Abstract
While studies have demonstrated the impact of asthma symptoms on quality of life, very few studies have investigated the relationship between detailed asthma symptoms, as reported by the patient, and lung function and inflammation. A cross-sectional study was conducted on treated (ICS/LABA) adult (> 18 years) asthma patients recruited from the Liege University Hospital Asthma Clinic (Belgium) between 2018 and 2023 (n = 505). The intensity of asthma symptoms (dyspnea, wheezing, chest tightness, cough, and airway secretion) was measured using five-point Likert scales (5 expressing the greatest intensity). Multiple linear regression models including all independent variables were carried out to evaluate whether lung function and inflammatory parameters were independently associated with distinct symptoms. Cough associated with female gender (p < 0.05), smoking (p < 0.01), low FeNO (p < 0.05) and FEV1% pred. (p < 0.05), and high blood and sputum eosinophils (p < 0.05 for both). Airway secretion associated with smoking (p < 0.05). Chest tightness associated with young age (p < 0.001), female gender (p < 0.05) and low FEV1% pred. (p < 0.01). Dyspnea associated with female gender (p < 0.001), high BMI (p < 0.05), low FEV1% pred. (p < 0.0001) and high FEV1/FVC % (p < 0.01). Wheezing associated with young age (p < 0.01), high BMI (p < 0.05), smoking (p < 0.01), low FEV1% pred. (p < 0.0001) and high FEV1/FVC % (p < 0.05). Different respiratory symptoms are associated with distinct demographic, functional and inflammatory features paving the way for personalized therapeutic interventions.
Similar content being viewed by others
Introduction
Asthma, a chronic respiratory disease, is a huge public health problem affecting approximatively 358 millions of people worldwide and around 8% of the population in Europe1. This pathology has important socio-economic impact2. The social incidence of the disease is reflected especially in an impaired health-related quality of life (HRQL), a loss of work productivity and a job loss3,4. The economic impact of asthma includes significant direct (e.g., medical, and non-medical costs associated with day-to-day care) and indirect (e.g., absenteeism from work and loss of productivity) costs5,6.
The primary goal of asthma care is to reach an optimal control of the disease7. However, in Europe, the scientific literature reports 54% of asthmatics being not controlled8,9. The level of asthma control is expressed by the frequency and intensity of asthma symptoms, as well as their impact on daily activities over a period of 1 to 4 weeks7,10. While previous studies have demonstrated the impact of symptoms on HRQL11,12, few studies have investigated the relationship between detailed asthma symptoms as reported by the patient and lung function and inflammation.
Knowing which symptom is related to either lung function parameters or inflammatory parameters is important in order to better understand the disease status. For instance, symptoms related to lung function and/or inflammation could have an impact on treatment choice. Indeed, the expression of a symptom linked to inflammation could lead to use a inhaled corticosteroids while the expression of another symptom linked to the impaired function could lead to the use of bronchodilators13,14. The appropriate treatment choice is a public health issue given the cost of asthma medications15 and their potential harmful environmental effects when consumed inappropriately16.
Therefore, the main objective of this study was to assess the relationship between five asthma symptoms, as reported by the patient, and lung function and inflammatory parameters in a population of treated adult asthmatics.
Methods
Study design, setting, and participants
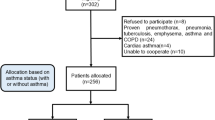
A cross-sectional study was conducted on adult (≥ 18 years) asthma patients, receiving ICS/LABA, recruited from the Liege University Hospital Asthma Clinic (Belgium) between 2018 and 2023. Asthma diagnosis was based on the presence of typical asthma symptoms (wheezing, dyspnea, chest tightness, cough and airway secretion) associated with a 12% and 200 ml forced expiratory volume in 1 s (FEV1) reversibility after inhalation of 400 µg salbutamol, and/or a provocative concentration of methacholine causing a 20% drop in FEV1 ≤16 mg/ml when FEV1 ≥ 70% predicted17. Of the asthmatics who completed symptom scales, only those with complete data including sputum cell counts were included. The sample size was 505 participants (Fig. 1).
