Abstract
Several observational studies have revealed that immune-mediated inflammatory diseases (IMIDs) are associated with an increased risk of peripheral artery disease (PAD). However, the causal association remains to be determined. To corroborate previous research, we conducted Mendelian randomization (MR) analysis with the aim of clarifying the associations of various IMIDs with PAD. two-sample MR analysis was conducted to investigate the potential causal association between eight common IMIDs (including rheumatoid arthritis (RA), Crohn’s disease (CD), ulcerative colitis (UC), systemic lupus erythematosus (SLE), ankylosing spondylitis(AS), psoriasis(PSO), multiple sclerosis(MS), and hashimoto thyroiditis(HT)) and PAD. Genome-wide association study (GWAS) was used to identify genetic variants associated with IMIDs and PAD. We employed the inverse variance weighted (IVW) method as the primary method to verify the causal relationship between exposures (IMIDs) and outcomes (PAD). In addition, heterogeneity test, horizontal pleiotropy test, and leave-one-out analysis were performed to evaluate the robustness of the MR results. The IVW model yielded evidence of a positive association between RA and PAD (OR = 1.059, 95% CI: 1.026–1.094, p<0.001), which was consistent with the results obtained from MR-Egger regression and weighted median analyses, indicating that the results of MR analysis were reliable. However, no statistically significant associations were observed between other IMIDs, including UC, CD, SLE, AS, PSO, MS, and HT, and PAD. Our analysis supported the causal association of RA with increased risks of PAD. Strengthening screening and prevention of PAD is of great significance in reducing the risk of PAD in populations with RA.
Similar content being viewed by others
Introduction
Peripheral artery disease(PAD) is characterized by peripheral circulatory dysfunction resulting from atherosclerosis of arteries other than the coronary arteries and aorta, primarily affecting the arteries of the lower extremities1,2. As the third most common cardiovascular disease after coronary heart disease (CHD) and stroke, PAD affects more than 200 million people worldwide3. Furthermore, with the rapid aging of the global population, the prevalence of PAD is expected to continue to rise4. The interaction of multiple risk factors causes PAD, and the effects of risk factors such as smoking, diabetes, hypertension and dyslipidemia on PAD have been commonly reported5,6. In recent years, it has been found that immune-mediated inflammatory diseases (IMIDs) may be associated with PAD, and as compared to the general population, IMIDs patients were significantly more likely to suffer from PAD7. IMIDs are mainly caused by autoimmune disorders, including rheumatoid arthritis (RA), Crohn’s disease (CD), ulcerative colitis (UC), systemic lupus erythematosus (SLE), ankylosing spondylitis(AS), psoriasis(PSO), multiple sclerosis(MS), and hashimoto thyroiditis(HT) and many other diseases8. Several observational studies have shown that patients with RA9, inflammatory bowel disease(including CD and UC)10, SLE11,12 and PSO13 have an independently higher risk of PAD than the general population. However, due to the influence of sample size and various risk factors, the results were prone to deviation, and further evidence is needed to prove their causality.
Mendelian randomization (MR) takes genetic variation as an instrumental variable (IV) to examine the relationship between IV, exposure factors and outcomes, thereby inferring the causal relationship between exposure factors and outcomes14. Because the genotypes of different genetic variables determine different intermediate phenotypes (exposures), MR Analyses substitute the causal effect of genotypes on disease for the causal effects of exposure on disease. Due to the fortuitous distribution of alleles of genetic variation during gamete formation, MR is less vulnerable to being affected by confounding factors and reverse causality15. Genome-wide association study(GWAS) is constantly improving and developing, providing a data source for MR analysis. Causality between blood pressure, lipoprotein, lifestyle behaviors, cardiovascular risk factors and PAD have been demonstrated16,17,18. However, no MR studies have hitherto explored the causal effects of IMIDs on PAD. To corroborate previous research, we conducted a two-sample MR analysis with the aim of clarifying the associations of various IMIDs (including RA, UC, CD, SLE, AS, PSO, MS, and HT) with PAD.
