Abstract
Resolvin D1 (RvD1) is an endogenous anti-inflammatory mediator that modulates the inflammatory response and promotes inflammation resolution. RvD1 has demonstrated neuroprotective effects in various central nervous system contexts; however, its role in the pathophysiological processes of intracerebral hemorrhage (ICH) and the potential protective mechanisms when combined with exercise rehabilitation remain unclear. A mouse model of ICH was established using collagenase, and treatment with RvD1 combined with three weeks of exercise rehabilitation significantly improved neurological deficits, muscle strength, learning, and memory in ICH mice while reducing anxiety-like behavior. RvD1 combined with exercise rehabilitation upregulated anti-inflammatory factors, inhibited the inflammatory state, and activated the BDNF/TrkB/PI3K/AKT pathway. TUNEL staining confirmed a decrease in residual apoptotic neurons, while transmission electron microscopy showed an increase in mitochondrial autophagosomes with combined treatment. Mendelian randomization and molecular docking further confirmed the association of RvD1 with targets related to mitophagy and inflammatory factors, clarifying the mechanism of RvD1 involvement. In summary, RvD1 combined with exercise rehabilitation activates the BDNF/TrkB/PI3K/AKT signaling pathway, effectively reduces neuronal apoptosis and inflammatory responses following ICH in mice, and participates in mitochondrial autophagy-related states. This comprehensive therapeutic strategy promotes neurological recovery and provides insights for clinical management of this condition.
Similar content being viewed by others
Introduction
Intracerebral hemorrhage (ICH) is defined as the non-traumatic rupture of blood vessels within brain tissue, resulting in bleeding either in the brain itself or in the subarachnoid space.ICH is a highly severe condition with poor recovery rates, and mortality rates ranging from 35 to 52% within the first 30 days post-onset. Many survivors experience lasting disabilities, often unable to regain independence in daily living skills even six months after the hemorrhagic event1. The secondary injury associated with ICH is driven by complex pathological mechanisms, including inflammation, autophagy, oxidative stress, mitochondrial dysfunction, blood-brain barrier disruption, and neuronal apoptosis. Together, these processes aggravate the primary injury, leading to extensive functional and structural neuronal damage2.Unlike other types of stroke, no definitive treatments exist for ICH. Clinical data suggest that interventions such as minimally invasive surgery and surgical clot removal mainly alleviate mechanical compression of adjacent brain tissue, with limited efficacy in reducing primary brain injury.Furthermore, these surgical approaches rarely yield substantial improvements in neurological recovery, and the recurrence rate remains high3.
Resolvin D1 (RvD1) is a critical endogenous anti-inflammatory mediator derived from the metabolism of docosahexaenoic acid via 5-lipoxygenase. RvD1 plays a pivotal role in controlling inflammation, actively promoting its resolution and maintaining local homeostasis. It has demonstrated protective effects in various central nervous system (CNS) disorders, including stroke4, Alzheimer’s disease5,traumatic brain injury6, cognitive dysfunction7and Parkinson’s disease8.The brain, as an energy-demanding organ, relies on mitochondrial metabolism to support physiological functions. ICH damages mitochondria, disrupting the electron transport chain (ETC), reducing ATP production, and increasing reactive oxygen species (ROS) generation9. Mildly damaged mitochondria can compensate for dysfunction through fusion or fission with healthy mitochondria, while severely damaged mitochondria are cleared via mitophagy10. Mitophagy selectively engulfs damaged mitochondria in autophagosomes, which then fuse with lysosomes to fully degrade them11. Numerous studies have shown that mitochondrial dysfunction is a major contributor to CNS diseases12. However, research on mitophagy in the context of ICH is still in its early stages. In recent years, modulating mitophagy has emerged as a promising approach for ICH treatment. Pharmacological regulation of mitophagy has demonstrated potential in reducing ICH-induced inflammation and oxidative stress, offering a more convenient and effective alternative to current therapies13. Currently, only a limited number of drugs have been identified to effectively regulate mitophagy in ICH, underscoring the need to explore new therapeutic strategies.
Most patients with ICH experience persistent physical impairments, with motor dysfunction accounts for the majority. Exercise rehabilitation therapy is a clinical non-invasive treatment method aimed at improving sensorimotor function and promoting the recovery of neurological function14. Existing clinical trial data and animal models consistently show that exercise rehabilitation significantly enhances motor recovery following ICH, reducing disability rates and improving quality of life15,16. Kinoshita et al.17reported that six weeks of treadmill preconditioning promotes neurological recovery in ICH mice by reducing lesion volume and increasing the number of phagocytic microglia. Furthermore, exercise rehabilitation has been shown to boost neural activity in the hippocampus, reduce neuronal damage and apoptosis, and alleviate inflammation around the hematoma. It also promotes collateral nerve circulation, facilitates the formation of new synaptic connections, enhances neuronal excitability and plasticity, and supports tissue reorganization around the lesion. These effects collectively aid in compensatory processes within contralateral brain regions, contributing to the recovery of motor and cognitive functions18.