Studied variables
All the variables described below were collected as part of the patient routine examination.
Patient-reported asthma symptoms intensity scales (dependent variable)
The intensity level of the 5 classic asthma symptoms17 including dyspnea (breathlessness), wheezing, chest tightness, cough, and sputum production were measured using five-point Likert scales (from 1 to 5), where the level 1 means that the symptom is not present, and level 5 expresses the greatest intensity of the symptom concerned.
Demographic and disease characteristics (independent variables)
Demographic characteristics were age, gender, BMI and smoking status. Smoking status was divided in three categories: never-smoker, ex-smoker (quit smoking at least 6 months previously) and current smokers. Disease characteristics were lung function and systemic and airway inflammation. Lung function testing was performed by spirometry (PFT spirostick, Geratherm, Germany), according to the ERS/ATS standard18. Inflammatory parameters included FeNO, sputum cell counts, blood cell counts, and systemic markers. FeNO was measured at a flow rate of 50 ml/s (NIOX; Aerocrine, Solna, Sweden) before spirometry. Sputum induction and processing were performed as previously described19,20. C-reactive protein (CRP), fibrinogen, (blood and sputum) eosinophils and neutrophils counts, and total serum IgE were determined by routine laboratory analysis at Liège University Hospital.
Statistical analysis
The normality of the distribution of the quantitative data was evaluated numerically by comparing mean and median and graphically by using a histogram and quantile-quantile plot. The Shapiro-Wilk test for normality was used to complete this assessment. Quantitative variables were summarized accordingly using median and interquartile range (P25–P75), while counts and percentages were calculated for qualitative variables.
The associations between quantitative variables and each symptom intensity scale were first determined using the Spearman correlation coefficient. To take into account the possibility of an important variable which could have not come out as significant because of confounding factors from univariate analyses, multiple linear regression models including all independent variables were carried out to evaluate how lung function and inflammatory parameters were independently associated with each patient-reported asthma symptoms21.
All statistical analyses were performed using GraphPad Prism software (version 9.4.1) at a significance level of 0.05.
Ethics
This study was approved by the Liège University Hospital ethics committee. Signed informed consent was obtained from patients as soon as they entered the asthma clinic. They agreed to allow their clinical data and the health outcomes they reported in the routine setting to be used for research purpose. Moreover, all methods were performed in accordance with the relevant guidelines and regulations. More specifically, this study was conducted in accordance with the “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement”, that are guidelines for reporting observational studies.
Results
Characteristics of the study population
The demographic characteristics of the study population are presented in Table 1. The majority of patient were female (58%) with a median age of 54 years. Non-smokers represented 50% of the population, while ex-smokers and smokers represented 29% and 21% respectively. The median BMI was 27, with an interquartile range from 23 to 30, i.e. 25% of our population was obese. Median FEV1% pred. was 83 and median FeNO was 22 ppb. Only 26% of the study population had well controlled asthma, as measured by an asthma control test (ACT) ≥ 20. Median (IQR) values of other lung function and inflammatory parameters are shown in Table 1.
In the study population, dyspnea was the symptom displaying the highest intensity with a mean (±SD) value of 3.68 (±1.1), followed by cough, chest tightness, wheezing and airway secretion with mean values of 3.10 (±1), 2.91 (±1.1), 2.68 (±1.1) and 2.61 (±1.2) respectively (Fig. 2). Figure 3 showed that the strongest correlations were found between dyspnea and chest tightness (rs = 0.55) and dyspnea and wheezing (rs = 0.53) while the lowest correlation was found between chest tightness and airway secretion (rs = 0.17).