Methods
Data sources and study design
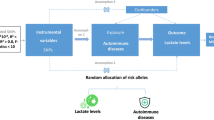
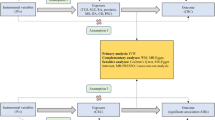
Two-sample MR method was used to study the potential causal relationship between IMIDs and PAD. This paper mainly discusses eight main IMIDs: RA, UC, CD, SLE, AS, PSO, MS, and HT. This study followed three hypotheses of MR analysis: (1) instrumental variables are closely related to exposure (IMIDs in this study); (2) instrumental variables are independent of confounding factors; (3) instrumental variables only affect the outcome via exposure (PAD in this study)19.
The outcome summary statistics for PAD was obtained from the R5 release of the FinnGen study (https://r5.finngen.fi/). The corresponding phenotypic codes obtained was “I9_PAD” (7,098 cases and 206,541 controls). The diagnosis of these cases was based on the International Classification of Diseases, Eighth and Ninth Revision(ICD-9/10) codes. GWAS data of IMIDs was obtained from the website of IEU OpenGWAS Database Project(https://gwas.mrceiu.ac.uk/). All of the participants were of European ancestry. GWAS datasets utilized for RA (14,361 cases and 43,923 controls) and SLE (5,201 cases and 9,066 controls) were obtained from GWAS Catalog(https://www.ebi.ac.uk/gwas/). The summary data of UC and CD was extracted from a commen GWAS study by de Lange KM et al.20, which included 40,266 participants (12,366 UC cases and 33,609 control samples, with 9,474,559 SNPs; 12,194 CD cases and 28,072 control samples, with 9,457,998 SNPs). Summary statistics for AS and PSO were derived from the UK Biobank (http://www.nealelab.is/uk-biobank/), including 9,851,867 and 10,894,596 SNPs, respectively. Genetic data for MS (n = 15,283) and HT (n = 395,640) were severally obtained from the results conducted by Andlauer et al.21 and Sakaue et al.22. The overview of GWAS employed for exposures and outcomes was shown in Table 1.
Instrumental variable selection
To satisfy hypothese (1) of MR analysis, we selected genetic variants that passed GWAS threshold (P < 5 × 10− 8) as instrumental variables (IVs). Moreover, to ensure independence between IVs, we set the linkage disequilibrium (LD) threshold for grouping to r2 < 0.001 and a window size of 10,000 kb23. The PhenoScanner database (http://www.phenoscanner.medschl.cam.ac.uk/) was used to examine the associated phenotypes of each genetic variant. Specifically, we systematically screened for and excluded SNPs that are associated with common comorbid conditions such as diabetes, hypertension, BMI, and HDL cholesterol. By filtering out SNPs linked to these confounders, we aimed to reduce the bias introduced by these factors and ensure that the observed associations between IMIDs and PAD were not driven by underlying comorbidities. MR-PRESSO was executed to exclude any outliers with potential pleiotropy to ensure the reliability of our MR estimates24,25. Additionally, some palindromic SNPs were manually removed by using the TwoSampleMR packages. We calculated the F-statistic to quantify the strength of IVs, with F > 10 as the strong correlation criterion. The calculation of the F-statistic was conducted using the formula: F = R2(N-K-1)/[K(1-R2)], where N indicates the sample size of the exposure database, K indicates the number of SNPs, and R2 denotes the proportion of variation explained by SNPs in the exposure database. The calculation of R2 was performed using the formula: R2 = 2×EAF×(1-EAF)×β2, where EAF represents the effect allele frequency and β represents the allele effect value. The information of the eight IMIDs and the PAD were summarized separately, while the SNPs directly related to PAD were excluded (P<5 × 10− 8).
Statistical analyses
We used the inverse variance weighted (IVW) method as the primary method of analysis, which incorporated Wald ratios to obtain consistent estimates of the causal impact of exposure on outcomes26. In addition, other MR analysis methods, including MR-Egger regression27 and weighted median28, were used to verify the causal relationship between exposures (IMIDs) and outcomes (PAD). The major difference between the MR-Egger and the IVW is the addition of intercept term, which is mainly used to determine the presence of horizontal pleiotropy. Only when at least 50% of the SNPs are valid IVs or valid IVs have more than half of weights can the weighted median method be used to estimate of the causal effect of exposures on outcomes. In addition, false discovery rate(FDR) correction was performed to account for multiple-level testing, with FDR-corrected q < 0.05 set as the predetermined significance threshold29. The TwoSampleMR and MR-PRESSO packages of R software (version 4.2.3) were used for statistical analysis.