Brain-derived neurotrophic factor (BDNF) plays a critical role in neurodevelopment by promoting neuronal survival and differentiation, as well as regulating axonal and dendritic growth and morphology19. Alomari et al.20reported that four weeks of exercise training in rats resulted in increased BDNF expression around the hematoma and improved motor function. Studies have indicated that following ICH, BDNF expression in the hippocampus decreases during the acute and subacute phases (up to 16 days), which is associated with elevated levels of neuroinflammatory markers and neuronal apoptosis21. In addition, Lee et al.22demonstrated that BDNF stem cell transplantation increased neuronal survival by approximately threefold at two and eight weeks after ICH, effectively promoting neurological recovery.Tropomyosin receptor kinase B (TrkB) is a high-affinity receptor for BDNF. When a ligand binds to the cell surface, TrkB dimerizes, selectively activating phosphoinositide 3-kinase (PI3K) and promoting neurogenesis, gliogenesis, neurite growth, and enhanced neuronal survival23. Furthermore, the BDNF/TrkB signaling pathway is critical not only for the treatment of ICH but also represents a significant therapeutic target in various neurodegenerative diseases such as Alzheimer’s disease24. PI3K is a critical lipid kinase that induces the phosphorylation and activation of Akt. Activated Akt regulates multiple cellular processes, including proliferation, survival, apoptosis, and metabolism, by phosphorylating various downstream target proteins25. The PI3K/Akt pathway serves as a prototypical anti-apoptotic signaling cascade; when activated by BDNF and TrkB, it upregulates Akt phosphorylation, thereby activating anti-apoptotic mechanisms, decreasing levels of pro-apoptotic proteins, and inhibiting apoptosis26. Xu et al.27 demonstrated that this pathway is essential for promoting neuronal survival and plasticity, providing critical protective effects for neuronal function during subarachnoid hemorrhage, while also exhibiting anti-inflammatory, anti-apoptotic, and anti-oxidative stress effects. Hitoshi et al.28reported that the classical mitophagy pathway, involving AKT signaling, plays a pivotal role in mediating inflammatory responses and can be regulated clinically to protect nerve cells in brain tissue.
In this study, we aim to investigate whether RvD1 combined with exercise rehabilitation training can protect against neurological deficits, improve cognitive function, and reduce reducing neuronal apoptosis and inflammation in mice with ICH by activating the BDNF/TrkB/PI3K/AKT signaling pathway, and to explore its relationship with mitophagy.
Results
ICH and grouped mortality
After the preliminary experiment, we performed brain dissections on the mice and observed that the ICH was primarily localized to the Willis ring and abdominal regions. Subsequently, spiral computed tomography (CT) scans and tissue slices were conducted to confirm the successful establishment of the ICH model and to identify its location within the right caudate nucleus. Measurements using Mimics software indicated that the area of the ICH region was approximately 4.68 mm2 (Fig. 1a).In the Sham group, the ICH group, and the ICH + Vehicle + exercise training, no mice died (0/10). In contrast, the mortality rates in the ICH + CCCP group, ICH + Mdivi-1 + exercise training group, and ICH + RvD1 + exercise training group were 3/13, 5/15, and 1/11, respectively.
Rehabilitation training combined with RvD1 improved neurological functions after ICH
Compared with Sham group, all ICH animals showed significant neurological deficits.Compared to the Sham group, the neurological deficit in the ICH group were significantly higher on day 1 (P = 0.0004), day 5 (P < 0.0001), day 10 (P = 0.0002), day 14 (P = 0.0003), and day 21 (P = 0.0010) post-ICH (Fig. 1b). On day 1 post-surgery, there were no statistically significant differences in neurological deficit scores among the groups other than the Sham group (P > 0.05).Mice in the ICH group that began rehabilitation training on day 3 showed some improvement in neurological function compared to the ICH model group, but only on days 14 (P = 0.0183) and 21 (P = 0.0369) did the neurological deficit scores show statistical significance.The neurological deficit scores in the ICH + CCCP group decreased compared to the ICH group, with statistically significant changes on day 10 (P = 0.0059) and day 14 (P = 0.0179). The ICH + RvD1 + exercise training group showed the most significant improvement in neurological deficits, with statistically significant changes on day 5 (P = 0.0427), day 10 (P = 0.0022), day 14 (P = 0.0248), and day 21 (P = 0.0071).The ICH + Mdivi-1 + exercise training group also demonstrated improvements in neurological deficits, with statistically significant changes on day 10 (P = 0.0035), day 14 (P = 0.0149), and day 21(P = 0.0049).On day 21 post-surgery, neurological function testing showed significant improvements compared to the ICH group in the following groups: ICH + Vehicle + exercise training group(P = 0.0369), ICH + CCCP (P = 0.0407), ICH + RvD1 + exercise training group (P = 0.0071), and ICH + Mdivi-1 + exercise training group (P = 0.0274, Fig. 1c). In the muscle strength test, the ICH + RvD1 + exercise training group (P = 0.0077) and the ICH + Mdivi-1 + exercise training group (P = 0.0239) showed significant statistical differences compared to the ICH group, indicating a notable improvement in muscle strength (p < 0.05, Fig. 1d).
