Abstract
The present investigation assessed the viability of utilizing a powdered clam shell in continuous adsorption to eliminate nickel ions from simulated wastewater. The breakthrough curves (BTC) were analyzed by altering the Q (inlet flow rate) in a glass column (ID 5 cm, H 35 cm) with a multi-port and filled with the powdered clamshell adsorbent (PCSA). The PCSA’s nickel adsorption efficiency was maximum (87.68%) with Q = 8 mL/min at a bed length (H) of 25 cm with 1.05 mg/g adsorption capacity. Moreover, the mass transfer zone (MTZ) and idle bed length (Lu) were estimated from the corresponding BTC. The values of MTZ and Lu demonstrated fluctuations in response to changes in bed length, suggesting the presence of non-ideal circumstances. The validity of the Thomas model for predicting column dynamics was established, and the associated model parameters were assessed. Additionally, the parameters of the BDST model were assessed in order to aid in calculating the sufficient depth for a packed bed column (PBC) while scaling up. Therefore, a metal removal process from industrial effluent can be efficiently achieved by utilizing a PBC of powdered clamshell adsorbent.
Similar content being viewed by others
Introduction
The global concern of preserving sustainability in the water ecosystem has become increasingly prevalent. The primary determinant was the uninterrupted discharge of processed or partially processed wastewater into aquatic systems, resulting in a more significant environmental impact. As a result, the government has implemented several strict regulations in order to address the issue of water resource pollution. In addition, the toxicity level of heavy metals has garnered significant attention in recent decades for their elimination from the aqueous environment1. Nickel is a toxic heavy metal due to their persistence and non-biodegradability nature2, which comes from industrial sources like electroplating, silver refining, battery and alloy production3. Furthermore, human exposure to nickel causes various adverse health effects, including anaphylaxis, alopecia, hemolysis, liver diseases, lung cancer, and nephrotoxicity4. Therefore, to mitigate the potential health and environmental hazards, developing an appealing treatment methodology for the retrieval of nickel metal is imperative. As a result, a highly effective removal strategy is necessary for eliminating the nickel pollutant beyond its hindering features.
Numerous challenges confront industries as they strive to mitigate nickel discharge, minimize water usage, and reduce energy consumption5. Therefore, various advanced technologies have been established to safeguard the sustainability of the environment, which have evolved into a significant area of research5,6,7. Each technological advancement comes with a unique set of benefits and drawbacks in nickel waste management8. Ion exchange9, electrocoagulation10, adsorption11, and membrane filtration12 have been identified as effective methods for the disposal of nickel pollutants. However, adsorption remains advantageous over other advanced technologies for several reasons, including its simple and cost-effective operation, minimal labor and land requirements, reduced secondary pollutant generation, and high efficiency13,14.
The bivalve mollusk species known as Mercenaria mercenaria, commonly called clams15, exhibit a notable ability to undergo accelerated growth within a condensed reproductive cycle. These organisms inhabit coastal lagoons, lakes, and estuaries16. These clams are considered a palatable and appetizing cuisine in countries such as Nigeria, China, and Korea17. On the other hand, the clamshell is a by-product of aquaculture and is often thrown along with the remaining parts of the clam18. Consequently, their utilization is not advantageous in the selling market and contributes to environmental strain as a form of biowaste. Clamshells themselves are not toxic. It is a by-product of aquaculture that accumulates as biowaste when discarded in larger quantities. These discarded clam shells may contain residual meat and other organic matter, which can be decomposed by bacteria or algae over time and potentially release toxins into the environment. Thus, it leads to significant environmental contamination18. The primary constituents of the clamshell are calcium carbonate (CaCO3) and chitin19. Therefore, many scientists have been intrigued by the use of clamshells as adsorbents because of their high affinity for toxic heavy metals as well as their substantial calcium carbonate (CaCO3) content20,21. Moreover, it was reported that carbonized or functionally changed clamshells had been said to eliminate assorted pollutants, including dyes and harmful heavy metals22,23,24.
The present investigation employed uncarbonized and functionally unmodified powdered clamshell to eliminate nickel ions in column adsorption. Therefore, no toxic chemicals are utilized, and neither extremely advanced machinery nor trained labor is needed to prepare powdered clamshell adsorbent (PCSA). The PCSA preparation process necessitates merely milling machinery, and the expenses are contingent solely on the associated electricity costs. Since the clamshells are biowaste material, their reutilization (i.e., recycling) as an adsorbent minimizes waste accumulation.
In this study, the clamshells are milled into tiny pieces (i.e., fine particles) to prepare powdered clamshell adsorbent (PCSA). Then, they are packed tightly in a packed bed column. This study utilized a multi-port column facilitating the simultaneous sample collection at various bed depths (i.e., 5 cm intervals). It systematically investigates the dynamics and transfer of nickel solution passed through it gravitationally. Researchers widely employ a single outlet column; however, it has limitations, such as incomplete bed utilization at the inlet or outlet, massive adsorbent utilization, time-consuming and difficulty maintaining uniform conditions during each run. Compared to a single outlet column, the multi-port column remains convenient as it is a time-saver since the experiments can be carried out in a single run for varied bed depths. Similarly, it is a material saver as the consumption of adsorbent is limited. Moreover, it facilitates the distribution of nickel solution to pass through the column easily as it minimizes the flow obstruction. Also, it leads to more uniform adsorption due to enhanced bed utilization and only a negligible pressure drop exists. XRD, FTIR, XPS, SEM, EDS and TEM studies examined PCSA’s phase identity and morphological properties. Also, its ability to abate nickel ions (Ni2+) from simulated effluent was evaluated. The effect of the flow rate on the column’s design parameters was also assessed. The results from the column studies on Ni2+ uptake using PCSA are also verified using non-linear and linear regression models.
Methodology
Chemicals and reagents used
Nickel (II) chloride (Emsure), Hydrochloric acid (Emparta, 37%), and Sodium hydroxide pellets (Emplura) were obtained from Merck. The reagents required for the experiments were prepared using deionized water. The Nickel stock solution (1000 ppm) was arranged by spiking 5 g nickel chloride in 1 L of deionized water. The stock solutions were then appropriately diluted to prepare test solutions of preferred concentrations.