Sensitivity analyses
In this study, Cochran’s Q test was employed to evaluate the heterogeneity of the IVW model. The presence of heterogeneity was determined by a significance level of P < 0.05, prompting the utilization of the random-effects model of IVW for causal inference. The results were presented as odds ratios (OR) and confidence interval (CI) and were visualized and represented through scatter plots. MR-Egger regression analysis and funnel plots were employed to assess the potential bias resulting from genetic pleiotropy. The regression intercept of the MR-Egger analysis was used to estimate the magnitude of horizontal pleiotropy, with a value closer to 0 indicating a lower likelihood of horizontal pleiotropy. Moreover, the SNPs were eliminated one by one by performing the leave-one-out sensitivity analysis; the influence of each SNP on the results was observed by calculating remaining SNPs’ combined effect to evaluate the effectiveness and stability of the randomized results. Finally, we carried out reverse MR analysis to evaluate the evidence for reverse causal association.
Results
Selected IVs
According to the SNPs selection criteria(P < 5 × 10− 8, r2 < 0.001, kb = 10,000), independent SNPs for each IMIDs (RA 90, UC 62, CD 89, SLE 45, AS 7, PSO 21, MS 22, and HT 15) were filtered primordially (Supplementary Table S1). Next, we excluded SNPs potential pleiotropy and palindromic SNPs, ultimately 78, 48, 74, 39, 6, 20, 19, and 9 SNPs were selected as IVs for RA, UC, CD, SLE, AS, PSO, MS, and HT on PAD, respectively. According to the MR-PRESSO global test, no potential pleiotropy was detected (P > 0.05) (Table 2). The F-statistics of IVs were all largely > 10, indicating no evidence of weak instrument bias. The details of the IVs in MR analysis were represented in Supplementary Table S2.
Causal effects of IMIDs on PAD
Figure 1 presents the causal effects of IMIDs on PAD based on IVW, MR-Egger, and weighted median. The IVW model yielded evidence of a positive association between RA and PAD (OR = 1.059, 95% CI: 1.026–1.094, p<0.001), which was consistent with the results obtained from MR-Egger regression and weighted median analyses, indicating that the results of MR analysis were reliable. However, no statistically significant associations were observed between UC(OR = 0.952, 95% CI: 0.906–1.001, p = 0.054), CD(OR = 0.988, 95% CI: 0.957–1.020, p = 0.457), SLE(OR = 1.003, 95% CI: 0.981–1.026, p = 0.771), AS(OR = 0.039, 95% CI: 0.001–1.540, p = 0.084), PSO(OR = 1.128, 95% CI: 0.063–20.124, p = 0.935), MS(OR = 1.027, 95% CI: 0.974–1.083, p = 0.331), HT(OR = 1.068, 95% CI: 0.943–1.209, p = 0.302) and PAD. And after FDR adjustment, the causal effect between RA and the increased risk of PAD was still significant(pFDR−corrected =0.002, Fig. 1). Moreover, as shown in Supplementary Table S3, the reverse causality of this study was found only for PAD and CD, however, it did not affect the results for RA. Due to the scarcity of IVs in HT(only one), reverse MR cannot be carried out.
Sensitivity analyses
The results of the MR-Egger regression indicated the absence of significant directional pleiotropy in the analysis, (P > 0.05) (Table 2), which was in line with the results from the scatter plot in Supplementary Figure S1. In the heterogeneity test, the outcome of UC and HT showed substantial heterogeneity based on Cochran’s Q value. Consequently, a random-effects model was employed to minimize the impact of heterogeneity, while the remaining IVW analyses were performed using a fixed-effects model (Table 2, Supplementary Figure S2). The leave-one-out sensitivity analysis depicted in Supplementary Figure S3 did not identify any individual SNP that exerted a strong influence on the causal effect of IMIDs on PAD.
Discussion
This study employed a two-sample MR method to evaluate the causal relationship between IMIDs and PAD. We obtained robust evidence for a causal relationship between RA with increased risk of PAD, supported by consistent effect estimates and directions from various MR methods (including IVW, MR-Egger, Weighted median). It will help us to better understand the genetic impact of RA on PAD. However, no significant causal effects of UC, CD, SLE, AS, PSO, MS, and HT on PAD risk were observed in this analysis.