RvD1 combined with exercise rehabilitation training mitigates neurologic deficits and motor function after ICH. (a) Localization map of the caudate nucleus and representative tissue sections and CT scans of mice with ICH.Cerebral hemorrhage was evaluated at D1, D5, D10, D14, and D21 using the modified mNSS score(b,c) and muscle strength tests (d) to assess neurological dysfunction in each group. The results demonstrated that RvD1 combined with exercise rehabilitation training significantly improved neurological function in mice with cerebral hemorrhage at D5, D10, D14, and D21 (n = 10, data presented as mean ± SEM, *significant vs. ICH group, p < 0.05).
Rehabilitation training and RvD1 improve anxiety-like behaviors after ICH
Compared to the Sham group, mice in the ICH group exhibited significantly reduced movement trajectories in the open field(Fig. 2a). and a lower center-to-total (C/T) distance ratio (P < 0.0001,Fig. 2c), indicating anxiety-like behaviors. Mice in the groups that received rehabilitation training (including the ICH + Vehicle + exercise training group, ICH + Mdivi-1 + exercise training group, and ICH + RvD1 + exercise training group) showed increased movement distance(Fig. 2b) and a higher C/T distance ratio(Fig. 2c), along with improved speed, indicating a reduction in anxiety-like behaviors (p < 0.05, Fig. 2d). Additionally, the average speed of mice in the ICH + Vehicle + exercise training group increased compared to the ICH group (P = 0.0224), and further improvement in movement ability was observed with the addition of Mdivi-1 treatment (P = 0.0030). The ICH + RvD1 + exercise training group showed a more significant increase in average speed (P = 0.0009), leading to a marked improvement in movement ability, with almost no significant difference compared to the Sham group, demonstrating the effectiveness of RvD1 combined treatment.
RvD1 combined with rehabilitation training improves spatial learning and memory function after ICH
Representative trajectories of mice in each group during the water maze test are shown (Fig. 2f). Compared with the Sham group, the difference in the time to find the hidden platform in the ICH group gradually increased from 22 to 26 days after the operation (p < 0.05,Fig. 2i). Mice in the intervention exercise rehabilitation training showed significant improvement. Additionally, mice in the ICH + RvD1 + exercise training group, ICH + CCCP group, and ICH + Mdivi-1 + exercise training group had a significantly shorter time to find the hidden platform compared to the ICH model group (p < 0.05, Fig. 2i). After the platform was removed on day 27, mice in the RvD1 combined exercise training group and the CCCP group crossed the quadrant of the target platform and exploration time significantly more times than those in the ICH model group (p < 0.05, Fig. 2g and h). The speed of each group of mice was also prolonged, but the difference was not statistically significant (Fig. 2e).
RvD1 combined with exercise rehabilitation training improves spatial learning and memory ability and reduces anxiety in mice after ICH. The open field test and water maze test were used to study the motor activity and spatial learning and memory of the six groups of mice. (a) Representative movement trajectories of each group of mice in the open field test. The open field test showed that RvD1 combined with exercise rehabilitation training improved activity, as indicated by total distance (b), center/total(C/T) distance (c), and average speed (d). The water maze test showed that RvD1 combined with exercise rehabilitation training enhanced spatial learning and memory in mice. (f) Representative swimming trajectories of mice in each group in the water maze test. Mice were assessed for escape latency (i), speed (e), number of crossings over the target platform (g), and exploration time in the target quadrant (h). Data are presented as mean ± SEM. (* significant vs. ICH group, p < 0.05).
RvD1 combined with rehabilitation training plays a protective role in neuroinflammation after ICH
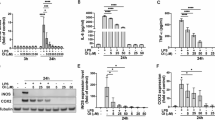
To verify whether RvD1 Combined with Rehabilitation Training affects ICH-related inflammatory responses, we selected anti-inflammatory factors (CD206 and IL-10) and inflammation-related factors (IL-1β and TNF-α) for RT-qPCR analysis. Our results confirmed that, compared to mice with only cerebral hemorrhage, the expression levels of anti-inflammatory factors were significantly increased(p < 0.05, Fig. 3a and b), while the mRNA expression levels of inflammation-related factors were significantly decreased (p < 0.05, Fig. 3c and d) in mice treated with RvD1 combined with exercise rehabilitation training.To investigate the role of RvD1 in promoting inflammation resolution, we further used Mendelian randomization (MR) to examine the effects of RvD1 on circulating inflammatory factors (Table S2). The results show that RvD1 can inhibit the expression of the pro-inflammatory factor IL20RA (P < 0.05, Fig. 3e) and increase the expression of the anti-inflammatory relative factors CCL25 and LIFR (P < 0.05, Fig. 3e). In conclusion, RvD1, as a pro-resolving mediator, in combination with exercise rehabilitation training, can promote inflammation resolution following cerebral hemorrhage in mice.