Protocol for powdered clam shell preparation
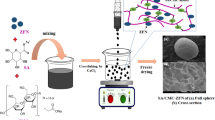
The clamshell specimens were freshly collected from Pullicat Lake, nearby Chennai, India. The detailed protocol for preparing a powered clamshell is demonstrated in Fig. 1.
Instrumentation
The structural and phase identity of the powdered clam shell was examined using an Empyrean X-ray diffractometer (XRD, Malvern PANalytical). The diffractogram is generated by a Cu X-ray source (λ = 1.54Å) that works with 40 kV. The functional region and significant constituents of PCSA were determined using a Fourier Transform Infrared Spectrometer (FTIR, PerkinElmer Spectrum Two). The infrared spectrum is operated over a temperature range of 5–45℃ under the wavenumber of 8300–350 cm− 1. The oxidation state of PCSA was analyzed using X-ray Photoelectron Spectrometer (XPS, Thermo Scientific ESCALAB 250 XI). The XPS spectrum is generated using an Al Kα monochromator source and pass energy of 200 eV. The surface texture and morphology of the powered clamshell were visualized using JEOL Scanning Electron Microscope (JSM-7600 F) and PHILIPS Transmission Electron Microscope (CM 200). The SEM micrograph is magnified under 10,000 X by applying 10 kV accelerating voltage, while the TEM image is magnified up to 2.4 Å resolution by operating under 20–200 kV voltage. The elemental composition of PCSA was obtained using Energy Dispersive Spectroscopy (EDS, Ametek Octane-T-Plus). The Ni2+ concentration was analyzed using a SYSTRONICS UV-visible spectrophotometer. The digital pH meter assessed the Ni2+ solution pH.
Column adsorption of Ni2+ using PCSA
The experimental investigations utilized a packed bed column with multi ports (PBCM) (Fig. 2) to examine the behavior of Ni2+ adsorption in continuous operation. The experimental PBCM is a glass support of 35 cm in height with an internal radius of 2.5 cm. The PBCM is designed with uniformly distributed ports at 5 cm intervals up to 25 cm from its bottom.
The setup includes an Influent tank to hold the nickel solution, a multi-port column hinged to a burette stand, an effluent tank to collect treated nickel solution, and a dripper to regulate the flow distribution. The dripper’s spike end was connected to the bottom of the influent tank and the needle end was dropped at the open end of the PBCMA. The influent tank is positioned at an elevation sufficient to maintain a significant head for achieving the desired flow of nickel solution. A specified quantity of powdered clamshell was filled within the PBCM, and the bed was upheld by glass drops (2 cm) and glass wool (1 cm). The package of glass beads enables a consistent flow of Ni2+ solution, while the glass wool prevents the movement of PCSA. The Ni2+ solution of a predetermined concentration (C0 = 50 mg/L) was introduced downwards into PBCM at a specified flow rate by means of a dripper. The flow rate is adjusted from 8 to 12 mL/min by adjusting the roller clamp of the dripper. The desired flow rate is converted to drops per minute, and the necessary adjustments are made by adjusting the roller clamp. The desired flow rate was ensured by counting the droplets in the drip chamber and the liquid samples collected for 1 min. The liquid flow is driven by gravity, and the column features multi-ports, enabling the efficient dispersion of Ni2+ ions that easily traverse the column. Therefore, the liquid flow was readily regulated using the dripper. The treated Ni2+ effluents were systematically obtained from the lower portion of PBCM at consistent time intervals, and the residual Ni2+ concentrations (Ce) were quantified. The experiments were sustained until they attained a concentration of 49.5 mg/L or greater.
Before the nickel adsorption, a trial run was preceded with distilled water to ensure the following: (a) the absence of air trapped between the PCSA particles and (b) the even distribution of PCSA. It was observed that the distilled water initially traversed the PCSA bed, and the bed was thoroughly wetted. Once it reached the concerned sampling port, the water samples were collected at each port. The PBCM’s dynamic behaviour was examined with their breakthrough curve generated while operating it under various flow rates (8–12 mL/min) and varied column lengths (5–25 cm).
The time required for the emergence of breakthroughs and their corresponding shape has significant importance in column design25,26. The time required for PBCM to adsorb 10% of C0 (i.e., Ct/C0 = 0.1) provides the breakthrough time (tb)27, while the time it takes to get saturated (i.e., Ct/C0 = 0.9) provides the exhaustion time (te)27. The experimental runs were performed in triplicates, and the error bars were provided with a 5% standard deviation (SD).
The total adsorbed Ni2+ ions (qtotal), maximum adsorption capacity at exhaustion time (qb), the total quantity of Ni2+ ions entering PBCM (Mtotal) and total nickel removal, were estimated using Eq. (1) to (4), respectively28.
where Cads is Ni2+ ion concentration adsorbed onto PCSA (mg/L) and ttotal is the complete duration for Ni2+ adsorption in PBCM.
where m is the quantity of PCSA packed in PBCM.
When the Ni2+ solution enters the PBCM, the Ni2+ ions’ transference initially occurs in the vicinity of the feed entrance within the PCSA’s bed29. Upon reaching saturation of PCSA with Ni2+ ions, MTZ (Eq. (5))30 progresses through the column until its withdrawal.
where Z is the PBCM’s loading height.
The approach of estimating the unused bed length (Lu) (Eq. (6)) of PBCM could be utilized while designing a scale-up packed bed column31,32.
Results and discussion
Phase formation of PCSA
The diffraction pattern of PCSA was used to examine their crystalline phase formation, which can be seen in Fig. 3(a). It was evident that hexagonal calcium carbonate was present, more specifically in their aragonite phase. The existence of planar indices (111), (021), (002), (012), (022), (211), (220), (122), (132), (113) and (114) confirmed the aragonite phase of PCSA which was consistent with JCPDS 41-147533,34. In addition, the Debye-Scherrer formula35 (d = 0.9λ/βcos θ) was utilized to determine the crystallite size of PCSA, and the result was determined to be 66 nm.