Factors associated with cough intensity
Results of spearman correlation coefficient (univariate analysis) are given in Table 2. FeNO (rs: − 0.18), FEV1% pred. (rs: − 0.14), blood neutrophils (rs: 0.11), fibrinogen (rs: 0.12) and CRP (rs: 0.10) were significantly correlated to cough intensity. In multivariate analysis, gender, smoking status, FeNO, FEV1% pred., blood eosinophils and sputum eosinophils were significantly and independently associated with cough intensity (Table 3; Fig. 4). Female gender was associated with increased cough intensity (p < 0.05). Being a smoker in comparison to ex-smokers and never-smokers associated with increased cough intensity (p < 0.01 for both). FeNO decreased with increasing cough intensity (p < 0.05). FEV1% pred. decreased with increasing cough intensity (p < 0.05). Blood eosinophils and sputum eosinophils increased with increasing cough intensity (p < 0.05 for both).
Factors associated with airway secretion intensity
Results of spearman correlation coefficient (univariate analysis) are given in Table 2. Only blood eosinophils (rs: 0.14) were significantly correlated to airway secretion intensity. In multivariate analysis, smoking status was significantly and independently associated with airway secretion intensity (Table 3). Being a smoker in comparison to ex-smokers associated with increased airway secretion intensity (p < 0.05).
Factors associated with chest tightness intensity
Results of spearman correlation coefficient (univariate analysis) are given in Table 2. Only age (rs: − 0.15), FeNO (rs: − 0.13), FEV1% pred. (rs: − 0.14), were significantly correlated to chest tightness intensity. In multivariate analysis, age, gender and FEV1% pred were significantly and independently associated with chest tightness intensity (Table 3; Fig. 4). Age decreased with increasing chest tightness intensity (p < 0.001). Being a female associated with increased chest tightness intensity (p < 0.05). FEV1% pred. decreased with increasing chest tightness intensity (p < 0.001).
Factors associated with dyspnea intensity
Results of spearman correlation coefficient (univariate analysis) are given in Table 2. Only BMI (rs: 0.17), FeNO (rs: − 0.15), FEV1% pred. (rs: − 0.26), sputum eosinophils (rs: − 0.11), blood neutrophils (rs: 0.13), fibrinogen (rs: 0.10), CRP (rs: 0.18) and total IgE (rs: − 0.11) were significantly correlated to dyspnea intensity. In multivariate analysis, gender, BMI, FEV1% pred. and FEV1/FVC % were significantly and independently associated with dyspnea intensity (Table 3; Fig. 4). Being a female associated with increased dyspnea intensity (p < 0.001). BMI increased with increasing dyspnea intensity (p < 0.05). FEV1% pred. decreased with increasing dyspnea intensity (p < 0.0001), while FEV1/FVC % increased with increasing dyspnea intensity (p < 0.01).
Factors associated with wheezing intensity
Results of spearman correlation coefficient (univariate analysis) are given in Table 2. Only age (rs: − 0.12), BMI (rs: 0.14), FEV1% pred. (rs: − 0.26), blood neutrophils (rs: 0.12) and CRP (rs: 0.12) were significantly correlated with wheezing intensity. In multivariate analysis, age, BMI, smoking status, FEV1% pred. and FEV1/FVC % were significantly and independently associated with wheezing intensity (Table 3; Fig. 4). Age decreased with increased wheezing intensity (p < 0.01). BMI increased with increasing wheezing intensity (p < 0.05). Being a smoker in comparison to never-smokers associated with increased wheezing intensity (p < 0.01). FEV1% pred. decreased with increasing wheezing intensity (p < 0.0001), while FEV1/FVC % increased with increasing wheezing intensity (p < 0.05).
Discussion
This study provides evidence that, in a large cohort of asthmatics treated with ICS/LABA, different respiratory symptoms are associated with specific demographic, functional and inflammatory features. While some associations were expected like smoking history with cough and airway secretion or BMI with dyspnea and wheezing, others such as the opposite relationship that sputum eosinophils and FeNO have with cough is an original finding.