RvD1 inhibited the mRNA expression of anti-inflammatory related factors (CD206 and IL-10) and inflammation-related states(IL-1β and TNF-α) after ICH.Expression levels of CD206 (a), IL-10 (b), IL-1β (c), and TNF-α (d) mRNAs.Data are presented as mean ± SEM. (* significant vs. ICH group, p < 0.05).(e) Volcano plot of causality between RvD1 and circulating inflammatory factors.
Effects of RvD1 combined with rehabilitation training on the expression of BDNF, PI3K, AKT and TrkB related proteins in mice with ICH
Western blot analysis showed that the expression of AKT was relatively consistent across all six groups, without significant differences. Compared to the sham group, the levels of BDNF, PI3K, p-AKT, and TrkB protein expression were significantly reduced in the ICH model mice (p < 0.05, Fig. 4a-e). Compared to the ICH group, the expression levels of BDNF, PI3K, p-AKT, and TrkB increased after exercise rehabilitation training but did not show statistically significant differences (p > 0.05). Mice treated with intraperitoneal injections of Mdivi-1 in combination with exercise rehabilitation training also showed some increase in the expression levels of these proteins, but again without statistical significance. Mice treated with intraperitoneal injections of CCCP showed a significant increase in the expression levels of BDNF, PI3K, p-AKT, and TrkB (p < 0.05). Additionally, ICH + RvD1 + exercise training group on day 28 significantly promoted the expression levels of BDNF, PI3K, p-AKT, and TrkB, comparable to the levels seen with intraperitoneal injections of CCCP, showing statistical significance compared to the ICH group (p < 0.05).
Effects of RvD1 combined with rehabilitation training on the mRNA expression of BDNF, PI3K, AKT, and TrkB in mice with ICH
Compared with the Sham group, the mRNA expression levels of BDNF, PI3K, AKT, and TrkB in the model control group were significantly down-regulated (P < 0.05; Fig. 4f-i). Compared with the ICH group, the mRNA expression levels of BDNF, PI3K, AKT, and TrkB were up-regulated following exercise rehabilitation treatment, although the differences were not statistically significant (P > 0.05). While the mRNA expression levels of BDNF, PI3K, AKT, and TrkB were marginally elevated after intraperitoneal injection of Mdivi-1 in conjunction with exercise rehabilitation treatment, only PI3K (P = 0.0108) and AKT (P = 0.0144) showed statistical significance. When mice received intraperitoneal injections of CCCP without any additional treatments, the mRNA expression levels of BDNF, PI3K, AKT, and TrkB were significantly increased (P < 0.05). In mice that received a combination of RvD1 and exercise training, the mRNA expression levels of BDNF, PI3K, AKT, and TrkB were also significantly increased (P < 0.05).
RvD1 combined with exercise rehabilitation training can activate BDNF/TrκB/p-Akt/PI3K related pathway and increase its protein and mRNA expression. (a) Western blot analysis was used to detect the expression levels of BDNF, TrκB, PI3K proteins, and both phosphorylated and total Akt. (b-d) Quantitative analysis of BDNF, TrκB, and PI3K protein expressions in brain tissue adjacent to the injection site on the ipsilateral side after the experiment. (e) Semiquantitative measurements of the ratio of phosphorylated to total Akt. (f-i) Expression levels of BDNF (f), TrκB (g), PI3K (h), and Akt (i) mRNAs at the injection site were determined. Western blot and RT-qPCR analyses revealed that the expression levels of BDNF, TrκB, p-Akt, and PI3K in the ICH + RvD1 + exercise training group were significantly higher compared to those in the ICH group. (*Significant vs. ICH group, p < 0.05).
RvD1 combined with rehabilitation training reduces neuronal apoptosis after ICH
TUNEL staining was employed to assess the changes in apoptotic neurons surrounding the injection sites across six experimental groups of mice. The results indicated that, in comparison to the Sham group, there was a significant increase in the number of TUNEL-positive apoptotic neurons in the ICH group (Fig. 5). Rehabilitation training alone led to a reduction in the number of TUNEL-positive neurons within the ICH group. Furthermore, the addition of RvD1 and Mdivi-1 resulted in an even greater decrease in TUNEL-positive neuron counts (p < 0.05). Additionally, the number of apoptotic neurons in CCCP-treated mice was also greatly improved compared with the ICH group (p < 0.05).
RvD1 Combined with Exercise Rehabilitation Training Reduces Neuronal Apoptosis Post-ICH. (a) Representative TUNEL/NeuN micrographs of different groups (scale bar = 50 μm). Fluorescence colors: TUNEL (green) and NeuN (blue). The box in (b) indicates the area where apoptosis was measured. (c) Quantification of the number of TUNEL/NeuN-positive cells in each group. (*Significant vs. ICH group, p < 0.05).