Functional groups of PCSA
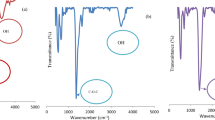
The infrared spectrum of PCSA is depicted in Fig. 3(b), which displays the stretching vibration of the -OH group at 3682.81 cm− 1. Similarly, it showed the occurrence of C = O groups at 1789.45 cm− 1 and C-OH groups at 1085.75 cm− 1. Furthermore, the characteristic peaks determining the existence of CO32− in calcium carbonate are identified from the peaks belonging to 1475.66, 862.23 and 708.02 cm− 136. These peaks exhibit significant strength. The aragonite’s asymmetric stretching is responsible for the prominent absorption peak observed at 1475.66 cm− 137. The FTIR analysis of PCSA revealed that their major constituent is aragonite calcium carbonate, which is reliable with XRD analysis. The adsorption of Ni2+ ions resulted in the displacement of some functional groups of PCSA towards lower intensity. An observed reduction in the peak intensity at their respective wavelengths can be visualized in the Fig. 3(b). The major functional groups crucial in Ni2+ ion removal were -OH and –CO32. It revealed the chemical bond formation between the Ni2+ ions and functional groups of the PCSA.
Surface area and pore distribution of PCSA
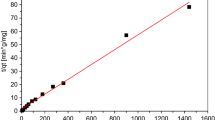
The BET analysis assessed the surface area properties of PCSA. Figure 4(a) depicted a hysteresis loop showing a Type IV isotherm with an H3 hysteresis loop. Furthermore, the hysteresis loop indicated that PCSA had a wedge-shaped structure and was mainly composed of mesopores. In addition, the PCSA has a surface area of 13.37 m2/g, a mean pore radius of 20.076 Å, and an overall pore volume of 0.002 cm3/g. Furthermore, their pores were evenly dispersed (Fig. 4(b)) within the 12 to 25 Å range.
Oxidation state and surface chemistry of PCSA
The XPS spectrum of PCSA established their valence state and composition of surface elements present in it, which was depicted in Fig. 5. The survey scan of PCSA (Fig. 5a) established that their primary constituents are C (carbon), O (oxygen) and Ca (calcium). The XPS spectra of PCSA (Fig. 5b) displayed multiple overlaid C1s peaks (C1-C2). The C1 peak observed at 285 eV was assigned to amorphous carbon. On the other hand, the C2 peak observed at 288.6 eV is typically associated with the binding energy of metal carbonate MCO3 and was therefore identified as CaCO3.
Similarly, the peak observed at 532.11 eV in the O1s spectra (Fig. 5c) was assigned to the presence of carbonate (CO32–) ions in calcium carbonate (CaCO3). Correspondingly, the Calcium peak (Fig. 5d) displayed at 346.8 eV was attributed to CaCO318. Therefore, the major constituents of PCSA were further confirmed with their XPS spectrum, which was calcium carbonate.
Surface texture and elemental composition of PCSA
The surface texture, geometric structure, and form of PCSA were examined using an SEM micrograph at a magnification of X 10,000, as shown in Fig. 6a. The geometric arrangement of PCSA, which can be seen in Fig. 6a, represented that their forms were majorly irregular, comprising of calcium material, and their surface textures were rather rough. In addition, the particles of PCSA were not dispersed consistently and consisted of spherical and rod-shaped crystals. Their size appeared to be bigger with the presence of carbonate as well as it is non-porous. Moreover, it is possible to see the PCSA particles clumping together into aggregates. The surface of Ni-absorbed PCSA has undergone slight changes in its surface roughness.
Figure 6b has exhibited that the PCSA particles were agglomerated due to Ni2+ ions’ adsorption on their surface. Moreover, Fig. 6b revealed the surface roughness of the particles of PCSA after the adsorption with Ni2+ ions. Therefore, the chemical bond formation between the Ni2+ ions and active sites/ functional groups of the PCSA was further confirmed.
The morphological features of PCSA were further examined using TEM analysis. Figure 6c exhibited the TEM image of PCSA measured by a scale bar of 500 nm. According to the findings, the particles of PCSA were distributed in 50–450 nm, as represented in Fig. S1 (see supplementary section). It further verified the heterogeneous size distribution comprised of spherical and rod-shaped particles and clustered configuration (i.e., agglomeration) of the powered clamshell38. The results obtained from TEM images exhibited conformity with those obtained from SEM images.
The elemental composition of PCSA is depicted in Fig. 6d. It represented the presence of Ca, O and C with the corresponding composition of 32.04%, 53.50% and 14.08%. It also showed a limited composition of 0.37%, which belongs to Na (sodium). These results revealed conformity with those obtained from XRD, FTIR and XPS.
Evaluation of design parameters of PBCM
The present study evaluated the efficacy of PCSA in a PBCM for Ni2+ ion removal while considering a bed depth spanning from 5 to 25 cm. The Q’s influence on Ni2+ adsorption was deliberated with 50 mg/L of Ni solution (C0) by changing the Q from 8 to 12 mL/min using a dripper. The effluent was collected at each bed length of PBCM every 10 min and was measured for residual Ni2+ concentration (Ce). The mass transfer zone of the multi-port column is analyzed using the breakthrough curve analysis. Figure 7 illustrates the breakthrough curves (i.e., BTC) observed during the process of Ni2+ utilizing PCSA (Error bar included with 5% SD), while the rate of influent rate was varied. As observed from Fig. 7, it exhibited a standard S-shaped profile, with their fluctuations contingent upon the PCSA’s dose and the flow rate.
The values for the column designing are computed from the associated BTC for Ni2+ uptake and are shown in Table 1. The computed parameters exposed the higher values of Ni2+ removal percentage and adsorption capacity for a lower flow rate of 8 mg/L and at a higher loading of PCSA (i.e., 25 cm). Conversely, these parameters showed a declining trend with an increasing flow rate and declined PCSA loading.
Higher loading of PCSA occurring with lengthening of column bed resulted in higher tb and te values (Table 1). Subsequently, the qtotal enhanced from 22.03 to 52.61 mg and percent removal from 57.98 to 87.68%, at 8 mg/L. These outcomes might have occurred due to increased exposure to nickel ions with PCSA. Similarly, the rest of the flow rates showed comparable results for design parameters.
