Abstract
This study aims to comprehensively evaluate the clinical utility of five diffusion models, including conventional mono-exponential (Mono), intravoxel incoherent motion (IVIM), diffusion kurtosis imaging (DKI), stretched exponential (SEM), and continuous-time random-walk (CTRW), for preoperatively predicting of breast lesion pathology, prognostic biomarkers, and molecular subtypes. We retrospectively analyzed 132 patients with pathologically verified breast lesions (41 benign and 91 malignant) who underwent a full protocol preoperative breast MRI protocol, including a diffusion-weighted imaging (DWI) sequence with nine b values (0 to 2000 s/mm2) on a 3.0T MR scanner. The diffusion parameters from each model—Mono (ADC), IVIM (D, D*, f), DKI (MD, MK), SEM (DDC, α) and CTRW (Dm, α, β)—were quantitatively calculated and compared between benign and malignant breast lesions, as well as across different prognostic biomarker statuses in breast cancer, using Mann–Whitney U-tests. For molecular subtypes comparisons, we employed the Kruskal-Wallis test followed by Bonferroni. All parameters, except IVIM-D*, significantly differentiated benign from malignant lesions. Notably, IVIM-D and DKI-MK values were significantly different between estrogen receptor (ER)-positive and ER-negative tumors. Progesterone receptor (PR)-positive cancers exhibited lower Mono-ADC, IVIM-D, DKI-MD, SEM-DDC, CTRW-Dm, and CTRW-α values, alongside higher DKI-MK value compared to PR-negative cancers (p < 0.05). Significant differences in IVIM-D, IVIM-D*, and DKI-MK values were observed between human epidermal growth factor receptor 2 (HER2)-negative and HER2-positive tumors. Furthermore, higher SEM-α and CTRW-β values, along with lower DKI-MD and SEM-DDC values, were noted in the high Ki-67 expression group compared to the low Ki-67 group (p < 0.05). All five diffusion models proved valuable for breast cancer diagnosis, with the CTRW model exhibiting the highest diagnostic performance, although the difference was not statistically significant. The diffusion parameters derived from these models can effectively assist in distinguishing prognostic factors and molecular subtypes of breast cancer.
Similar content being viewed by others
Introduction
Breast cancer characterized by significant tumor heterogeneity1, which necessitates highly individualized treatment strategies and survival prognosis based on molecular subtypes2. Clinically, breast cancer is classified into four intrinsic subtypes according to key prognostic factors, including the expression of estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki-67 proliferation index3. Additionally, the assessment of histological grades and axillary lymph node metastasis (ALNM) is crucial for accurate staging and therapeutic planning4. However, the identification of these clinic prognostic factors generally relies on invasive histopathological examination5.
Dynamic contrast-enhanced MRI (DCE-MRI) is recognized for its high sensitivity in detecting breast cancer and plays a vital role in clinical practice6,7. However, it often suffers from variable specificity and raises concerns regarding the use of gadolinium contrast agents8,9,10. In contrast, diffusion-weighted imaging (DWI) is a non-invasive technique that exploits the diffusion of water molecules as an endogenous contrast, and it has been extensively investigated as an important complementary approach for detecting and characterizing breast cancer11,12,13,14,15. The apparent diffusion coefficient (ADC), a commonly used metric derived from the mono-exponential (Mono) model, is limited by its reliance on the simple Gaussian diffusion assumption, which may not be accurately reflect water diffusion in the presence of microperfusion and biological complexity16.
To address these limitations, more advanced diffusion models have been developed to better correlate diffusion parameters with biologically relevant microstructures17,18,19,20,21. The intravoxel incoherent motion (IVIM) model distinguishes between microcirculatory perfusion and water molecule diffusion, yielding quantitative parameters such as tissue diffusion coefficient (D), pseudo diffusion coefficient (D*) and perfusion fraction (f)22. The diffusion kurtosis imaging (DKI) model enhances our understanding of tissue heterogeneity and complexity through the calculation of the mean diffusion coefficient (MD) and dimensionless kurtosis coefficient (MK)23. The stretched exponential model (SEM) accounts for intravoxel heterogeneity and distributed diffusion, providing metrics such as the distributed diffusion coefficient (DDC) and heterogeneity index (α)24. The continuous-time random-walk (CTRW) model, a novel diffusion framework, captures intravoxel heterogeneity in both time and space dimensions, offering parameters α and β, alongside a derived metric Dm that is sensitive to tissue cellularity25.
While preliminary research has highlighted the potential of these diffusion models in providing valuable insights into breast lesions, a systematic comparison of these diverse diffusion imaging approaches regarding their ability to identify breast lesion pathology, prognostic biomarkers, and molecular subtypes is lacking. The purpose of this study is to comprehensively compare and evaluate the clinical value of various diffusion parameters derived from the Mono, IVIM, DKI, SEM, and CTRW models, with the goal of determining which metrics are most effective for the preoperative prediction of breast lesion pathology, prognostic biomarkers, and molecular subtypes.