RvD1 combined with exercise rehabilitation training can affect mitophagy in mice
Transmission electron microscopy (TEM) was used to observe the ultrastructural changes in cells surrounding the hemorrhage in the Sham group, ICH group, ICH + CCCP group, ICH + exercise training group, ICH + RvD1 + exercise training group, and ICH + Mdivi-1 + exercise training group (Fig. 6). The formation of early autophagic mitochondrial autophagosomes can be identified by the characteristic double membrane and cristae of mitochondria, while the fusion of mitochondrial autophagosomes with lysosomes can be roughly identified by their single membrane or residuals after digestion.In all six groups, neuronal cells exhibited varying degrees of damage. The cell membranes showed different levels of rupture, and the cytoplasm exhibited edema, becoming sparse and dissolved. Organelles were notably swollen, mitochondria were enlarged with significant matrix dissolution, and the cristae showed clear breakage and disappearance, with severe cases exhibiting vacuolation. Additionally, the quantity of rough endoplasmic reticulum decreased, local membrane damage was observed, and ribosomes were visible on the surface; the Golgi apparatus showed expansion of some cisternae.
Different numbers of autophagic lysosomes were observed across the groups. Neuronal cells in the Sham group displayed only mild structural damage, with typical autophagic structures not observed. In the ICH group, neuronal cells showed more pronounced damage, with only one autophagic lysosome structure observed. The ICH + exercise training group displayed two autophagic lysosome structures. The ICH + CCCP group had a higher number of autophagic lysosomes, with four observed. In the ICH + RvD1 + exercise training group, three autophagic lysosome structures were visible, while in the ICH + Mdivi-1 + exercise training group, one autophagic lysosome structure was noted.By comparing the differences between the groups, it can be inferred that RvD1 is involved in activities related to mitochondrial autophagy.
Effects of RvD1 combined with exercise rehabilitation training on mitophagy in mice after intracerebral hemorrhage. TEM was used to observe the mitochondrial morphology of neurons in the Sham group, ICH group, ICH + CCCP group, ICH + exercise training group, ICH + RvD1 + exercise training group, and ICH + Mdivi-1 + exercise training group. Significant changes in autophagy levels were observed in these groups. N = Nucleus, Nu = Nucleolus, M = Mitochondria, RER = Rough endoplasmic reticulum, Go = Golgi apparatus, Lib = Lipofuscin.
Mendelian randomization analysis reveals the causal relationship between mitophagy-related targets in the caudate basal ganglia of the brain and ICH
We identified MR-related targets located in the Brain Caudate basal ganglia. To further explore the relationship between mitochondrial autophagy and ICH, we employed Mendelian randomization methods to analyze the causal relationship between the relevant targets and ICH. However, due to the lack of data or valid instrumental variables, this study included a total of 8 relevant targets to assess their relationship with ICH. We used the random-effects IVW (IVW-MRE) method as the primary analysis, and the causal relationships between each core target and the relevant outcomes are illustrated in the figure, with the targets supporting causal relationships including MFN2, RAB7B, and TOMM7 (Fig. 7A, p < 0.01). Subsequently, we assessed the sensitivity of the results using MR Egger, weighted median, simple mode, weighted mode, and inverse variance weighted methods. The results showed that the findings for RAB7B were consistent, while the results for TOMM7 were supported by all methods except MR Egger(Fig. 7b-d). The funnel plot of causal effects appeared largely symmetrical (Figure S1a-c). Leave-one-out analysis demonstrated that removing each single nucleotide polymorphism (SNP) still produced consistent results in the remaining Mendelian randomization analyses, thus supporting the robustness of the findings ( Figure S1 d-e). Additionally, we did not find significant heterogeneity in our analysis. MR-Egger regression analysis indicated that there was no evidence of horizontal pleiotropy (p > 0.05; Table S3). In summary, this study clarified the relationship between mitochondrial autophagy-related targets and ICH outcomes through Mendelian randomization. Interestingly, this finding is consistent with our previous experimental results, suggesting that RvD1 may influence mitochondrial autophagy, thereby alleviating neuronal damage following ICH.
(a)Mendelian Randomization Results for Mitophagy-Related Targets in the Brain Caudate basal ganglia on ICH. nSNP = number of SNPs included in the analysis; OR = odds ratio; CI = confidence interval; ICH = Intracranial hemorrhage.Scatter plots for the causal association between MFN2, RAB7B, and TOMM7 and ICH. SNP effects were plotted into lines for the inverse variance-weighted test (light blue line), multiplicative random effects (dark blue line), MR Egger (light green line), Simple mode (dark green line), Weighted median (light red line) and Weighted mode(dark red line).The slope of the line corresponded to the causal estimation. (b)MFN2,(c)RAB7B, (d)TOMM7.