Observational studies have demonstrated the protective effects of RA on PAD studies30,31,32,33. However, the causal relationship between RA and PAD and its extent remains to be determined. Rinco´n et al.30 conducted a multicenter study involving 234 RA patients and 102 controls to assess the peripheral arterial function by measuring the ankle-brachial index(ABI). The study revealed significantly higher rates of peripheral arterial incompressibility and obstruction in RA patients compared to healthy individuals of the same age and sex, and peripheral arterial dysfunction was also more prevalent in patients with RA. Interestingly the odds ratio for incompressibility in rheumatoid arthritis versus control approached unity upon inclusion of clinical manifestations of RA in the adjustment model, while it decreased slightly upon inclusion of cardiovascular risk factors. It suggested that clinical manifestations, rather than cardiovascular risk factors, were the primary drivers of the increased frequency of arterial abnormalities in RA. In addition, they also speculated that the degree of peripheral arterial impairment was correlated to the severity of RA. A systematic review involving 11 observational studies suggested RA was an independent risk factor for PAD, and the disease manifestations of greater severity increased RA patients’ susceptibility to PAD development34, which was consistent with Rinco´n’s study. Our MR findings aligned with previous studies, supporting a causal relationship between RA and increased risk of PAD. Meanwhile, in contrast to conventional observational studies, MR analysis is less susceptible to confounding factors and reverse causality, rendering it a dependable approach for inferring associations alongside randomized controlled trials (RCTs)35. As such, our findings furnished substantiation for comprehending the genetic risk factors of PAD, established a foundation for subsequent molecular investigations, and hold considerable significance for the prevention and management of PAD in RA patients.
The exact mechanisms of RA and increased risk of PAD have yet to be clarified. Several potential hypotheses have been posited to elucidate the impact of RA on PAD. Firstly, the systemic inflammatory response elicited by RA represents a significant risk factor for PAD. Systemic inflammation was considered to act as a pivotal mediator of endothelial dysfunction. It has been proved that CD4 + CD28- T lymphocytes and inflammatory cytokines such as osteoprotegerin (OPG), tumor necrosis factor α, are known to increase in RA patients, which drive localized inflamation of the synovium as well as the arterial wall, ultimately resulting in endothelial dysfunction(ED) and arterial stiffness36,37. OPG concentrations were independently related to early atherogenesis and atherosclerosis in patients with severe RA who had not experienced cardiovascular events38. Research has demonstrated that OPG concentrations were increased in patients with RA who have CVD compared to those who do not and disease severity was relate to large OPG concentrations39. Secondly, A potential correlation between heightened intimal medial thickness (IMT) and augmented risk of PAD in patients with RA has been identified. IMT has been established as a marker for PAD40. In a study conducted by Stametelopoulos et al.31, femoral IMT was examined in 80 patients with RA who exhibited no apparent cardiovascular risk factors. Their study revealed IMT in the femoral arteries was increased in the patients with RA compared to age-matched controls. Notably, they also demonstrated that the extent of the inflammatory process in patients with RA was directly proportional to the increase in IMT. This might be associated with elevated Galectin-3 levels in RA patients. Galectin-3 is a member of a β-galactosidase binding lectin family which plays an active role in inflammatory response pathways41. A cohort study by Anyfanti et al.42 revealed that the expression of Galectin-3 was increased in RA patients and were correlated with markers of arterial stiffness and cIMT. Thirdly, it is plausible that oxidative stress may exacerbate the burden of atherosclerosis in RA patient. It is due to the heightened formation of reactive oxygen species and elevated levels of markers of protein and lipid oxidation, which can impair peripheral vasodilatory capacity, promote early ED, ultimately accelerate arterial wall stiffening and increase plaque burden43,44.
