretion that increase hatching success. J. Anim. Ecol. 83, 1289–1301. https://doi.org/10.1111/1365-2656.12243 (2014).
Ruiz-Rodríguez, M. et al. Symbiotic bacteria living in the hoopoe’s uropygial gland prevent feather degradation. J. Exp. Biol. 212, 3621–3626. https://doi.org/10.1242/jeb.031336 (2009).
Ruiz-Rodríguez, M., Martinez-Bueno, M., Martin-Vivaldi, M., Valdivia, E. & Soler, J. J. Bacteriocins with a broader antimicrobial spectrum prevail in enterococcal symbionts isolated from the hoopoe’s uropygial gland. FEMS Microb. Ecol. 85, 495–502 (2013).
Martín-Vivaldi, M. et al. Acquisition of uropygial gland microbiome by hoopoe nestlings. Microb. Ecol. 76, 285–297. https://doi.org/10.1007/s00248-017-1125-5 (2018).
Ruiz-Rodríguez, M. et al. Environmental factors shape the community of symbionts in the uropygial gland of hoopoes more than genetic factors. Appl. Environ. Microbiol. 80, 6714–6723 (2014).
Martínez-García, A. et al. The microbiome of the uropygial secretion in hoopoes is shaped along the nesting phase. Microb. Ecol. 72, 252–261 (2016).
Rodríguez-Ruano, S. et al. Seasonal and sexual differences in the microbiota of the hoopoe uropygial secretion. Genes 9, 407 (2018).
Riley, M. A. & Wertz, J. E. Bacteriocines: evolution, ecology, and application. Ann. Rev. Microbiol. 56, 117–137 (2002).
Sánchez-Hidalgo, M. et al. AS-48 bacteriocin: Close to perfection. Cell. Mol. Life Sci. 68, 2845–2857. https://doi.org/10.1007/s00018-011-0724-4 (2011).
Moreno, J. et al. Beneficial effects of cloacal bacteria on growth and fledging size in nestling pied flycatchers (Ficedula hypoleuca) in Spain. Auk 120, 784–790 (2003).
Franz, C. M. A. P., Holzapfel, W. H. & Stiles, M. E. Enterococci at the crossroads of food safety?. Int. J. Food Microbiol. 47, 1–24 (1999).
Montalbán-López, M., Sánchez-Hidalgo, M., Valdivia, E., Martínez-Bueno, M. & Maqueda, M. Are bacteriocins underexploited? Novel applications for OLD antimicrobials. Curr. Pharma. Biotechnol. 12, 1205–1220. https://doi.org/10.2174/138920111796117364 (2011).
Foulquié Moreno, M. R., Sarantinopoulos, P., Tsakalidou, E. & De Vuyst, L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 106, 1–24 (2006).
Martínez-Renau, E. et al. Microbial infection risk predicts antimicrobial potential of avian symbionts. Front. Microbiol. 13 (2022).
Ibáñez-Álamo, J. D., Rubio, E. & Soler, J. J. Evolution of nestling faeces removal in avian phylogeny. Anim. Behav. 124, 1–5. https://doi.org/10.1016/j.anbehav.2016.11.033 (2017).
Martín-Vivaldi, M., Doña, J., Romero-Masegosa, J. & Soto-Cárdenas, M. In Enciclopedia Virtual de los Vertebrados Españoles (eds A. Salvador & M.B. Morales) Ch. http://www.vertebradosibericos.org/, (Museo Nacional de Ciencias Naturales, 2014).
Møller, A. P. & Jennions, M. D. How much variance can be explained by ecologists and evolutionary biologists?. Oecologia 132, 492–500 (2002).
Liu, J., Zhang, F., Liu, Y. & Liang, W. Egg recognition and nestling discrimination in the Crested Myna (Acridotheres cristatellus): Size matters. Avian Res. 14, 100111–100111. https://doi.org/10.1016/j.avrs.2023.100111 (2023).
Ruiz-Castellano, C., Ruiz-Rodríguez, M., Tomás, G. & Soler, J. J. Antimicrobial activity of nest-lining feathers is enhanced by breeding activity in Avian nests. FEMS Microbiol. Ecol. 95, fiz05. https://doi.org/10.1093/femsec/fiz052 (2019).
Pinowski, J., Barkowska, M., Kruszewicz, A. H. & Kruszewicz, A. G. The causes of the mortality of eggs and nestlings of Passer Spp. J. Biosci. 19, 441–451 (1994).
Hubalek, Z. An annotated checklist of pathogenic microorganisms associated with migratory birds. J. Wildl. Dis. 40, 639–659 (2004).
Benskin, C. M. H., Wilson, K., Jones, K. & Hartley, I. R. Bacterial pathogens in wild birds: a review of the frequency and effects of infection. Biol. Rev. 84, 349–373 (2009).
Dell-Inc. STATISTICA (data analysis software system), version 13. (software.dell.com, 2015).
Acknowledgements
Jinmei Liu, Yuran Liu, Yidong Wei and Baisalbayeva Rakhima, helped us with the field work, and their contribution contacting local people asking them for hoopoe nests, was essential for collecting the samples used in the present work. Cristina Soler-Zamora and Javier Martín-Vivaldi also helped us in the field and with the laboratory work. Bingyu Cai and Zhufen Gao helped us in the lab plating bacterial samples. The Spanish part of the research group received funds from the projects PID2020-117429GB-C21 and PID2020-117429GB-C22, funded by the Ministerio de Ciencia e Innovación/Agencia Estatal de Investigación/10.13039/501100011033.
Author information
Authors and Affiliations
Contributions
MMV, JJS, with the help of WL designed the study and, with the help of MDB, EMR and LZ, updated the laboratory protocol. MMV and JJS, carried out the fieldwork and, with the help of LZ, the laboratory work too. JJS performed the statistical analyses and wrote a first version of the manuscript. All the authors substantially contributed to the final version of the manuscript and approved the submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Soler, J.J., Barón, M.D., Martínez-Renau, E. et al. Nesting hoopoes cultivate in their uropygial gland the microbial symbionts with the highest antimicrobial capacity. Sci Rep 14, 30797 (2024). https://doi.org/10.1038/s41598-024-81062-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81062-1
Keywords
This article is cited by
-
Exploring the associations between preen oil bacterial, chemical and proteomic profiles of passerines
Antonie van Leeuwenhoek (2025)