The relationship between obesity and the symptoms of dyspnoea and wheezing on the one hand22,23, and the one between smoking history and the symptoms of cough and airway secretion on the other hand are well documented, the latter supporting the concept of tobacco induced chronic bronchitis24. The inverse relationship between age and some symptoms intensity has important public health consequences as it would suggest that physicians have to be careful about the possibility of some kind underreporting in elderly asthmatics. This in line with a review conducted by Battaglia et al.25 related to asthma in the elderly, where the authors showed that age-related cognitive decline contribute to an impairment of the perception of asthma symptoms. This would suggest asking physicians to use cognitive assessment tools to avoid negative consequences of an underestimated asthma in the elderly26. While females experiencing a greater dyspnea intensity than males has been largely demonstrated in the literature27,28, the reasons of this difference is complex and controversial. A potential explanation shared by several authors is that anxiety and depression, traits more prevalent in women, are two recognized predictors of dyspnea29,30. Another valuable explanation might be the lower airway size associated with smaller lung volumes in females31.
The expiratory flow rate measured by the FEV1 is the parameter that showed the strongest association with symptoms, being significantly related to all asthma symptoms except for the airway secretion. Interestingly, in the univariate analysis, the symptom of dyspnea was correlated with reduced FEV1 but not the FEV1/FVC ratio, often considered as the gold standard of airway obstruction. However, in the multivariate analysis, the ratio FEV1/FVC positively associated with the intensity of dyspnea which indicates that dyspnea was maximal when spirometry showed a restrictive pattern featuring a reduction in FVC that surpasses the reduction in FEV1. This is an original and somewhat unexpected finding. Of course, reduced FVC are likely to reflect increased residual volume as a consequence of air trapping caused by distal airway obstruction since total lung capacity is generally preserved in our cohorts of asthmatics32. Previous studies have highlighted the critical role of distal airway obstruction in lack of asthma control33.
Airway inflammation has been considered of paramount importance in asthma and its understanding has driven much of the pharmacological progress in the disease. Here, eosinophilic trait, both at the systemic and the airway level, associates with cough intensity but not with other symptoms. This finding strengthens the role of eosinophils in generating the symptom of cough and is in keeping with the recognized existence of chronic eosinophilic bronchitis, a pathological entity that shares some clinical features with asthma but differs from the latter by the absence of dyspnea and bronchial hyperresponsiveness34. Surprisingly the multivariate model shows that FeNO levels were in fact inversely associated with cough intensity. This is the demonstration that molecular pathways leading to either eosinophilia or raised FeNO may actually impact disease expression in a very different manner, and it would suggest that elevated FeNO by itself might actually confer some protection to the patients against excessive cough. Since this association emerged after multivariate analysis including smoking as an independent variable the relationship between low FeNO and high cough intensity cannot be accounted by the smoking habit, known to dramatically reduce FeNO levels35,36. A high FeNO level has traditionally been seen as detrimental in asthmatics. Indeed FeNO levels were found to correlate with sputum eosinophils35,37 and high FeNO levels combined to high blood eosinophils in moderate to severe asthmatics is a risk factor for future exacerbation38 and in particular for viral induced exacerbation39. However, other studies found that a low FeNO level was an independent factor associated with poor quality of life and, in particular, in the activity dimension40. Furthermore, a longitudinal study has shown that a rise in FeNO may increase the chance to achieve a good quality of life (AQLQ > 6) in asthmatics not treated with biologics20. Finally, patients receiving anti-IL-5 and anti-IL5(R) have not shown convincing fall in FeNO despite major clinical improvement41,42. Whether residual high FeNO without eosinophilia may accelerate lung function decline is a key issue that needs to be answered in long term studies although the recent SHAMAL study suggest it might be the case43.
The results of this study may help the clinician better understand the disease based on the patient complains and therefore better choose a treatment according to the symptom reported by the patient. This is in line with the recent integration of the patient perspective, using patient-reported outcome measures, in chronic disease management as a way to refine personalized medicine44,45. The latter aims to help healthcare providers to individualized patient treatment based on biomarkers, genetics and demographic characteristics44,45. In patients receiving ICS/LABA as maintenance treatment, our study would suggest that an increase in perceived cough intensity could lead the patient to prioritize the use of inhaled corticosteroid therapy13,14. Conversely, due to their independent relationship with lung function parameters, an increase in the perceived intensity of dyspnea and/or wheezing could lead the patient to prioritize bronchodilating agents to improve airway flow rates13,14. As a result, disseminating asthma symptom scales in family practices could be a simple, quick and inexpensive way to better manage asthma patients in routine, where spirometry and FeNO measurement are rarely available46,47.