Materials and methods
Patients
The institutional review board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology approved this retrospective clinical study and written informed consent was waived. All methods were performed in accordance with the relevant guidelines and regulations. From July 2021 through November 2022, one hundred and sixty-three consecutive female patients who underwent a complete breast MRI examination at our institution for suspicious breast lesions detected by mammography or ultrasonography were preliminarily recruited. The inclusion criteria were as follows: (1) mass lesion greater than 1 cm; (2) no biopsy, surgery, radiotherapy or chemotherapy before the MRI scanning; and (3) histopathological confirmation of either malignant or benign breast lesions following the initial MRI examination within 2 weeks. Thirty-one patients were excluded for the following reasons: (1) insufficient quality of DWI images for further processing (n = 6); (2) lesion diameter smaller than 10 mm (n = 9); (3) presence of nonmass-like enhancing lesion on DCE-MRI (n = 12); and (4) incomplete immunohistochemical results after scanning (n = 4). Only the largest tumor was included when multifocal or multicentric mass was present in either breast. Consequently, a total of 132 patients were included in this study.
MRI protocol
A 3T MR scanner (uMR 790; United Imaging Healthcare, Shanghai, China) equipped with a dedicated bilateral breast array coil consisting of 10 channels was used for all MRI examinations. The imaging protocol involved the sequential acquisition of T2-weighted, DWI, and DCE-MRI. The multi b value DWI data were acquired using echo-planar imaging (EPI) technique with the following parameters: repetition time (TR), 2000 ms; echo time (TE), 61.5 ms; field of view (FOV), 320 × 160 mm2; flip angle (FA), 90°; matrix size, 192 × 192; slice thickness, 5 mm; pixel bandwidth, 1500 Hz/Px; b-values, 0, 50, 100, 250, 500, 750, 1000, 1500, and 2000 s/mm2; gradient directions, 3 orthogonal directions; acquisition time, 5min33s.
Image analysis
All DWI data were analyzed using the Mono, IVIM, DKI, SEM, and CTRW models. Parametric maps derived from the five diffusion models were all reconstructed with MATLAB R2018b (MathWorks Inc., Natick, MA, USA).
-
1.
The ADC value was obtained using the Mono model26:
$$\:Sb/{S0}=exp(-bADC)$$ -
2.
Maps of the pure diffusion coefficient (D), perfusion-related incoherent microcirculation (D*), and microvascular volume fraction (f) were derived using the IVIM model22:
$$\:Sb/S0=(1-f)\:exp\:(-bD)\:+\:f\:exp\:[-b\:(D^{*}+D\left)\right]$$ -
3.
Mean kurtosis (MK, which quantifies the deviation of water diffusion distribution from a Gaussian pattern) and mean diffusivity (MD, similar to ADC) maps were calculated based on the DKI model23:
$$\:Sb/S0=exp\:(-bMD\:+\:1/6\:\times\:\:{b}^{2}\:\times\:\:{MD}^{2}\:\times\:\:MK)$$ -
4.
Maps of the diffusion coefficient (DDC) and empirical heterogeneity index (α) were fitted using the SEM model24:
$$\:Sb/S0=\text{e}\text{x}\text{p}[-{\left(bDDC\right)}^{\alpha\:}]$$ -
5.
The CTRW model provided maps of temporal diffusion heterogeneity (α), spatial diffusion heterogeneity (β), and diffusion coefficient (Dm)21:
$$\:Sb/S0={E}_{\alpha\:}\:[-{\left(bD_{m}\right)}^{\beta\:}]$$
S0 represents the signal intensity when b-values of 0 s/mm2, and Sb is the signal intensity at a certain b-value on the DWI image. The Mono model was calculated using b-values of 0 and 750 s/mm2 as utilized by Pereira et al. previously, whose study demonstrated that the ADC calculated from b = 0 and 750 s/mm2 was slightly better than the other b value combinations27. The IVIM model utilized b-values of 0, 50, 100, 250, 500, 750, and 1000 s/mm2 due to low b-value region particularly sensitive to tissue vascularity28. The DKI, SEM, and CTRW models were calculated using all available b-values. The Mittag-Leffler function is denoted by Eα. In the fitting process, Dm was first estimated using diffusion images with all b-values. After determining Dm, α, and β were subsequently obtained from all DWI images (b-values ranging from 0 to 2000 s/mm2) using an iterative Levenberg-Marquardt method, as detailed in Karaman et al.21
Two readers, with 11 years (T.A.) and 5 years (Y.Q.H.) of experience in breast MRI respectively, independently interpreted and performed volume of interest (VOI) measurements encompassing the entire tumor (including necrotic or cystic areas). Both readers were blinded to the pathological results. The VOIs were drawn on DWI images (b = 750 s/mm2) using ITK snape (version 3.8.0; http://www.itksnap.org), guided by the T2WI and DCE-MRI images. The VOI was then copied onto all five model parameter maps for each patient. Finally, the parametric values calculated by two radiologists were averaged as the final results for quantitative statistical analyses in our study.