The MTZ (i.e., mass transfer zone) depends on the changes in residence time; the increase in column depth results in increased residence time and vice versa. Therefore, the values of MTZ showed an inclined trend with the rise in the time available for nickel ions to contact the PCSA, which improved the treatment efficiency. For instance, the values of MTZ ranged from 3.75 to 14.84 cm for an 8 mL/min influent flow rate. Consequently, the bottom layer of the multi-port column (i.e., H = 25 cm) had the highest adsorption loading (i.e., MTotal 60 mg), and the bottom layer (i.e., H = 5 cm) owned the maximum adsorption capacity (i.e., qb 2.20 mg/g).
The 8 mL/min flow rate yielded a Ni2+ uptake of 1.05 mg/g with a removal efficiency of 87.68%. A change in Ni2+ absorption was observed, and a decrease from 1.05 to 0.92 mg/g was recorded as the flow elevated at a rate of 8 to 12 mL/min. The active surface area of PCSA does not become fully saturated with Ni2+ ions due to limited contact time between the PCSA and Ni2+. Consequently, equilibrium is not attained. Similar results were observed for every bed length. Mavinkattimath et al.. (2023) have also observed a decrease in adsorption capacity with an advanced flow that can be credited to the minimal period of the dye molecule’s interaction inside the bed31,39,40,41,42,43.
The Ni2+ ions diffusion involves initial permeation through larger pores, followed by subsequent penetration through macropores and ultimately into micropores31. The duration of time that a substance spends in the column is inversely proportional to the flow. At advanced flow, the permeation of Ni2+ ions over the pores and their occupation of dynamic sites on internal pore surfaces may be hindered due to insufficient time44. The Ni2+ adsorption might have occurred more frequently on the PCSA’s exterior surface than their pores when the residence time is more rapid. Therefore, a significant proportion of active sites on the interior pores of PCSA’s surface may remain unused while increasing the flow rate. The Ni2+ ions could be surpassed the column beforehand they can penetrate the PCSA’s internal pores and become adsorbed as a result of the reduced internal diffusion rate. Consequently, the available active sites within the interior pores do not adsorb the Ni2+ ions, resulting in their passage out of the column. Therefore, at elevated flow rates, the adsorption capacity experiences a decrease45.
Table 1 illustrated that the values of tb (65 to 45 min) and te (150 to 125 min) exhibited a decline with an upsurge in the Q. As illustrated in Fig. 7, it is apparent that the breakthrough transpired at a relatively accelerated pace with an increase in flow rate, resulting in a reduced time engaged to attain the breakthrough46. At elevated stream rates, the mass transfer rate is augmented, resulting in an escalated rate of Ni2+ adsorption and consequently hastening the saturation process47.
On the flip side, the elevated mass transfer rate favored the increased uptake of the Ni2+ ions onto PCSA, as is apparent from the higher values of qtotal (52.61 to 46.01 mg) while increasing the flow rate48 (Table 1). Similarly, the velocity of nickel ions that traverse through the PBCM bed increased with the corresponding increase in flow rate, leading to a more rapid saturation of the PBCM bed. Subsequently, there may be a reduction in the treated effluent volume within the column, resulting in a reduced adsorption zone and a consequent reduction from 87.68 to 61.35% nickel removal.
The impacts of radial mixing as well as turbulence while increasing the flow rate, might have accelerated the saturation of the PBCM bed height. As a result, the MTZ span gets reduced from 14.84 to 14.42 cm. Similarly, the PBCM’s unused bed length reduced from 10.2 to 8.8 cm as a result of an increased flow rate, which suggested that the resistance to the mass transfer becomes less significant as the flow rate increases49. Moreover, the lower values of Lu indicated a higher utilization rate of available PBCM beds. Since the mass transfer is more effective at advanced flow rates, the utilization of PBCM bed efficiency improves31,50. Consequently, the PBCM’s bed was utilized most effectively for Ni2+ adsorption when the inflowing flow was 8 mL/min.
In contrast, the impact of PBCM’s bed length exhibited a distinct pattern on design parameters, as presented in Table 1. The packing of PCSA increases with the extended length of PBCM, leading to increased PCSA’s surface sites51. Moreover, the duration of PCSA-nickel ions interaction might get increased. Therefore, the time taken for breakthrough (tb) and exhaustion (te) lengthened from 25 to 65 min and 95 to 150 min, respectively, at 8 mL/min. Sun et al. (2014) have reported a comparable result in the values of tb and te with elevated bed depth for phosphate removal52. The MTZ values are contingent upon alterations in residence time. The lengthened PBCM’s bed leads to a corresponding surge in the residence time, and conversely, a decrease in column length results in a decrease in residence time53. As resulted in Table 1, the MTZ values exhibited a positive correlation with the duration of exposure between Ni2+ ions and PCSA. The MTZ values revealed a range of 3.75 to 14.84 cm when subjected to an influent flow of 8 mL/min. The consequences substantiated that the PBCM column has been predominantly employed at its maximum bed length of 25 cm. In conclusion, the best operating condition for removing Ni2+ ions in PBCM was a lower flow rate (8 mL/min) and higher bed depth (15 cm).
Analysis of BTC using a non-linear regression approach
The validation of the efficacy of the PBCM column bed for the Ni2+ uptake was carried out through dynamic modeling using a non-linear regression approach, explicitly employing the Yoon-Nelson model54 and the Thomas model54. The Yoon-Nelson model (Eq. (7)) postulates a linear relationship between the reduced probability of nickel ions being adsorbed and their probability on the adsorbent’s breakthrough curve55.
where KYN is the concerned rate constant and τ is the period necessary to achieve half saturation. The Thomas model (Eq. (8)) incorporates axial dispersion to account for the interaction between the PCSA and the nickel ion54.
where KTh is Thomas model’s rate constant. The dynamic modeling parameters were derived by generating a non-linear regression plot depicting Ct/C0 versus t.
Error analysis is used to assess whether or not the method of non-linear regression is adequate for use in dynamic modeling56. Error Analysis (EA) and correlation coefficient (R2) values were used in an evaluation to determine which column adsorption model was the most appropriate. The following error analysis methodologies (Eq. (9) to (12)) such as RMSE (Root Mean Square Error)56, χ2 (Pearson’s Chi-square)56 and SSE (Sum of Squares of errors)56, are utilized in order to determine the nickel concentration after adsorption using PCSA.