In our real-life study, it appears that the majority of patients treated with ICS/LABA remain symptomatic with an ACT score below 20. The reasons for that are probably plural. First, a poor adherence is likely to contribute in some patients48. Second, some comorbidities like smoking and obesity may impair the response to ICS by generating respiratory symptoms not directly related to asthma itself49. Third there might also be true pharmacological resistance to ICS either because the disease was T2 low before starting ICS or because ICS was unable to fully control T2 high disease as demonstrated by the fact that 50% of patients still exhibit sputum eosinophil count of at least 2%.
Strengths and limitations of the study
One strength is the fact that it is one of the first real-life study to investigate the relationship between detailed asthma symptoms and lung function and inflammatory parameters. Another strength is the inclusion of asthma patients for whom the diagnosis has been made according to the recently published ERS guidelines on asthma diagnosis50. However, this study has some limitations. First, because of its cross-sectional design, the cause and effect of the demonstrated associations cannot be established. A prospective interventional study to see if change in management strategy according to symptoms could be efficient would be needed. Second, this study did not include patient occupation in the multivariate model, a factor which is known to have an impact on respiratory symptoms51. Third, data on comorbidities—such as rhinosinusitis or gastroesophageal reflux—would have been useful as it is known that they may influence asthma symptoms52.
Conclusion
This study demonstrates that, in a large cohort of asthmatics treated with ICS/LABA, distinct patient-reported respiratory symptoms are associated with specific demographic, functional and inflammatory features. In particular, while dyspnea, wheezing and chest tightness are associated with impairment of spirometric indices, cough rather relates to eosinophilic inflammation. These findings pave the way for a new personalized medicine based on patient perspective, which could lead to a better management of asthma in primary care where spirometry and FeNO measurement are rarely available. To fully support a concept of symptoms-guided treatment a prospective longitudinal study would be warranted.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the privacy of certain patient data but are available from the corresponding author on reasonable request.
References
Soriano, J. B. et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the global burden of Disease Study 2015. Lancet Respir. Med. 5(9), 691–706. https://doi.org/10.1016/S2213-2600(17)30293-X (2017).
Nunes, C., Pereira, A. M. & Morais-Almeida, M. Asthma costs and social impact. Asthma Res. Pract. 3, 1. https://doi.org/10.1186/s40733-016-0029-3 (2017).
Nocon, A. & Booth, T. The social impact of asthma. Fam. Pract. 8(1), 37–41. https://doi.org/10.1093/fampra/8.1.37 (1991).
Tarlo, S. M. & Lemiere, C. Occupational asthma. N. Engl. J. Med. 370(7), 640–649. https://doi.org/10.1056/NEJMra1301758 (2014).
Bousquet, J., Bousquet, P. J., Godard, P. & Daures, J. P. The public health implications of asthma. Bull. World Health Organ. 83(7), 548–554. https://doi.org/10.1590/S0042-96862005000700016 (2005).
Weiss, K. B., Gergen, P. J. & Hodgson, T. A. An economic evaluation of asthma in the United States. N. Engl. J. Med. 326(13), 862–866. https://doi.org/10.1056/NEJM199203263261304 (1992).
Global Strategy for Asthma Management and Prevention Updated 2020. http://www.ginasthma.org (Accessed 21 August 2020) (2020).
Demoly, P. et al. Prevalence of asthma control among adults in France, Germany, Italy, Spain and the UK. Eur. Respir. Rev. 18(112), 105–112. https://doi.org/10.1183/09059180.00001209 (2009).