Histopathologic analysis
Within 2 weeks after the MRI scan, histopathologic analyses were performed by two pathologists (with 5 and 13 years of experience) independently. Malignant tumors were confirmed by the excised specimens through surgery, while benign lesions were evaluated by surgery or core needle biopsy. Histopathologic information was obtained from detailed pathological reports. The negativity status of estrogen receptor (ER) or progesterone receptor (PR) was indicated by stained nuclei in < 1% of cancer cells29. Human epidermal growth factor receptor 2 (HER2) negativity was defined when membrane immunostaining was scored 0, 1+, or when there was no HER2 gene amplification in fluorescence in situ hybridization (FISH)30. The threshold value to discriminate low/high expression of Ki-67 was set at 20%31. Histological grades were categorized according to the World Health Organization (WHO) classification as low (WHO I and II) and high (WHO III)32. Positive axillary lymph node metastasis (ALNM) was confirmed by biopsy or surgery33. Molecular subtypes were defined as follows: Luminal A, Luminal B, HER2-positive, and Triple-negative (TN)34.
Statistical analysis
All statistical analyses were conducted with SPSS (version 26; SPSS, Inc., Chicago, IL, USA) and MedCalc (version 15.0; MedCalc, Mariakerke, Belgium). The inter-reader reliability of all diffusion parameters was evaluated using the intra-class correlation coefficient (ICC), which is considered excellent agreement if greater than 0.8. The diffusion parameters of lesion types (benign vs. malignant), ER/PR/HER2 expressions (negative vs. positive), Ki-67/histological grades (low vs. high), and ALNM status (negative vs. positive) were compared using Mann–Whitney U-tests. Among the molecular subtypes, the Kruskal-Wallis test was used, followed by post-hoc Bonferroni test. Sensitivities and specificities between lesion types were calculated using receiver operating characteristic (ROC) curves, and the threshold values were determined using the Youden Index. The IVIM model (D + D* + f), DKI model (MK + MD), SEM model (DDC + α), and CTRW model (Dm + α + β) were combined using logistical analysis. The area under the ROC curve (AUC) comparisons among different parameters and models were also performed using the Delong test. A p-value < 0.05 was considered statistically significant.
Results
Clinical characteristics
Among the 132 breast lesions confirmed by histopathologic analysis, 41 were classified as benign, while 91 were determined to be malignant. The benign lesions consisted of 23 fibroadenomas, 16 adenoses, and 2 intraductal papillomas. Among the malignant lesions, 13 patients were diagnosed with ductal carcinoma in situ (3 low-grade, 6 intermediate-grade, and 4 high-grade), and 78 had invasive ductal carcinomas. The age of patients with malignant lesions was found to be significantly higher than that of patients with benign lesions (p = 0.018). More detailed clinical characteristics of the breast lesions are presented in Table 1.
Comparisons of different parameters and models between Benign and malignant breast lesions
The inter-observer agreement was excellent for all quantitative parameters (ICC range: 0.969–0.998). The results of the Mann–Whitney U-test are presented in Table 2; Fig. 1, indicating that, apart from IVIM-D*, all diffusion parameters exhibited significant differences in distinguishing benign and malignant breast lesions. Specifically, Mono-ADC, IVIM-D, IVIM-f, DKI-MD, SEM-DDC, SEM-α, CTRW-Dm, CTRW-α, and CTRW-β values were significantly higher in benign lesions compared to malignant ones (all p < 0.005), whereas the DKI-MK value was significantly lower in benign lesions than in malignant ones (p < 0.001). Figures 2 and 3 depict a case of a malignant lesion and a benign lesion, respectively.
Violin graphs showing the significant quantitative metrics from Mono (a), IVIM (b-d), DKI (e-f), SEM (g-h), and CTRW (i-k) diffusion models between malignant and benign lesions. IVIM-f, DKI-MK, SEM-α, CTRW-α, and CTRW-β are dimensionless. The units for Mono-ADC, IVIM-D, IVIM-D*, DKI-MD, SEM-DDC, and CTRW-Dm are mm2/s. * = P < 0.01, ** = P value < 0.001.
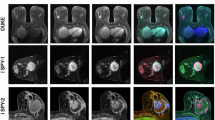
Invasive breast cancer in the right breast of a 26-year-old woman. Maps (a-k) showing Mono-ADC, IVIM-D, IVIM-D*, IVIM-f, DKI-MD, DKI-MK, SEM-DDC, SEM-α, CTRW-Dm, CTRW-α, and CTRW-β values calculated from multi-b-values DWI. The mean values for the malignant lesion were 0.99 × 10− 3 mm2/s, 0.49 × 10− 3 mm2/s, 0.02 mm2/s, 0.38, 1.07 × 10− 3 mm2/s, 0.94, 0.82 × 10− 3 mm2/s, 0.75, 0.78 × 10− 3 s/mm2, 0.97, and 0.79, respectively.