Molecular docking was used to analyze the binding affinity between RvD1 and the core target of mitophagy after ICH
To assess the binding affinity between RvD1 and its core targets, we conducted molecular docking analyses using Autodock Vina v.1.2.2. Based on the positive results with P < 0.01 from the MR analysis, we selected the relevant proteins as core targets for simulated docking. The binding forms and interactions of RvD1 with MFN2 and RAB7B are shown (Fig. 8). The findings indicated that RvD1 can form stable complexes with the target proteins through hydrogen bonding. Additionally, the binding energies of MFN2, RAB7B, and TOMM7 with RvD1 were − 7.1 kcal/mol, -5.3 kcal/mol, and − 4.4 kcal/mol, respectively, indicating a certain stability of these molecular interactions.In summary, these results suggest that RvD1 may alleviate damage caused by ICH by modulating key proteins involved in mitochondrial autophagy-related pathways.
Discussion
In this study, we evaluated the potential neuroprotective effects of RvD1 combined with exercise rehabilitation in a mouse model of ICH and explored the possible biological mechanisms. The main observations are as follows: (1)After ICH, treatment with RvD1 combined with rehabilitation training exerted neuroprotective effects by reducing neurological deficits, neuronal apoptosis, and inflammation.(2)RvD1 combined with exercise rehabilitation training significantly increased the expression of BDNF/TrkB/PI3K/AKT proteins and mRNA in the right basal ganglia region of the brain hemorrhage site.(3)These protective effects of RvD1 combined with exercise rehabilitation training may be associated with the promotion of mitochondrial autophagy.(4)RvD1 combined with exercise rehabilitation training reduced anxiety-like behavior and enhanced spatial learning and memory abilities in ICH mice.Therefore, RvD1 combined with exercise rehabilitation training may represent a potential therapeutic strategy for ICH.
The inflammatory response following ICH can cause a series of secondary pathological changes, including disruption of the blood-brain barrier, apoptosis, oxidative stress, and mitochondrial dysfunction29. Numerous studies have shown that significant neuroinflammatory responses are observed from days 3 to 21 after ICH in various animal models30,31. Reports have indicated that apoptosis and neuronal loss were observed at 3, 7, and 14 days after ICH32. These results suggest that neuroinflammation and apoptosis may occur early after ICH and persist throughout the acute and even chronic phases. Traditionally, the resolution of inflammation has been considered a passive process, but recent studies have shown that specialized pro-resolving lipid mediators (SPMs) can actively mediate the resolution of inflammation33. At the early stage of neuroinflammation, the endogenous regulatory program for the active resolution of inflammation is initiated. RvD1, as one of the important SPMs, has a key impact on the severity and duration of inflammation by regulating the development of inflammation, promoting its active resolution, and maintaining the stability of the local microenvironment34. Wei et al.35 confirmed that in an animal model simulating SAH, RvD1 intervention treatment could effectively reduce inflammation-mediated blood-brain barrier (BBB) injury and the development of brain edema, thereby improving neurological dysfunction in the SAH rat model.To evaluate the effects of RvD1 combined with exercise rehabilitation training on reducing neuroinflammation, we measured the mRNA expression of anti-inflammatory factors (CD206 and IL-10) and inflammation-related factors (IL-1β and TNF-α). Using qPCR, we found that, compared to mice with only cerebral hemorrhage, the treatment with RvD1 combined with exercise rehabilitation training significantly increased the mRNA expression of anti-inflammatory factors and decreased the mRNA expression of inflammation-related factors. Additionally, we used MR as a tool to predict the relationship between 91 circulating inflammatory factors and RvD1. The results show that RvD1 can inhibit the expression of the pro-inflammatory factor IL20RA and increase the expression of the anti-inflammatory relative factors CCL25 and LIFR.Therefore, we conclude that this combined treatment effectively promotes the resolution of inflammation.
Functional exercise rehabilitation training after cerebral hemorrhage is a common clinical treatment method that can improve somatic function recovery and reduce disability rates in patients36. Motor training also contributes to the recovery of motor function in mice with ICH, inhibits the expression of proinflammatory factors, reduces neuronal death and dendritic degeneration in the sensorimotor cortex, and improves dendritic morphology (including increasing the number and length of dendrites) in the striatum, thereby alleviating neurological deficits37.BDNF is an important neurotrophic factor that promotes the growth, development, and survival of neurons38. Exercise rehabilitation training has been shown to enhance neural activity and further upregulate BDNF expression, thereby promoting neuronal growth, development, and survival, which is essential for recovery after ICH39. Kılınç et al.40 confirmed that exercise rehabilitation training can not only improve BDNF levels but also significantly enhance cognitive function.Takamatsu et al.2 found that BDNF expression was decreased after ICH, and this decrease was associated with the occurrence of neurological dysfunction.In line with this, our experimental results showed that BDNF expression was significantly decreased after ICH. The results of the neurological function test, water maze test, and open field test indicated that ICH impaired neurological function, spatial learning and memory abilities, and increased anxiety behavior in mice. BDNF expression levels in the striatum hematoma area decreased significantly after cerebral hemorrhage, and exercise rehabilitation training could effectively reverse the declining trend of BDNF levels caused by cerebral hemorrhage, thereby preventing secondary injury and neurological dysfunction2. Our study further extended this conclusion: compared with the ICH group, the mice in the rehabilitation training group had significantly upregulated BDNF expression and showed better rehabilitation effects in the neurological function test, water maze test, and open field test. These effects were further significantly enhanced by the addition of RvD1.