Demoly, P., Annunziata, K., Gubba, E. & Adamek, L. Repeated cross-sectional survey of patient-reported asthma control in Europe in the past 5 years. Eur. Respir. Rev. 21(123), 66–74. https://doi.org/10.1183/09059180.00008111 (2012).
Juniper, E. F., O’Byrne, P. M., Guyatt, G. H., Ferrie, P. J. & King, D. R. Development and validation of a questionnaire to measure asthma control. Eur. Respir. J. 14(4), 902–907. https://doi.org/10.1034/j.1399-3003.1999.14d29.x (1999).
Louis, G. et al. How respiratory symptoms impact asthma-related quality of life in mild asthmatics. Respir. Med. 207, 107098. https://doi.org/10.1016/j.rmed.2022.107098 (2023).
Louis, G. et al. Back to the roots of medicine: It’s severe asthma patient-reported symptoms that matter! Pulmonology 29, S92–S95. https://doi.org/10.1016/J.PULMOE.2023.10.005 (2023).
Louis, R., Schleich, F. & Barnes, P. J. Corticosteroids: still at the frontline in asthma treatment? Clin. Chest Med. 33(3), 531–541. https://doi.org/10.1016/J.CCM.2012.05.004 (2012).
Pavord, I. D. et al. After asthma: redefining airways diseases. Lancet 391(10118), 350–400. https://doi.org/10.1016/S0140-6736(17)30879-6 (2018).
Domínguez-Ortega, J., Phillips-Anglés, E., Barranco, P. & Quirce, S. Cost-effectiveness of asthma therapy: a comprehensive review. J. Asthma 52(6), 529–537. https://doi.org/10.3109/02770903.2014.999283 (2015).
Wilkinson, A. & Woodcock, A. The environmental impact of inhalers for asthma: a green challenge and a golden opportunity. Br. J. Clin. Pharmacol. 88(7), 3016–3022. https://doi.org/10.1111/BCP.15135 (2022).
Gater, A. et al. Assessing asthma symptoms in adolescents and adults: qualitative research supporting development of the asthma daily symptom diary. Value Health 19(4), 440–450. https://doi.org/10.1016/j.jval.2016.01.007 (2016).
Graham, B. L. et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am. J. Respir. Crit. Care Med. 200(8), E70–E88. https://doi.org/10.1164/rccm.201908-1590ST (2019).
Demarche, S. F. et al. Effectiveness of inhaled corticosteroids in real life on clinical outcomes, sputum cells and systemic inflammation in asthmatics: a retrospective cohort study in a secondary care centre. BMJ Open 7(11). https://doi.org/10.1136/bmjopen-2017-018186 (2017).
Louis, G. et al. Predictors of change in asthma-related quality of life: a longitudinal real-life study in adult asthmatics. Qual. Life Res. 1, 3. https://doi.org/10.1007/s11136-022-03339-0 (2023).
Sun, G. W., Shook, T. L. & Kay, G. L. Inappropriate use of bivariable analysis to screen risk factors for use in multivariable analysis. J. Clin. Epidemiol. 49(8), 907–916. https://doi.org/10.1016/0895-4356(96)00025-X (1996).
Sin, D. D., Jones, R. L. & Paul Man, S. F. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch. Intern. Med. 162(13), 1477–1481. https://doi.org/10.1001/ARCHINTE.162.13.1477 (2002).
Kasteleyn, M. J. et al. Pulmonary function, exhaled nitric oxide and symptoms in asthma patients with obesity: a cross-sectional study. Respir. Res. 18 (1), 205. https://doi.org/10.1186/S12931-017-0684-9/TABLES/5 (2017).
Zhang, J. et al. Risk factors for chronic cough in adults: a systematic review and meta-analysis. Respirology 27(1), 36–47. https://doi.org/10.1111/RESP.14169 (2022).
Battaglia, S., Benfante, A., Spatafora, M. & Scichilone, N. Asthma in the elderly: a different disease? Breathe 12(1), 18. https://doi.org/10.1183/20734735.002816 (2016).