Figure S2-S4 (see supplementary section) illustrates the graphical representation of the Yoon-Nelson and Thomas model utilized for the purpose of analyzing the Ni2+ adsorption onto PCSA under varying flow rates ranging from 8 to 12 mL/min. Table 2 provides the corresponding parameters (τ, KYN, KTh and qe) and R2 values. In addition, the EA values were summarized in Table S1 (see supplementary section).
The Yoon-Nelson and the Thomas models exhibited comparable correlation coefficient values (Table 2) and EA values (Table S1 (see supplementary section)). However, Thomas model possessed a little higher value (0.9906 − 0.9693) and EA values appeared to be comparatively lower, signifying their optimal fitness over the other. Additionally, it exhibited a higher degree of agreement between their estimated and experimental qe values in comparison to the Yoon-Nelson model. Thus, the results of the Thomas model provided evidence for its superior suitability in facilitating the Ni2+ adsorption using PCSA.
The present study revealed that the KTh values negatively correlated with increased PBCM’s bed length and reduced flow rate. This is because, during the higher length of PBCM, the mass transfer rate of Ni2+ ions into the column reduces, resulting in decreased KTh values. Vilvanathan and Shanthakumar (2017) reported that lower values of KTh were obtained with a lower flow rate and lengthened bed depth46. Also, the highest qe may be attained by decreased PBCM’s bed length while increasing the flow rate. Hence, it can be inferred that the Ni2+ sequestration is not solely restricted by external and internal diffusions57.
The trend observed in the τ values indicated an increase with increasing PBCM’s bed length and a declined flow58. The heightened bed lengths are associated with a greater abundance of adsorption sites, resulting in elevated τ values. Similarly, the instantaneous saturation of the PBCM transpired at heightened flow rates, which can be ascribed to the diminished values of τ59. The KYN values exhibited a decline with lengthened PBCM’s bed and reduced flow.
Analysis of BTC using a linear regression approach
where N0 is the PCSA’s adsorption capability, U is influent velocity (cm/min), kAB is the concerned rate constant and Cb is the Ni2+ absorption at breakthrough (mg/L).
The plot (not shown here) depicting the correlation of PBCM’s bed length with service time is drawn to estimate the N0 and kAB. Specifically, the plot examined the service time at various fractions of the C0 (i.e., Ct/C0 = 0.2, 0.4, 0.6, and 0.8). Table 3 presents the BDST parameters, namely N0 and kAB.
The BDST model’s R2 values exhibited values ranging from 0.9897 to 0.8000, indicating strong proximity to unity. The values of N0 and kAB exhibited an upward trend in tandem with the escalation of the breakthrough point. The decreased active sites are directly proportional to the increased breaking point. As a result, the PBCM becomes saturated, potentially leading to high N0 values61,62.
Desorption and regeneration studies
The practicality and cost-effectiveness of an adsorbent depend on its capacity to desorb adsorbed molecules from their surface and replenish itself. The desorption of Ni ions (Fig. 8a) from loaded PCSA was achieved by utilizing desorbing agents such as chelating agent (i.e., EDTA), organic solvent (i.e., Ethanol), acids (Hydrochloric acid and Nitric acid), and alkalies (Sodium hydroxide and Sodium chloride). The concentration of all the desorbing agents was maintained at one molar concentration. Hydrochloric acid and nitric acid have shown better performance among the chosen desorbing agents, with respective desorption efficiencies of 90% and 95%. This phenomenon may be due to the protonation of the PCSA surface in acidic environments, which enabled the release of cations (i.e., Ni2+ ions)63,64. (Steinhaus et al. 2022; Isaac et al. 2023). Therefore, it is evident that PCSA can be desorbed effectively using nitric acid.
In the regeneration study (Fig. 8b), the nickel-loaded PCSA was eluted with 1 M HNO3 solution for one hour and neutralized with a distilled water wash. The refreshed PCSA was used for the next adsorption cycle after drying. The PCSA has retained significant nickel adsorption capacity after the three cycles. The nickel removal declined from 45% after three cyclic runs. The PCSA regeneration could alleviate the burden of the sludge treatment, though PCSA regeneration was unsatisfactory.
Comparison studies on dynamic adsorption of Ni2+ ions
In this study, the adsorption capacity of powdered clam shell adsorbent was evaluated using fixed-bed technology and compared to other adsorbents for treating Ni2+ ions from simulated wastewater (Table 4). The utilization of powdered clam shell adsorbent can be regarded as an economical and sustainable resource for application in the remediation of industrial wastewater. Nonetheless, conducting a precise comparison between the various adsorbents is challenging due to the utilization of distinct experimental conditions.
Significance of PCSA for metal remediation
The significance of PCSA is depicted in Fig. 9. Using PCSA as an adsorbent has shown remarkable performance in removing nickel while being cost-effective, environmentally friendly, and biocompatible. Thus, it can function as an alternative adsorbent for treating industrial effluents.
Conclusion
The PCSA’s packed bed column has exhibited efficacy in adsorbing Ni2+ ions from simulated wastewater, with a maximum Ni2+ removal of 87.68% while at 8 mL/min (Q) utilizing a 25 cm (Z). The design parameters of PBCM were analyzed using the related breakthrough curves. The breakthrough is delayed, Ni2+ uptake is enlarged and the removal efficiency is increased due to reduced flow rate and lengthened PBCM bed. The results endorsed the successful procedure of dynamic adsorption and the effective usage of PCSA in PBCM for Ni2+ uptake. The applicability of Thomas model has suggested that Ni2+ adsorption in PBCM may entail exterior mass transfer as well as internal diffusional resistances. The utilization of PCSA as an adsorbent has demonstrated significant efficacy in removing nickel while simultaneously exhibiting cost-effectiveness, eco-friendliness, and biocompatibility. Therefore, it can serve as a substitute adsorbent for the purpose of treating industrial effluents.
Data availability
The original data in this article will be shared upon reasonable request to the corresponding authors.