Benign fibroadenoma in the left breast of a 29-year-old woman. Maps (a-k) showing Mono-ADC, IVIM-D, IVIM-D*, IVIM-f, DKI-MD, DKI-MK, SEM-DDC, SEM-α, CTRW-Dm, CTRW-α, and CTRW-β values calculated from multi-b-values DWI. The mean values for the benign lesion were 2.17 × 10− 3 mm2/s, 1.62 × 10− 3 mm2/s, 0.02 mm2/s, 0.21, 2.14 × 10− 3 mm2/s, 0.18, 2.09 × 10− 3 mm2/s, 0.92, 1.90 × 10− 3 mm2/s, 0.99, and 0.95, respectively.
The results of ROC analyses for individual diffusion parameters and each model are listed in Table 2 and displayed in Fig. 4. Among these individual parameters, CTRW-Dm exhibited the highest AUC in the differentiation (AUC = 0.93; Table S1). In addition, the CTRW model demonstrated significantly higher AUC for the classifications, compared to either Mono or IVIM models (AUCs = 0.93, 0.88, and 0.87, respectively; Table S2).
As shown in Fig. 5, strong correlations were found between Mono-ADC, IVIM-D, DKI-MD, DKI-MK, SEM-DDC, CTRW-Dm, and CTRW-α (all p < 0.01). Particularly, a strong positive correlation was observed between SEM-DDC and CTRW-Dm (r = 0.969, p < 0.01).
Multiple parameters among different prognostic factor subgroups of breast Cancer
Table 3 presents the results of the analysis of various diffusion parameters among different molecular prognostic factors of breast cancer. It was observed that ER-positive lesions exhibited significantly lower IVIM-D values and higher DKI-MK values compared to ER-negative lesions (p = 0.018 and p = 0.041, respectively). PR-positive lesions showed significantly lower values of Mono-ADC, IVIM-D, DKI-MD, SEM-DDC, CTRW-Dm, and CTRW-α, while demonstrating higher DKI-MK values in comparison to PR-negative lesions (all p < 0.05). Additionally, HER2-positive lesions demonstrated significantly higher IVIM-D and IVIM-D* values, along with a lower DKI-MK value compared to HER2-negative lesions (p = 0.041, p = 0.031, and 0.012, respectively). Furthermore, the DKI-MD, SEM-DDC, SEM-α and CTRW-β values showed significant differences between lesions with low and high Ki-67 expression (all p < 0.05). However, no significant differences were observed in multiple diffusion parameters among breast cancers with different histological grades and ALNM subgroups (all p > 0.05).
Multiple parameters among different molecular subtypes of breast cancers
The analysis results of the association between quantitative diffusion parameters and different molecular subtypes are presented in Table 4; Fig. 6. The Kruskal-Wallis test indicated that Mono-ADC, IVIM-D, DKI-MD, SEM-DDC, CTRW-Dm, and CTRW-α exhibited significant differences among the four aforementioned subtypes (all p < 0.05; Table 4). Further analysis using post-hoc Bonferroni correction revealed that TN had significantly higher values of IVIM-D, DKI-MD, SEM-DDC, CTRW-Dm, and CTRW-α compared to Luminal B (all p < 0.05; Fig. 6). Luminal A exhibited higher values of Mono-ADC, IVIM-D, DKI-MD, SEM-DDC, and CTRW-Dm compared to Luminal B (all p < 0.05; Fig. 6).
Discussion
In this study, we utilized the Mono, IVIM, DKI, SEM, and CTRW models to characterize the diffusion signal attenuation behavior in the tissue microenvironment of breast lesions and evaluate the effectiveness of the functional diffusion parameters in the preoperative prediction of breast lesion pathology, prognostic biomarkers, and molecular subtypes. From a quantitative perspective, different parameters provide distinct biological information related to cellularity, perfusion, and tissue heterogeneity. Specifically, Mono-ADC, IVIM-D, DKI-MD, SEM-DDC, and CTRW-Dm provide insights into cellularity, while IVIM-D* and IVIM-f offer information about perfusion. Furthermore, DKI-MK, SEM-α, CTRW-α, and CTRW-β provide insights into tissue heterogeneity. All parameters derived from the five models exhibited higher inter-observer agreement, indicating reliable and objective tumor analysis.
Differential diagnosis of breast lesions
Previous studies have extensively investigated the use of multiple diffusion-weighted imaging (DWI) models for the differential diagnosis of breast lesions24,35,36,37. Our findings revealed that malignant lesions had lower diffusion parameters, including Mono-ADC, IVIM-D, DKI-MD, SEM-DDC, and CTRW-Dm. These values decrease when there is limited space for the diffusion of free water molecules and increased cellularity in malignant tumors38. Huang et al. demonstrated decreased ADC values in malignant lesions30; and Suo et al. showed lower values for Mono-ADC, IVIM-D, DKI-MD, and SEM-DDC in malignant lesions39, which align with our results. In addition, Ji et al. observed lower CTRW-Dm values in malignant groups compared to benign ones, consistent with our study40.