Tuch, G. et al. Cognitive assessment tools recommended in geriatric oncology guidelines: a rapid review. Curr. Oncol. 28(5), 3987. https://doi.org/10.3390/CURRONCOL28050339 (2021).
Eagan, T. M. L., Bakke, P. S., Eide, G. E. & Gulsvik, A. Incidence of asthma and respiratory symptoms by sex, age and smoking in a community study. Eur. Respir. J. 19(4), 599–605. https://doi.org/10.1183/09031936.02.00247302 (2002).
Chhabra, S. K. & Chhabra, P. Gender differences in perception of dyspnea, assessment of control, and quality of life in asthma. J. Asthma 48(6), 609–615. https://doi.org/10.3109/02770903.2011.587577 (2011).
Neuman, Å. et al. Dyspnea in relation to symptoms of anxiety and depression: a prospective population study. Respir. Med. 100(10), 1843–1849. https://doi.org/10.1016/J.RMED.2006.01.016 (2006).
McLean, C. P., Asnaani, A., Litz, B. T. & Hofmann, S. G. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 45(8), 1027–1035. https://doi.org/10.1016/J.JPSYCHIRES.2011.03.006 (2011).
Yamamoto, T. et al. Management reality of female patients with COPD: a multicenter cross-sectional CAP study in Japan. Int. J. Chron. Obstruct. Pulmon. Dis. 19, 1123–1130. https://doi.org/10.2147/COPD.S455397 (2024).
Schleich, F. N. et al. Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. Eur. Respir. J. 44(1), 97–108. https://doi.org/10.1183/09031936.00201813 (2014).
Kraft, M. et al. The role of small airway dysfunction in asthma control and exacerbations: a longitudinal, observational analysis using data from the ATLANTIS study. Lancet Respir. Med. 10(7), 661–668. https://doi.org/10.1016/S2213-2600(21)00536-1 (2022).
Brightling, C. E., Ward, R., Goh, K. L., Wardlaw, A. J. & Pavord, I. D. Eosinophilic bronchitis is an important cause of chronic cough. Am. J. Respir. Crit. Care Med. 160(2), 406–410. https://doi.org/10.1164/AJRCCM.160.2.9810100 (1999).
Schleich, F. N. et al. Exhaled nitric oxide thresholds associated with a sputum eosinophil count ≥ 3% in a cohort of unselected patients with asthma. Thorax 65(12), 1039–1044. https://doi.org/10.1136/thx.2009.124925 (2010).
Verleden, G. M., Dupont, L. J., Verpeut, A. C. & Demedts, M. G. The effect of cigarette smoking on exhaled nitric oxide in mild steroid-naive asthmatics. Chest 116(1), 59–64. https://doi.org/10.1378/CHEST.116.1.59 (1999).
Berry, M. A. et al. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin. Exp. Allergy 35(9), 1175–1179. https://doi.org/10.1111/J.1365-2222.2005.02314.X (2005).
Couillard, S. et al. Derivation of a prototype asthma attack risk scale centred on blood eosinophils and exhaled nitric oxide. Thorax 77(2), 199–202. https://doi.org/10.1136/THORAXJNL-2021-217325 (2022).
Bjerregaard, A. et al. High fractional exhaled nitric oxide and sputum eosinophils are associated with an increased risk of future virus-induced exacerbations: a prospective cohort study. Clin. Exp. Allergy 47(8), 1007. https://doi.org/10.1111/CEA.12935 (2017).
Louis, G. et al. Predictors of asthma-related quality of life in a large cohort of asthmatics: a cross‐sectional study in a secondary care center. Clin. Transl. Allergy 11(7), e12054. https://doi.org/10.1002/clt2.12054 (2021).
Schleich, F. et al. Real-world experience with mepolizumab: does it deliver what it has promised? Clin. Exp. Allergy 50(6), 687–695. https://doi.org/10.1111/CEA.13601 (2020).