TrkB is a member of the neurotrophin tyrosine kinase receptor family and a specific receptor for BDNF. BDNF activates the PI3K/Akt pathway by binding to its high-affinity receptor TrkB, thereby preventing neuronal degeneration and death and promoting neuronal differentiation, survival, synaptic plasticity, and both embryonic and adult neurogenesis in the CNS. Akt is closely related to cell growth and differentiation and plays an important role in neuronal branching, elongation, and diameter increase, which is essential for maintaining cellular function41. Akt regulates various biological processes such as cell survival, proliferation, metabolism, and apoptosis through its phosphorylation state and promotes neuronal growth and the formation of neuronal polarity by interacting with specific proteins42.Our results showed that RvD1 combined with exercise rehabilitation training could up-regulate the protein and mRNA expression of BDNF/TrkB/PI3k/AKT and activate related pathways to exert its neuroprotective effect.
Mitochondria serve as the energy factories of cells, and the autophagy process of removing damaged mitochondria helps maintain cellular homeostasis during ICH, which is a selective autophagy process43. Zhang et al.44confirmed in a mouse model that promoting mitophagy through the injection of mitophagy receptors could effectively reduce ICH-induced inflammatory responses in mice. Liu et al.45also confirmed in the ICH model that scalp acupuncture could alleviate brain injury in rats by enhancing mitophagy and reducing neuroapoptosis. These results suggest that mitophagy can alleviate neurological damage after ICH, which is consistent with our findings.By comparing TEM images from different groups and combining MR and molecular docking analyses, we found that RvD1 combined with exercise rehabilitation training can alleviate neural damage after ICH by affecting mitochondrial autophagy. In TEM observations, the number of autophagosomes in samples treated with RvD1 combined with exercise rehabilitation training was comparable to that observed with the mitochondrial autophagy activator CCCP, further supporting the notion that RvD1 may alleviate mitochondrial dysfunction after ICH by promoting mitochondrial autophagy. Through Mendelian randomization studies, we determined the relationship between mitophagy-related targets and ICH-related outcomes, and further molecular docking analysis revealed high binding stability between RvD1 and its mitophagy-related targets. This suggests that RvD1 may influence mitochondrial autophagy through targets such as MFN2, RAB7B, and TOMM7, thereby alleviating neural damage after ICH. Interestingly, this finding is consistent with our previous experimental results. However, this conclusion still requires further validation.
Conclusion
In this study, we found that RvD1 combined with exercise rehabilitation activated BDNF/TrkB/PI3K/AKT signaling pathway, ameliorated neurological deficits, neuroapoptosis and neuroinflammation after ICH, and possibly promoted mitophagy. Due to the different methods of intracerebral hemorrhage modeling, the universality of the results may be affected. However, the findings still provide valuable evidence for subsequent in vitro experiments and clinical trials aimed at treating intracerebral hemorrhage.Compared with previous studies, this study for the first time proposed the strategy of RvD1 combined with exercise rehabilitation training, improved the downstream signaling pathway mechanism of BDNF, and explored the relationship between mitophagy, a key process, and ICH(Fig. 9).Therefore, RvD1 can be regarded as a potential new drug candidate, and its mechanism of action may involve the BDNF/TrkB/PI3K/AKT signaling pathway, providing an important pharmacological target for the clinical treatment of ICH.
Methods
Animals
Seventy adult male C57BL/6J mice, aged 8–10 weeks and weighing between 23 and 25 g, were obtained from Kunming Shuangxin Biotechnology Co., Ltd. After one week of routine feeding to acclimate to the environment, the mice underwent one week of preoperative adaptive exercise training, and then underwent intracerebral hemorrhage surgery to induce Intracerebral hemorrhage model.The mice were randomly assigned to six experimental groups using a random number table: Sham group、ICH group、ICH + Vehicle + exercise training group、ICH + CCCP group、ICH + RvD1 + exercise training group、ICH + Mdivi-1 + exercise training group.Each animal was uniquely coded to ensure blinding of both experimenters and evaluators throughout the study. All experimental protocols were approved by the Ethics Committee of Dali University and adhered to the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals.The experimental timeline is illustrated in Fig. 10.
Intracerebral hemorrhage model
Surgical procedure
Mice were fasted for 12 h and deprived of water for 4 h prior to anesthesia. Initial anesthesia was induced using 1.5% isoflurane and maintained at 1% throughout the procedure. The mice were positioned in a prone orientation on a stereotaxic frame. A small hole (approximately 1 mm in diameter) was drilled at the coordinates of the right striatum (0.8 mm anterior to bregma, 2 mm to the right of the midline and 3.5 mm deep). A solution containing 0.75 U of type IV collagenase and 7 U/µL heparin in PBS (0.4 µL) was injected at a rate of 0.2 µL/min over 5 min. The needle was left in place for an additional 10 min following the injection before withdrawal. The scalp was then sutured, and the mice were allowed to recover in a warm, ventilated environment. In the Sham group, an equal volume of saline was injected, with all other procedures remaining identical. Any mice that died within 24 h post-surgery were replaced with mice from the same batch.