Our study has furnished initial evidence for plausible causal relationships between RA and PAD. However, it should be noted that the occurrence of IMIDs and PAD is not solely dependent on genetic factors; rather, their onset is the consequence of both genetic and environmental factors. For example, the dysbiosis that occurs due to inflammatory bowel disease(IBD) is also implicated in atherosclerosis and arterial stiffness development. Alterations in the number of gut microbiota and the metabolites it secretes lead to escalated inflammation of vascular endothelium and increased plaque formation in the arteries45,46. Agüero et al.7 carried out a pooled analysis of individual data obtained from two large cross-sectional projects, They found that people who were diagnosed with chronic immune-mediated inflammatory diseases(CIID), including IBD, RA, and SLE, etc., had a significantly higher prevalence of PAD compared to those without CIID. However, when the analysis was adjusted for age, sex, and other potential confounders or intermediate variables, the association between IBD, SLE and PAD disappeared, which suggested that environmental factors play a crucial role in the pathogenesis of IMIDs. Additionally, The potential impact of medications utilized for the treatment of IMIDs is also an important confounder. Biologics, such as TNF-α inhibitors, may reduce PAD risk in patients with IMIDs by reducing the inflammatory response. Some studies have suggested that TNF-α inhibitors may have a cardiovascular protective effect, thereby reducing the incidence of PAD. However, this protective effect may vary depending on the patient’s specific condition and duration of treatment47. Conventional immunosuppressants, such as methotrexate, control the disease by suppressing the immune response, but the effect on PAD risk is unclear. It was reported that methotrexate may reduce the risk of cardiovascular events, but its specific effects on PAD require further study48. On the other hand, long-term use of medications such as glucocorticoids may increase the risk of blood pressure, blood sugar, and lipid abnormalities, thereby indirectly increasing the risk of PAD49.
The current investigation offered several merits. First, in contrast to randomized controlled trials, MR analysis is more expedient and economical, conferring an edge in scrutinizing and filtering potential causal associations. Additionally, MR studies mitigate confounding bias and circumvent reverse causality. Second, this study implemented rigorous quality control measures and rational analytical techniques, such as the utilization of multiple models to assess causal effects. As a result, the findings of this study are dependable and consistent. Third, we used data from a large GWAS, which provided a sufficient sample size for high statistical efficacy.
At the same time, there were some limitations. First of all, almost GWASs data were mainly from European populations, and the findings of this study couldn’t be applied to other populations. Secondly, given the limitation of statistical approaches and interference of other confounding factors, it is impractical to fully adhere to the three assumptions of MR analysis. Thirdly, IVs associated with the traits under investigation accounted for a limited proportion of the variability in risk factors, and the relationship between them remained theoretical. Due to the limited variability in SNPs explanations, the estimated causal effects may deviate from the true effect size. While acknowledging the limitations of the study design and potential biases, the selection of SNPs in this analysis remains a robust approach to identifying genetic factors that are strongly associated with key exposure variables, although the findings should be interpreted with caution given the inherent limitations of the methodology. Given the above considerations, a large cohort study will be necessary in the future to more comprehensively evaluate the relationship between IMIDs and the prevalence of PAD. To minimize the impact of confounders such as diabetes, hypertension, BMI, and cholesterol on the findings, sophisticated logistic regression models which adjusting for these confounders will be employed, thereby enhancing the robustness and accuracy of the results.
Conclusion
In conclusion, this study employed two-sample MR analysis method to examine the association between IMIDs and the risk of PAD. Our analysis supported the causal association of RA with PAD which help enhance the clinical management and prevention of PAD in populations with RA. However, no conclusive evidence was found for causal associations between other IMIDs, including UC, CD, SLE, AS, PSO, MS, and HT, and PAD. Further studies are warranted to corroborate our findings and to unravel molecular mechanisms.
References
Tran, B. Assessment and management of peripheral arterial disease: what every cardiologist should know. Heart (British Cardiac Society). 107 (22), 1835–1843 (2021).
Halliday, A. & Bax, J. J. The 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (ESVS). Eur. J. Vascular Endovascular Surgery: Official J. Eur. Soc. Vascular Surg. 55 (3), 301–302 (2018).
Heidemann, F. et al. A health insurance claims analysis on the effect of female sex on long-term outcomes after peripheral endovascular interventions for symptomatic peripheral arterial occlusive disease. J. Vasc. Surg. 74 (3), 780–787e787 (2021).
Song, P. et al. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Global Health. 7 (8), e1020–e1030 (2019).
Campia, U., Gerhard-Herman, M., Piazza, G. & Goldhaber, S. Z. Peripheral artery disease: past, Present, and future. Am. J. Med. 132 (10), 1133–1141 (2019).