The values derived from various diffusion-weighted imaging (DWI) models, such as DKI-MK, SEM-α, CTRW-α, and CTRW-β, reflect microstructure heterogeneity in distinct ways. Previous studies have established DKI-MK as an indicator of tissue heterogeneity26. Consistent with these findings, our study demonstrated higher DKI-MK values in malignant lesions compared to benign lesions, which is in line with previous research26,39,41. An elevated DKI-MK value in malignant breast lesions suggests a greater deviation from Gaussian distribution due to dense cellularity and high heterogeneity, leading to altered water diffusion characteristics30. The empirical heterogeneity index SEM-α enables the assessment of intravoxel water diffusion heterogeneity19. Previous studies have shown that the α value can effectively differentiate between breast lesions, which is consistent with our study results39. Our study found lower CTRW-α and CTRW-β values in malignant breast lesions. Breast cancers exhibit complex pathological heterogeneity compared to benign lesions due to the presence of cancer nests, necrosis, intraductal components, and other factors42. The lower values of CTRW-α and CTRW-β in malignant lesions indicate that water molecules diffuse through a more heterogeneous environment in both time and space43.
Although multiple parameters derived from the five models are interrelated, they offer distinct information. In our study, the area under the curve (AUC) for heterogeneity parameters (SEM-α, CTRW-α, and CTRW-β) was lower than that of diffusion parameters (Mono-ADC, IVIM-D, DKI-MD, SEM-DDC, and CTRW-Dm). This suggests that the heterogeneity between malignant and benign lesions is not as significant as the difference in diffusion coefficient. Logistic analysis demonstrated that all five models exhibited good sensitivity and specificity, with the CTRW model displaying a higher AUC compared to the other four models, although the difference was not statistically significant.
Classification of prognostic biomarker status
Regarding prognostic factors, our study revealed that ER-positive tumors exhibited lower IVIM-D values compared to ER-negative tumors. This finding is consistent with the results reported by Suo et al., suggesting that overexpression of ER inhibits angiogenesis and restricts water diffusion39. Similarly, PR-positive tumors also demonstrated lower IVIM-D values, likely due to similar effects of hormone receptors. Additionally, PR-positive tumors displayed lower Mono-ADC, DKI-MD, SEM-DDC, and CTRW-Dm values, which could be attributed to higher cellularity and increased cell membrane permeability15. Yang et al. identified significant differences in DKI-derived parameters based on ER and PR status26. Our results indicated that both ER- and PR-positive tumors had higher DKI-MK values, consistent with the findings of Kang et al.23. While a CTRW-α value was able to differentiate PR status, it failed to discriminate ER status, which is partially in line with our previous study. The discrepancy may be due to variations in the numbers and selection of b-values used to calculate the CTRW model. HER2-positive tumors, characterized by increased cell density, may exhibit higher IVIM-D values. Zhao et al. reported higher IVIM-D* values in HER2-positive tumors44, while Yang et al. found lower DKI-MK values in HER2-positive tumors26, which aligns with our study. These findings suggest that HER2-positive lesions tend to display more proliferation and angiogenesis. Furthermore, our study demonstrated lower DKI-MD values in tumors with high Ki-67 expression compared to those with lower Ki-67 expression, aligning with the study conducted by Sun et al.41. This observation may be attributed to enhanced cellularity in tumors with high Ki-67 expression30.
Classification of molecular subtypes
Although the role of various noninvasive parameters should not be overlooked when evaluating breast cancer, extensive research has focused on DCE-MRI parameters in molecular subtypes. Our study revealed that IVIM-D, DKI-MD, SEM-DDC, CTRW-Dm, and CTRW-α exhibited higher values in triple-negative (TN) subtypes compared to Luminal B subtypes. TN subtypes are known to exhibit higher levels of VEGF, promoting angiogenesis and resulting in increased microvessel density, which could explain the observed elevation in diffusion parameters (IVIM-D, DKI-MD, SEM-DDC, and CTRW-Dm) and the heterogeneity parameter (CTRW-α) in TN subtypes34. Notably, our study also demonstrated significantly lower values of Mono-ADC, IVIM-D, DKI-MD, SEM-DDC, and CTRW-Dm in Luminal B subtypes compared to Luminal A subtypes. This suggests a higher cellular density in Luminal B subtypes as supported by previous studies45,46, albeit with potential variations in specific values.