Schleich, F. et al. Benralizumab in severe eosinophilic asthma in real life: confirmed effectiveness and contrasted effect on sputum eosinophilia versus exhaled nitric oxide fraction—PROMISE. ERJ Open Res. 9, 6. https://doi.org/10.1183/23120541.00383-2023 (2023).
Jackson, D. J. et al. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study. Lancet 403(10423), 271–281. https://doi.org/10.1016/S0140-6736(23)02284-5 (2024).
Cella, D. & Wagner, L. Re-personalizing precision medicine: is there a role for patient-reported outcomes? J. Community Support. Oncol. 275, 7. https://doi.org/10.12788/jcso.0161 (2015).
Alemayehu, D. & Cappelleri, J. C. Conceptual and analytical considerations toward the use of patient-reported outcomes in personalized medicine. Am. Health Drug Benefits 5 (5), 310 (2012).
Díaz-Lobato, S., Mayoradas, S. & Buffels, J. Underuse of spirometry in primary care [4] (multiple letters). Chest 126(5), 1712–1713. https://doi.org/10.1378/chest.126.5.1712 (2004).
Yu, W. C. et al. Spirometry is underused in the diagnosis and monitoring of patients with chronic obstructive pulmonary disease (COPD). Int. J. Chron. Obstruct. Pulmon. Dis. 8, 389–395. https://doi.org/10.2147/COPD.S48659 (2013).
Sousa-Pinto, B. et al. Adherence to inhaled corticosteroids and long-acting β2-agonists in asthma: a MASK-air study. Pulmonology. https://doi.org/10.1016/J.PULMOE.2023.07.004 (2023).
Lazarus, S. C. et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N. Engl. J. Med. 380(21), 2009–2019 (2019).
Louis, R. et al. European Respiratory Society Guidelines for the diagnosis of asthma in adults. Eur. Respir. J. 1, 2101585. https://doi.org/10.1183/13993003.01585-2021 (2022).
Vermeulen, R., Heederik, D., Kromhout, H. & Smit, H. A. Respiratory symptoms and occupation: a cross-sectional study of the general population. Environ. Health 1(1), 1–7. https://doi.org/10.1186/1476-069X-1-5 (2002).
Rogliani, P., Laitano, R., Ora, J., Beasley, R. & Calzetta, L. Strength of association between comorbidities and asthma: a meta-analysis. Eur. Respir. Rev. 32, 167. https://doi.org/10.1183/16000617.0202-2022 (2023).
Funding
The study received support from a federal grant of the Belgian Government (EOS 0013618 F & EOS O.001422).
Author information
Authors and Affiliations
Contributions
GL, BP, RL, JB & FS contributed to the conception of the study. FS, & RL contributed to data acquisition. GL performed statistical analysis. GL, BP, FS, RL, EVG, BSP & JB drafted and critically revised the work. All authors gave final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Outside of this submitted work, RL received unrestricted research grants from GSK, AstraZeneca, Novartis and Chiesi and lecture or adboard fees from GSK, AZ, Novartis and Sonafi. Outside of this submitted work, FS received lecture or adboard fees from Chiesi, AZ, GSK, and Novartis. Outside this submitted work, JB reports personal fees (member of advisory boards, consultations, honoraria for meeting lectures) from Cipla, Menarini, Mylan, Novartis, Purina, Sanofi‐Aventis, Teva, Uriach. Shareholder of KYomed Innov and MASK-air-SAS. The rest of the authors declare that they have no relevant conflicts of interest.
Ethics approval and consent to participate
The Study was approved by the CHU Liège ethics committee. Signed informed consent was obtained from patients as soon as they entered the asthma clinic of the CHU Liège. They agreed that their clinical data and the health outcomes they reported in the routine setting would be used for the purposes of research.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Louis, G., Pétré, B., Sousa-Pinto, B. et al. When patient-reported respiratory symptoms shed light on pathophysiology in adult asthma: a cross-sectional study. Sci Rep 14, 29997 (2024). https://doi.org/10.1038/s41598-024-81745-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-81745-9