References
Tahir, M. B., Kiran, H. & Iqbal, T. The detoxification of heavy metals from aqueous environment using nano-photocatalysis approach: a review. Environ. Sci. Pollut. Res. 26(11), 10515–10528 (2019).
Rao, L. N. & Prabhakar, G. Removal of heavy metals by biosorption-an overall review. J. Eng. Res. Stud. 2(4), 17–22 (2011).
Kiran, S. et al. Valorization of wastes for the remediation of toxicants from industrial wastewater. Integrating Green. Chem. Sustainable Eng. 15, 473–525 (2019).
Carolin, C. F., Kumar, P. S., Saravanan, A., Joshiba, G. J. & Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J. Environ. Chem. Eng. 5(3), 2782–2799 (2017).
Guieysse, B. & Norvill, Z. N. Sequential chemical–biological processes for the treatment of industrial wastewaters: review of recent progresses and critical assessment. J. Hazard. Mater. 267, 142–152 (2014).
Noman, E. et al. Sustainable approaches for nickel removal from wastewater using bacterial biomass and nanocomposite adsorbents: a review. Chemosphere 291, 132862 (2022).
Kadhim, Z. A. & Abbar, A. H. Nickel removal from simulated wastewater using a novel bio-electrochemical cell with packed bed rotating cylinder cathode. Chem. Eng. Res. Des. 190, 157–173 (2023).
Qasem, N. A., Mohammed, R. H. & Lawal, D. U. Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean. Water 4(1), 36 (2021).
Ma, A., Abushaikha, A., Allen, S. J. & McKay, G. Ion exchange homogeneous surface diffusion modelling by binary site resin for the removal of nickel ions from wastewater in fixed beds. Chem. Eng. J. 358, 1–10 (2019).
Shaker, O. A., Safwat, S. M. & Matta, M. E. Nickel removal from wastewater using electrocoagulation process with zinc electrodes under various operating conditions: performance investigation, mechanism exploration, and cost analysis. Environ. Sci. Pollut. Res. 30(10), 26650–26662 (2023).
Naushad, M., Ahamad, T., AlOthman, Z. A. & Ala’a, H. Green and eco-friendly nanocomposite for the removal of toxic hg (II) metal ion from aqueous environment: adsorption kinetics & isotherm modelling. J. Mol. Liq. 279, 1–8 (2019).
Moradi, G., Zinadini, S., Rahimi, M. & Shiri, F. Efficient Zn2+, Pb2+, and Ni2+ removal using antifouling mixed matrix nanofiltration membrane with curcumin modified mesoporous Santa Barbara Amorphous-15 (Cur-SBA-15) filler. J. Environ. Chem. Eng. 10(3), 107302 (2022).
Bolisetty, S., Peydayesh, M. & Mezzenga, R. Sustainable technologies for water purification from heavy metals: review and analysis. Chem. Soc. Rev. 48(2), 463–487 (2019).
Jayalakshmi, R. & Jeyanthi, J. Simultaneous removal of binary dye from textile effluent using cobalt ferrite-alginate nanocomposite: performance and mechanism. Microchem. J. 145, 791–800 (2019).
Farhat, S. et al. Comparative analysis of the Mercenaria mercenaria genome provides insights into the diversity of transposable elements and immune molecules in bivalve mollusks. BMC Genom. 23(1), 1–23 (2022).
Gökoğlu, N. & Gökoğlu, N. Molluscan Shellfish. Shellfish Process. Preservation. 129–250. https://doi.org/10.1007/978-3-030-60303-8_3 (2021).
Iloamaeke, I. M., Nnaji, N. J., Okpala, E. C., Eboatu, A. N. & Onuegbu, T. U. Mercenaria mercenaria shell: coagulation-flocculation studies on colour removal by response surface methodology and nephlometric kinetics of an industrial effluent. J. Environ. Chem. Eng. 9(4), 105715 (2021).
Qu, T., Yao, X., Owens, G., Gao, L. & Zhang, H. A sustainable natural clam shell derived photocatalyst for the effective adsorption and photodegradation of organic dyes. Sci. Rep. 12(1), 2988 (2022).
Fitriani, N. et al. Visualization of the Microbial Community and Elemental Mapping of Anadara granosa media used in a slow sand Filter using a SEM-EDS. Asian J. Water Environ. Pollut. 17(3), 31–36 (2020).
Wibowo, Y. G., Safitri, H. & Ramadan, B. S. Adsorption test using ultra-fine materials on heavy metals removal. Bioresource Technol. Rep. 19, 101149 (2022).
Aly, H. M., Shama, S. A., Elsharkawy, A. M., Helal, A. I. & Khodir, A. M. Adsorption of Cu2+ ion from aqueous solution by Clam Shell Powder. Benha J. Appl. Sci. 1(1), 1–9 (2016).
Ma, J., Zou, J., Cui, B., Yao, C. & Li, D. Adsorption of Orange II dye from aqueous solutions using phosphoric-acid modified clam shell powder. Desalination Water Treat. 51(34–36), 6536–6544 (2013).
Pai, S., Kini, M. S., Rangasamy, G. & Selvaraj, R. Mesoporous calcium hydroxide nanoparticle synthesis from waste bivalve clamshells and evaluation of its adsorptive potential for the removal of Acid Blue 113 dye. Chemosphere 313, 137476 (2023).
Li, X., Wen, B. & Li, Y. Adsorption of the malachite green by magnetic clam shell powder. Pol. J. Environ. Stud. 30, 717–726 (2017).
Çelebi, H., Gök, G. & Gök, O. Adsorption capability of brewed tea waste in waters containing toxic lead (II), cadmium (II), nickel (II), and zinc (II) heavy metal ions. Sci. Rep. 10(1), 17570 (2020).
Bai, S., Li, J., Ding, W., Chen, S. & Ya, R. Removal of boron by a modified resin in fixed bed column: Breakthrough curve analysis using dynamic adsorption models and artificial neural network model. Chemosphere, 296, 134021. (2022).
Ramesh, S. T., Gandhimathi, R., Nidheesh, P. V., Badabhagni, N. & Bharathi, K. S. Breakthrough data analysis of adsorption of cd (II) on Coir pith column. Electron. J. Environ. Agricultural Food Chem. 10(8), 2638–2658 (2011).