Several limitations of this study should be noted. Firstly, this is a single-center study with relatively small sample size and unbalanced data distribution. Our findings need to be validated in future multicenter large cohort with a prior sample size calculation. Secondly, further investigation is required to determine the optimal number and selection of b-values for all diffusion models. Thirdly, while our results indicate statistically significant differences in the diffusion parameters between benign and malignant lesions, it is important to note that there is also substantial overlap in the measured values between the two groups, which limits the clinical use of the proposed threshold value. Future efforts to standardize the acquisition protocols and data postprocessing and larger multiple institutions studies are vital for the widespread implementation of quantitative breast DWI. Lastly, in this study, we solely relied on mean values to evaluate breast lesions. However, histogram analysis can offer additional insights into tumor heterogeneity. Therefore, future studies comparing multiple diffusion models of breast lesions using histogram analysis are warranted.
In conclusion, the results of this study showed that multiple diffusion parameters derived from these five models differed between malignant and benign breast lesions and are promising as supplementary imaging biomarkers for improving the diagnostic accuracy of breast lesions. Additionally, the functional parameters might be of use in the prediction of the prognostic factors and molecular subtypes of breast cancer, which would be helpful in the prognostic estimation and the formulation of personalized treatment strategies in clinical practice.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- Mono:
-
Mono exponential
- IVIM:
-
Intravoxel incoherent motion
- DKI:
-
Diffusion kurtosis imaging
- SEM:
-
Stretched exponential model
- CTRW:
-
Continuous-time random-walk
- ADC:
-
Apparent diffusion coefficient
- MD:
-
Mean diffusion
- MK:
-
Mean kurtosis
- DDC:
-
Distributed diffusion coefficient
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal growth factor receptor 2
- ALNM:
-
Axillary lymph node metastasis
- MRI:
-
Magnetic resonance imaging
- DWI:
-
Diffusion-weighted imaging
- DCE:
-
Dynamic contrast-enhanced
- EPI:
-
Echo-planar imaging
- VOI:
-
Volume of interest
- FISH:
-
Fluorescence in situ hybridization
- WHO:
-
World Health Organization
- TN:
-
Triple-negative
- ICC:
-
Intraclass correlation coefficient
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curves
References
Yeo, S. K. & Guan, J. L. Breast Cancer: multiple subtypes within a Tumor? Trends Cancer. 3, 753–760. https://doi.org/10.1016/j.trecan.2017.09.001 (2017).
Goto, W. et al. Significance of intrinsic breast cancer subtypes on the long-term prognosis after neoadjuvant chemotherapy. J. Transl Med. 16, 307. https://doi.org/10.1186/s12967-018-1679-0 (2018).
Schnitt, S. J. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod. Pathol. 23 (Suppl 2), 60–64. https://doi.org/10.1038/modpathol.2010.33 (2010).
Wang, Q. et al. Multi-modality radiomics model predicts axillary lymph node metastasis of breast cancer using MRI and mammography. Eur. Radiol. https://doi.org/10.1007/s00330-024-10638-2 (2024).
Ba, R. et al. Diffusion-time dependent diffusion MRI: effect of diffusion-time on microstructural mapping and prediction of prognostic features in breast cancer. Eur. Radiol. 33, 6226–6237. https://doi.org/10.1007/s00330-023-09623-y (2023).
Mann, R. M., Cho, N., Moy, L. & Breast, M. R. I. State of the art. Radiology 292, 520–536. https://doi.org/10.1148/radiol.2019182947 (2019).
Kataoka, M., Iima, M., Miyake, K. K. & Matsumoto, Y. Multiparametric imaging of breast cancer: an update of current applications. Diagn. Interv Imaging. 103, 574–583. https://doi.org/10.1016/j.diii.2022.10.012 (2022).
Wekking, D. et al. Clinical indications, recommendations, and future applications in breast Cancer diagnosis. Curr. Oncol. Rep. 25, 257–267. https://doi.org/10.1007/s11912-023-01372-x (2023). Breast MRI.
Leithner, D. et al. Abbreviated MRI of the breast: does it provide Value? J. Magn. Reson. Imaging. 49, e85–e100. https://doi.org/10.1002/jmri.26291 (2019).
Onishi, N. et al. Differentiation between subcentimeter carcinomas and benign lesions using kinetic parameters derived from ultrafast dynamic contrast-enhanced breast MRI. Eur. Radiol. 30, 756–766. https://doi.org/10.1007/s00330-019-06392-5 (2020).
Mendez, A. M. et al. Diffusion breast MRI: current standard and emerging techniques. Front. Oncol. 12, 844790. https://doi.org/10.3389/fonc.2022.844790 (2022).
Horvat, J. V. et al. Diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) mapping as a quantitative imaging biomarker for prediction of immunohistochemical receptor status, proliferation rate, and molecular subtypes of breast cancer. J. Magn. Reson. Imaging. 50, 836–846. https://doi.org/10.1002/jmri.26697 (2019).
Partridge, S. C. & McDonald, E. S. Diffusion weighted magnetic resonance imaging of the breast: protocol optimization, interpretation, and clinical applications. Magn. Reson. Imaging Clin. N Am. 21, 601–624. https://doi.org/10.1016/j.mric.2013.04.007 (2013).