Pohlman, F. W. et al. Impact of risk factor control on peripheral artery disease outcomes and health disparities . (2022). Vascular medicine (London, England), 27(4): 323–332 .
Agüero, F. et al. Prevalence of lower extremity peripheral arterial disease in individuals with chronic immune mediated inflammatory disorders.Atherosclerosis 242 (1), 1–7 (2015).
Mcinnes, I. B. & Gravallese, E. M. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat. Rev. Immunol. 21 (10), 680–686 (2021).
Chuang, Y. W. et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb. Haemost. 115 (2), 439–445 (2016).
Lin, T. Y., Chen, Y. G., Lin, C. L., Huang, W. S. & Kao, C. H. Inflammatory bowel disease increases the risk of peripheral arterial disease: a nationwide Cohort study. Medicine 94 (52), e2381 (2015).
Hassan, A. A., Habib, H. M. & Eissa, A. A. Peripheral arterial disease in patients with systemic lupus erythematosus: a prospective controlled study. Int. J. Rheum. Dis. 16 (3), 319–324 (2013).
Forte, F. et al. Association of systemic lupus erythematosus with peripheral arterial disease: a meta-analysis of literature studies. Rheumatol. (Oxford England). 59 (11), 3181–3192 (2020).
Charlton, R. et al. Risk of type 2 diabetes and cardiovascular disease in an incident cohort of people with psoriatic arthritis: a population-based cohort study. Rheumatol. (Oxford England). 58 (1), 144–148 (2019).
Hemani, G., Bowden, J. & Davey Smith, G. Evaluating the potential role of pleiotropy in mendelian randomization studies. Hum. Mol. Genet. 27 (R2), R195–r208 (2018).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. Jama 326 (16), 1614–1621 (2021).
Dikilitas, O., Satterfield, B. A. & Kullo, I. J. Risk factors for Polyvascular involvement in patients with peripheral artery disease: a mendelian randomization study. J. Am. Heart Association. 9 (24), e017740 (2020).
Hoek, A. G., Van Oort, S., Elders, P. J. M. & Beulens, J. W. J. Causal association of cardiovascular risk factors and lifestyle behaviors with peripheral artery disease: a mendelian randomization approach. J. Am. Heart Association. 11 (16), e025644 (2022).
Levin, M. G. et al. Prioritizing the role of Major Lipoproteins and subfractions as risk factors for peripheral artery disease. Circulation 144 (5), 353–364 (2021).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ (Clinical Res. ed), 362k601. (2018).
De Lange, K. M. et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 49 (2), 256–261 (2017).
Andlauer, T. F. et al. Müller-Myhsok B. (2016). Novel multiple sclerosis susceptibility loci implicated in epigenetic regulation. Science advances, 2(6): e1501678 .
Sakaue, S. et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 53 (10), 1415–1424 (2021).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife, 7 (2018).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat. Genet. 50 (5), 693–698 (2018).
Weng, H. et al. Association between uric acid and risk of venous thromboembolism in East Asian populations: a cohort and Mendelian randomization study. The Lancet regional health Western Pacific, 39(100848. (2023).
Burgess, S., Bowden, J., Fall, T., Ingelsson, E. & Thompson, S. G. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology (Cambridge, Mass), 28(1): 30–42. (2017).
Slob, E., a W, Groenen, P. J. F., Thurik, A. R. & Rietveld, C. A. A note on the use of Egger regression in mendelian randomization studies. Int. J. Epidemiol. 46 (6), 2094–2097 (2017).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in mendelian randomization with some Invalid instruments using a weighted median estimator. Genet. Epidemiol. 40 (4), 304–314 (2016).
Zuber, V. et al. High-throughput multivariable mendelian randomization analysis prioritizes apolipoprotein B as key lipid risk factor for coronary artery disease. Int. J. Epidemiol. 50 (3), 893–901 (2021).
Del Rincón, I., Haas, R. W., Pogosian, S. & Escalante, A. Lower limb arterial incompressibility and obstruction in rheumatoid arthritis. Ann. Rheum. Dis. 64 (3), 425–432 (2005).
Stamatelopoulos, K. S. et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis 212 (1), 305–309 (2010).
Crowson, C. S. et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis7748–54 (Annals of the rheumatic diseases, 2018). 1.