Oguz, E. Fixed-bed column studies on the removal of Fe3+ and neural network modeling. Arab. J. Chem. 10(3), 313–320 (2017).
Mesfer, M. K. A. et al. Continuous fixed bed CO2 adsorption: breakthrough, column efficiency, mass transfer zone. Processes 8(10), 1233 (2020).
Deokar, S. et al. Adsorptive column studies for removal of acid orange 7 dye using bagasse fly ash. Indian J. Chem. Technol. (IJCT). 28 (3), 319–327 (2021).
Mavinkattimath, R. G., Kodialbail, S., Srinikethan, G. & V., & Continuous fixed-bed adsorption of reactive azo dye on activated red mud for wastewater treatment-evaluation of column dynamics and design parameters. Environ. Sci. Pollut. Res. 30, 57058–57075 (2023).
Alan Gabelman. Adsorption Basics-Part 1. Gabelman Process Solutions, LLC. In: CEP-Back to Basics. American Institute of Chemical Engineers (AIChE), 48–53. (2017).
Chang, T. Y., Wang, C. M., Lin, T. Y. & Lin, H. M. Conversion of biowaste Asian hard clam (meretrix lusoria) shells into White-Emitting phosphors for use in neutral white LEDs. Materials 9(12), 979 (2016).
Li, T. & Zeng, K. Nano-hierarchical structure and electromechanical coupling properties of clamshell. J. Struct. Biol. 180(1), 73–83 (2012).
Jayalakshmi, R. & Jeyanthi, J. Synthesis and structural characterization of polymer-based cobalt ferrite nanocomposite with core–shell structure. J. Inorg. Organomet. Polym Mater. 28, 1286–1293 (2018).
Li, Y. et al. Synergistic effect of clam shell bio-filler on the fire-resistance and char formation of intumescent fire-retardant coatings. J. Mater. Res. Technol. 9(6), 14718–14728 (2020).
Wang, F., Liu, H. & Yan, L. Comparative study of fire resistance and char formation of intumescent fire-retardant coatings reinforced with three types of shell bio-fillers. Polymers 13(24), 4333 (2021).
Alif, M. F., Aprillia, W. & Arief, S. A hydrothermal synthesis of natural hydroxyapatite obtained from Corbicula moltkiana freshwater clams shell biowaste. Mater. Lett. 230, 40–43 (2018).
Alslaibi, T. M., Abustan, I., Ahmad, M. A. & Abu Foul, A. Comparative studies on the olive stone activated carbon adsorption of Zn2+, Ni2+, and Cd2 + from synthetic wastewater. Desalination Water Treat. 54(1), 166–177 (2015).
El-Azim, H. & Mourad, F. Removal of Heavy metals cd (II), Fe (III) and ni (II), from aqueous solutions by natural (clinoptilolite) zeolites and application to industrial wastewater. Asian J. Environ. Ecol. 7(1), 1–13 (2018).
Bartczak, P., Norman, M., Klapiszewski, Ł., Karwańska, N., Kawalec, M., Baczyńska,M., … Jesionowski, T. Removal of nickel (II) and lead (II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arabian Journal of Chemistry, 11(8), 1209–1222. (2018).
Adeli, M., Yamini, Y. & Faraji, M. Removal of copper, nickel and zinc by sodium dodecyl sulphate coated magnetite nanoparticles from water and wastewater samples. Arab. J. Chem. 10, S514–S521 (2017).
Sobhanardakani, S. & Zandipak, R. Synthesis and application of TiO2/SiO2/Fe3O4 nanoparticles as novel adsorbent for removal of cd (II), hg (II) and ni (II) ions from water samples. Clean Technol. Environ. Policy 19, 1913–1925 (2017).
Xavier, A. L. P. et al. Modeling adsorption of copper (II), cobalt (II) and nickel (II) metal ions from aqueous solution onto a new carboxylated sugarcane bagasse. Part II: optimization of monocomponent fixed-bed column adsorption. J. Colloid Interface Sci. 516, 431–445 (2018).
Gong, J. L., Zhang, Y. L., Jiang, Y., Zeng, G. M., Cui, Z. H., Liu, K., … Huan, S.Y. Continuous adsorption of Pb (II) and methylene blue by engineered graphite oxide coated sand in fixed-bed column. Applied Surface Science, 330, 148–157. (2015).
Vilvanathan, S. & Shanthakumar, S. Column adsorption studies on nickel and cobalt removal from aqueous solution using native and biochar form of Tectona grandis. Environ. Prog. Sustain. Energy. 36 (4), 1030–1038 (2017).
Mangaleshwaran, L., Thirulogachandar, A., Rajasekar, V., Muthukumaran, C. & Rasappan, K. Batch and fixed bed column studies on nickel (II) adsorption from aqueous solution by treated polyurethane foam. J. Taiwan Inst. Chem. Eng. 55, 112–118 (2015).
Yagub, M. T., Sen, T. K., Afroze, S. & Ang, H. M. Fixed-bed dynamic column adsorption study of methylene blue (MB) onto pine cone. Desalination Water Treat. 55(4), 1026–1039 (2015).
Qu, J., Song, T., Liang, J., Bai, X., Li, Y., Wei, Y., … Jin, Y. U. Adsorption of lead (II) from aqueous solution by modified Auricularia matrix waste: A fixed-bed column study. Ecotoxicology and Environmental Safety, 169, 722–729. (2019).
Hethnawi, A. et al. Metformin removal from Water using fixed-bed column of silica-alumina composite. Colloids Surf. A 597, 124814 (2020).
Simate, G. S. & Ndlovu, S. The removal of heavy metals in a packed bed column using immobilized cassava peel waste biomass. J. Ind. Eng. Chem. 21, 635–643 (2015).
Sun, X. et al. Adsorption of phosphate using calcined Mg3-Fe layered double hydroxides in a fixed-bed column study. J. Ind. Eng. Chem. 20, 3623–3630 (2014).