Ohlmeyer, S. et al. Ultra-high b-Value diffusion-weighted imaging-based abbreviated protocols for breast Cancer detection. Invest. Radiol. 56, 629–636. https://doi.org/10.1097/rli.0000000000000784 (2021).
Iima, M. et al. Diffusion MRI of the breast: current status and future directions. J. Magn. Reson. Imaging. 52, 70–90. https://doi.org/10.1002/jmri.26908 (2020).
Lin, M. et al. Contribution of mono-exponential, bi-exponential and stretched exponential model-based diffusion-weighted MR imaging in the diagnosis and differentiation of uterine cervical carcinoma. Eur. Radiol. 27, 2400–2410. https://doi.org/10.1007/s00330-016-4596-8 (2017).
Zhang, J. et al. Grading and proliferation assessment of diffuse astrocytic tumors with monoexponential, biexponential, and stretched-exponential diffusion-weighted imaging and diffusion kurtosis imaging. Eur. J. Radiol. 109, 188–195. https://doi.org/10.1016/j.ejrad.2018.11.003 (2018).
Ren, H. et al. Evaluating the clinical value of MRI multi-model diffusion-weighted imaging on liver fibrosis in chronic hepatitis B patients. Abdom. Radiol. (NY). 46, 1552–1561. https://doi.org/10.1007/s00261-020-02806-x (2021).
Fujimoto, K. et al. Comparison of mono-exponential, bi-exponential, and stretched exponential diffusion-weighted MR imaging models in differentiating hepatic hemangiomas from liver metastases. Eur. J. Radiol. 141, 109806. https://doi.org/10.1016/j.ejrad.2021.109806 (2021).
Wang, F. et al. Comparison between types I and II epithelial ovarian cancer using histogram analysis of monoexponential, biexponential, and stretched-exponential diffusion models. J. Magn. Reson. Imaging. 46, 1797–1809. https://doi.org/10.1002/jmri.25722 (2017).
Karaman, M. M., Zhang, J., Xie, K. L., Zhu, W. & Zhou, X. J. Quartile histogram assessment of glioma malignancy using high b-value diffusion MRI with a continuous-time random-walk model. NMR Biomed. 34, e4485. https://doi.org/10.1002/nbm.4485 (2021).
Cho, G. Y. et al. Evaluation of breast cancer using intravoxel incoherent motion (IVIM) histogram analysis: comparison with malignant status, histological subtype, and molecular prognostic factors. Eur. Radiol. 26, 2547–2558. https://doi.org/10.1007/s00330-015-4087-3 (2016).
Kang, H. S. et al. Diffusion kurtosis MR imaging of invasive breast Cancer: correlations with prognostic factors and molecular subtypes. J. Magn. Reson. Imaging. 56, 110–120. https://doi.org/10.1002/jmri.27999 (2022).
Ertas, G., Onaygil, C., Akin, Y., Kaya, H. & Aribal, E. Quantitative differentiation of breast lesions at 3T diffusion-weighted imaging (DWI) using the ratio of distributed diffusion coefficient (DDC). J. Magn. Reson. Imaging. 44, 1633–1641. https://doi.org/10.1002/jmri.25327 (2016).
Karaman, M. M. et al. Differentiating low- and high-grade pediatric brain tumors using a continuous-time random-walk diffusion model at high b-values. Magn. Reson. Med. 76, 1149–1157. https://doi.org/10.1002/mrm.26012 (2016).
Yang, Z. L. et al. Evaluation of suspicious breast lesions with diffusion kurtosis MR imaging and connection with prognostic factors. Eur. J. Radiol. 145, 110014. https://doi.org/10.1016/j.ejrad.2021.110014 (2021).
Pereira, F. P. et al. Assessment of breast lesions with diffusion-weighted MRI: comparing the use of different b values. AJR Am. J. Roentgenol. 193, 1030–1035. https://doi.org/10.2214/ajr.09.2522 (2009).
Tang, L. & Zhou, X. J. Diffusion MRI of cancer: from low to high b-values. J. Magn. Reson. Imaging. 49, 23–40. https://doi.org/10.1002/jmri.26293 (2019).
Li, Q. et al. Histogram analysis of quantitative parameters from synthetic MRI: correlations with prognostic factors and molecular subtypes in invasive ductal breast cancer. Eur. J. Radiol. 139, 109697. https://doi.org/10.1016/j.ejrad.2021.109697 (2021).
Huang, Y. et al. Diffusion kurtosis at 3.0T as an in vivo imaging marker for breast Cancer characterization: correlation with prognostic factors. J. Magn. Reson. Imaging. 49, 845–856. https://doi.org/10.1002/jmri.26249 (2019).
Feng, W. et al. Correlation between molecular prognostic factors and magnetic resonance imaging intravoxel incoherent motion histogram parameters in breast cancer. Magn. Reson. Imaging. 85, 262–270. https://doi.org/10.1016/j.mri.2021.10.027 (2022).