Yang, Y. et al. Stiffening of aorta is more preferentially associated with rheumatoid arthritis than peripheral arteries. Rheumatol. Int. 39 (10), 1711–1721 (2019).
Zoubi, T. & Gordon, H. Systematic review of associations between concomitant rheumatoid arthritis and peripheral arterial disease, health-related quality of life and functional capacity. Rheumatol. Int. 43 (2), 221–232 (2023).
Birney, E. Mendelian randomization. Cold Spring Harbor Perspect. Med. 12 (4). J (2022).
Figus, F. A., Piga, M., Azzolin, I., Mcconnell, R. & Iagnocco, A. Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmun. rev. 20 (4), 102776 (2021).
Mellana, W. M., Aronow, W. S., Palaniswamy, C. & Khera, S. Rheumatoid arthritis: cardiovascular manifestations, pathogenesis, and therapy. Curr. Pharm. Design. 18 (11), 1450–1456 (2012).
Dessein, P. H. et al. Independent relationship of osteoprotegerin concentrations with endothelial activation and carotid atherosclerosis in patients with severe rheumatoid arthritis. J. Rhuematol. 41 (3), 429–436 (2014).
López-Mejias, R. et al. Osteoprotegerin concentrations relate independently to established cardiovascular disease in rheumatoid arthritis. J. Rhuematol. 42 (1), 39–45 (2015).
Sedrakyan, S. et al. Evaluation of the risk of getting peripheral artery Disease in rheumatoid arthritis and the selection of appropriate diagnostic methods. Cureus 12 (8), e9782 (2020).
Harvey, A., Montezano, A. C., Lopes, R. A., Rios, F. & Touyz, R. M. Vascular fibrosis in aging and hypertension: Molecular mechanisms and clinical implications. Can. J. Cardiol. 32 (5), 659–668 (2016).
Anyfanti, P. et al. Association of galectin-3 with markers of myocardial function, atherosclerosis, and vascular fibrosis in patients with rheumatoid arthritis. Clin. Cardiol. 42 (1), 62–68 (2019).
Bassu, S. et al. Oxidative stress biomarkers and peripheral endothelial dysfunction in rheumatoid arthritis: a monocentric cross-sectional case-control study. Molecules (Basel Switzerland), 25(17): (2020).
England, B. R., Thiele, G. M., Anderson, D. R. & Mikuls, T. R. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. (2018). BMJ (Clinical research ed), 361k1036 .
Huć, T., Nowinski, A., Drapala, A., Konopelski, P. & Ufnal, M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol. Res. 130 (null), 172–179 (2018).
Maloy, K. J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474 (7351), 298–306 (2011).
England, B. R., Thiele, G. M., Anderson, D. R. & Mikuls, T. R. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ (Clinical Res. ed). 361 (null), k1036 (2018).
Golledge, J., Velu, R., Quigley, F., Jenkins, J. & Singh, T. P. Cohort study examining the association of immunosuppressant drug prescription with major adverse cardiovascular and limb events in patients with peripheral artery disease. Ann. Vasc. Surg. 78, 310–320 (2022).
Pujades-Rodriguez, M., Morgan, A. W., Cubbon, R. M. & Wu, J. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLoS Med. 17 (12), e1003432 (2020).
Ha, E., Bae, S. C. & Kim, K. Large-scale meta-analysis across east Asian and European populations updated genetic architecture and variant-driven biology of rheumatoid arthritis, identifying 11 novel susceptibility loci. Ann. Rheum. Dis. 80 (5), 558–565 (2021).
Bentham, J. et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat. Genet. 47 (12), 1457–1464 (2015).
Acknowledgements
The authors thank the GWAS Catalog, FinnGen biobank, and UK Biobank for making the data available.
Funding
This article was found by Health New Technology Product Research and Development Project of Zhejiang Province(2021PY030).
Author information
Authors and Affiliations
Contributions
LZ and QW designed the study. HW and DZ conducted most of the MR analysis and were major contributors in writing the manuscript. TW drawn tables and pictures. SL was responsible for revising the article and funded the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, H., Zheng, D., Zhou, L. et al. Genetic association between immune-mediated inflammatory diseases and peripheral artery disease: a Mendelian randomization study. Sci Rep 15, 3891 (2025). https://doi.org/10.1038/s41598-024-82987-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-82987-3