Hernandez-Eudave, M. T., Bonilla-Petriciolet, A., Moreno-Virgen, M. R., Rojas-Mayorga, C. K. & Tovar-Gómez, R. Design analysis of fixed-bed synergic adsorption of heavy metals and acid blue 25 on activated carbon. Desalination Water Treat. 57(21), 9824–9836 (2016).
Belat, B., Veli, S. & Isgoren, M. Modeling of linear alkyl benzene sulphonic acid removal from aqueous solution with fixed bed adsorption column: Thomas and Yoon–Nelson methods. J. Chem. Technol. Biotechnol. 97(7), 1771–1780 (2022).
Basu, M. & Guha, A. K. Separation of lead from aqueous phase by cucumber peel in column bioreactor: a phenomenon of interaction between biological and chemical system and its ecological importance. J. Environ. Manage. 337, 117738 (2023).
Jayalakshmi, R., Jeyanthi, J. & Sidhaarth, K. A. Versatile application of cobalt ferrite nanoparticles for the removal of heavy metals and dyes from aqueous solution. Environ. Nanatechnol. Monit. Manage. 17, 100659 (2022).
Mondal, S., Aikat, K. & Halder, G. Ranitidine hydrochloride sorption onto superheated steam activated biochar derived from mung bean husk in fixed bed column. J. Environ. Chem. Eng. 4(1), 488–497 (2016).
Chatterjee, S., Sivareddy, I. & De, S. Adsorptive removal of potentially toxic metals (cadmium, copper, nickel and zinc) by chemically treated laterite: single and multicomponent batch and column study. J. Environ. Chem. Eng. 5(4), 3273–3289 (2017).
Jayalakshmi, R. & Jeyanthi, J. Dynamic modelling of Alginate-Cobalt ferrite nanocomposite for removal of binary dyes from textile effluent. J. Environ. Chem. Eng. 9(1), 104924 (2021).
Pérez-Calderón, J., Santos, M. V. & Zaritzky, N. Experimental study and modelling of an azo colourant dynamic adsorption onto functional cross-linked chitosan/ceramic particles in a fixed bed column. Can. J. Chem. Eng. https://doi.org/10.1002/cjce.24852
Bishayee, B., Ruj, B., Nandi, S., Chatterjee, R. P., Mallick, A., Chakraborty, P.,… Chakrabortty, S. Sorptive elimination of fluoride from contaminated groundwater in a fixed bed column: A kinetic model validation based study. Journal of the Indian Chemical Society, 99(1), 100302. (2022).
Hernández-Flores, H., Pariona, N., Herrera-Trejo, M., Hdz-García, H. M. & Mtz-Enriquez, A. I. Concrete/maghemite nanocomposites as novel adsorbents for arsenic removal. J. Mol. Struct. 1171, 9–16 (2018).
Steinhaus, J., Pasel, C., Bläker, C. & Bathen, D. Adsorption of Mercury on Chlorine-modified activated Carbon: breakthrough curves and temperature-programmed desorption. ACS Omega 7(27), 23833–23841 (2022).
Isaac, R., Siddiqui, S., Aldosari, O. F. & Uddin, M. K. Magnetic biochar derived from Juglans regia for the adsorption of Cu2 + and Ni2+: characterization, modelling, optimization, and cost analysis. J. Saudi Chem. Soc. 27(6), 101749 (2023).
Mokokwe, G. & Letshwenyo, M. W. Investigation of clay brick waste for the removal of copper, nickel and iron from aqueous solution: batch and fixed–bed column studies. Heliyon, 8(7), e09963. (2022).
Sundhararasu, E. et al. Alkali-activated adsorbents from slags: column adsorption and regeneration study for nickel (II) removal. ChemEngineering 5(1), 13 (2021).
Sizirici, B. & Yildiz, I. Simultaneous removal of organics and metals in fixed bed using gravel and iron oxide coated gravel. Results Eng. 5, 100093 (2020).
Egbosiuba, T. C., Abdulkareem, A. S., Kovo, A. S., Afolabi, E. A., Tijani, J. O.,Bankole, M. T., … Roos, W. D. Adsorption of Cr (VI), Ni (II), Fe (II) and Cd (II) ions by KIAgNPs decorated MWCNTs in a batch and fixed bed process. Scientific reports, 11(1), 75. (2021).
Kapur, M. & Mondal, M. K. Design and model parameters estimation for fixed–bed column adsorption of Cu (II) and ni (II) ions using magnetized saw dust. Desalination Water Treat. 57(26), 12192–12203 (2016).
Acknowledgements
The authors thank the Head of the Department of Civil Engineering, Dean School of Mechanical and Construction, Dean Research Studies and Dean R & D, Registrar, Vice chancellor and Management of Veltech Rangarajan Dr. Sagunthala R&D Institute of Science and Technology for providing sufficient lab facilities to carry out our experiments. The author S.K. Lakkaboyana thanks to Vel Tech Rangarajan Dr. Sagunthala R&D institute of science and Technology provided by RDF and SEED funds. The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2025R301), King Saud University, Riyadh, Saudi Arabia. The authors are thankful to Dr. R. Jayalakshmi for her assistance in proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
S. Baskar: Formal analysis, Conducting experiments, Writing & editing, Methodology and reviewing; K. R. Aswin Sidhaarth: Conceptualization, Supervision, Methodology and reviewing Project Manager; L. Mangaleshwaran: Conceptualization, Reviewing & editing; Sivarama Krishna Lakkaboyana: Formal analysis, Reviewing & editing; Herri Trilaksana: Formal analysis, Reviewing & editing R.M.N.Kalla: Reviewing & editing; Jaewong Lee: Review & editing; Leonard I. Atanase: Writing- Review & editing; Mohsin Kazi: Review & editing, Fund manager, S.Praveenkumar: Writing- Review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Baskar, S., Sidhaarth, K.R.A., Mangaleshwaran, L. et al. Elimination of nickel ions in a packed column using clamshell waste as an adsorbent. Sci Rep 15, 32 (2025). https://doi.org/10.1038/s41598-024-82267-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82267-0
Keywords
This article is cited by
-
Innovative use of immobilized zinc oxide-impregnated activated carbon (ZnO@CB) for effective treatment of leachate: modeling and predictive assessment
Environmental Science and Pollution Research (2025)