Kim, T. H., Kang, D. K., Kim, J. Y., Han, S. & Jung, Y. Histologic Grade and decrease in Tumor dimensions affect Axillary Lymph Node Status after Neoadjuvant Chemotherapy in breast Cancer patients. J. Breast Cancer. 18, 394–399. https://doi.org/10.4048/jbc.2015.18.4.394 (2015).
Choi, S. Y. et al. Correlation of the apparent diffusion coefficiency values on diffusion-weighted imaging with prognostic factors for breast cancer. Br. J. Radiol. 85, e474–479. https://doi.org/10.1259/bjr/79381464 (2012).
Du, S. et al. Improved discrimination of molecular subtypes in invasive breast cancer: comparison of multiple quantitative parameters from breast MRI. Magn. Reson. Imaging. 77, 148–158. https://doi.org/10.1016/j.mri.2020.12.001 (2021).
Zhang, Q. et al. Multiple mathematical models of diffusion-weighted imaging for endometrial cancer characterization: correlation with prognosis-related risk factors. Eur. J. Radiol. 130, 109102. https://doi.org/10.1016/j.ejrad.2020.109102 (2020).
Wu, D. et al. Characterization of breast tumors using diffusion kurtosis imaging (DKI). PLoS One. 9, e113240. https://doi.org/10.1371/journal.pone.0113240 (2014).
Mao, X., Zou, X., Yu, N., Jiang, X. & Du, J. Quantitative evaluation of intravoxel incoherent motion diffusion-weighted imaging (IVIM) for differential diagnosis and grading prediction of benign and malignant breast lesions. Med. (Baltim). 97, e11109. https://doi.org/10.1097/MD.0000000000011109 (2018).
Gao, W. et al. Investigation of Synthetic Relaxometry and Diffusion measures in the differentiation of Benign and malignant breast lesions as compared to BI-RADS. J. Magn. Reson. Imaging. 53, 1118–1127. https://doi.org/10.1002/jmri.27435 (2021).
Suo, S. et al. Multiparametric diffusion-weighted imaging in breast lesions: Association with pathologic diagnosis and prognostic factors. J. Magn. Reson. Imaging. 46, 740–750. https://doi.org/10.1002/jmri.25612 (2017).
Ji, Y. et al. Application of advanced diffusion models from diffusion weighted imaging in a large cohort study of breast lesions. BMC Med. Imaging. 23, 52. https://doi.org/10.1186/s12880-023-01005-6 (2023).
Sun Kun, C. X. Breast Cancer: Diffusion Kurtosis MR Imaging-Diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 277, 46–55. https://doi.org/10.1148/radiol.15141625 (2015).
Li, T., Hong, Y., Kong, D. & Li, K. Histogram analysis of diffusion kurtosis imaging based on whole-volume images of breast lesions. J. Magn. Reson. Imaging. 51, 627–634. https://doi.org/10.1002/jmri.26884 (2020).
Zhong, Z. et al. High-spatial-resolution diffusion MRI in Parkinson Disease: lateral asymmetry of the Substantia Nigra. Radiology 291, 149–157. https://doi.org/10.1148/radiol.2019181042 (2019).
Zhao, M. et al. Intravoxel incoherent motion magnetic resonance imaging for breast cancer: a comparison with benign lesions and evaluation of heterogeneity in different tumor regions with prognostic factors and molecular classification. Oncol. Lett. 16, 5100–5112. https://doi.org/10.3892/ol.2018.9312 (2018).
Kawashima, H. et al. Differentiation between Luminal-A and Luminal-B breast Cancer using Intravoxel Incoherent Motion and Dynamic contrast-enhanced magnetic resonance imaging. Acad. Radiol. 24, 1575–1581. https://doi.org/10.1016/j.acra.2017.06.016 (2017).
Kim, E. J. et al. Histogram analysis of apparent diffusion coefficient at 3.0t: correlation with prognostic factors and subtypes of invasive ductal carcinoma. J. Magn. Reson. Imaging. 42, 1666–1678. https://doi.org/10.1002/jmri.24934 (2015).
Author information
Authors and Affiliations
Contributions
TA and YFZ contributed to conceptualization. LTH and FL contributed to manuscript writing and statistical analysis. LTH, FL, YJQ, YLL, QLH, and ZQL contributed to data collection and data curation. All authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, L., Li, F., Qin, Y. et al. Enhanced preoperative prediction of breast lesion pathology, prognostic biomarkers, and molecular subtypes using multiple models diffusion-weighted MR imaging. Sci Rep 15, 4704 (2025). https://doi.org/10.1038/s41598-024-81713-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81713-3
This article is cited by
-
Whole-lesion histogram analysis of multi-model diffusion-weighted imaging for characterization and molecular classification of breast lesions
La radiologia medica (2025)
-
Predicting Neoadjuvant Chemotherapy Response in Breast Cancer Using Multiparametric MRI-Based Radiomics and Machine Learning: A Systematic Review and Meta-Analysis of 1,469 Patients
Indian Journal of Surgical Oncology (2025)