CT scanning and slicing
Twenty-four hours post-surgery, the mice underwent cranial spiral computed tomography (CT) scans (SIEMENS, Germany) alongside neurological function assessments to confirm the successful establishment of the ICH model. The mice were rapidly anesthetized with 2–3% isoflurane and subsequently transferred to the CT scanning bed. The scanning parameters were configured as follows: slice thickness of 0.6 mm, tube voltage of 120 kV, and tube current of 210 mAs. The striatum was designated as the region of interest (ROI).The DICOM image data obtained from the CT scans were processed and analyzed using Mimics 21.0 software (Materialise, Belgium).
Drug administration
RvD1 was obtained from Cayman Chemical Company. Following the manufacturer’s instructions, the preservative ethanol was evaporated, and RvD1 was then dissolved in sterile phosphate-buffered saline (PBS). Starting from third day post-surgery, the ICH + RvD1 + exercise training group received 0.1 ml of a solution containing 3.0 µg/kg RvD1 through the tail vein daily while performing exercise rehabilitation training. To emphasize the role of mitochondrial quality control in the therapeutic effect of RvD1 on intracerebral hemorrhage, We evaluated mice with intracerebral hemorrhage treated with the Mitochondrial uncoupling agent carbonyl cyanide p-trichloromethoxyphenylhydrazone (CCCP) and the Mitochondrial Division Inhibitor (Mdivi-1).In ICH + CCCP group and ICH + Mdivi-1 + exercise training group, CCCP and Mdivi-1 were diluted with DMSO and injected intraperitoneally at a dose of 1 mg/kg and 25 mg/kg, respectively. The Sham group and ICH + Vehicle + exercise training group received daily intraperitoneal injection of 0.2 ml normal saline.
Exercise training
Prior to formal modeling, the mice underwent one week of pre-exercise training. On day one, the training duration was set at 10 min, increasing by 10 min each subsequent day until reaching 40 min on day four, which was maintained during days five and six.Seventy-two hours after modeling, once the hematoma had stabilized, the mice commenced exercise rehabilitation training: ① Cage rolling training device was used to train the walking and grasping function of mice; ② Net screen training device was used to train the forepaw grasping function of mice; ③ The balance beam training device was used to train the balance function of mice.The above three exercises were performed twice a day, 10 min each time, 6 days a week, for a total of 3 weeks. The mice in the Sham group、the ICH group and the ICH + CCCP group moved freely without exercise training.
Sensory and motor detection
Neurologic deficit scoring
Postoperative observations were conducted to assess the survival status, physical appearance, mental state, and responsiveness of the mice. The modified neurological severity score (mNSS) was employed to evaluate neurologic deficits at specific time points: 1 day, 5 days, 10 days, 14 days, and 21 days post-hemorrhage. The mNSS includes nine distinct tests that assess motor function, sensory test, beam balance, reflexes and abnormal movement. The scoring criteria for these assessments are provided in the Supplementary Table S1.
Muscle strength test
A wire with a diameter of 0.15 cm was horizontally mounted at a height of 70 cm above the ground, with a foam pad placed beneath it to ensure safety. Mice were allowed to place their forepaws on the wire before being released. Their behavior and hanging duration on the wire were observed and recorded. Scores were assigned based on limb placement and hanging time, as follows:3 points: Hanging on the wire for 0–2 s.2 points: Hanging on the wire for 3–4 s.1 point: Hanging on the wire for more than 5 s.0 points: Successfully hanging on the wire for more than 5 s while also placing hind limbs on the wire.The detailed scoring criteria are visually represented in the accompanying figure.
Morris water maze testing
The Morris water maze was employed to evaluate the spatial learning and memory capabilities of the animals. Training and testing were conducted in a circular water pool. During the training phase (days 22–26), mice were placed into the water from six different entry points each day and allowed 1 min to seek a submerged platform.The swimming data of mice were recorded by video camera to obtain parameters such as latency, swimming distance and trajectory. On the final training day, after the platform was removed, the number of times each mouse crossed the platform’s original platform quadrant within a 1-minute interval was recorded. This test assessed the mice’s spatial learning and memory abilities through analysis of their swim trajectories and navigational parameters.Statistical analyses were performed to determine differences between groups, with significance set at p < 0.05.
Open field test
The open field test was utilized to evaluate locomotor activity and anxiety-like behaviors in mice. Prior to the experiment, the mice were acclimatized to the testing room for 2 h to minimize environmental influences on the results. Each mouse was subsequently placed in an open arena and allowed to explore freely for a duration of 15 min. Behavior was recorded using a video tracking system that captured movement paths, speed, and the amount of time spent in distinct areas, including the center and periphery.
Brain tissue collection
After exposing mice to volatile anesthetic isoflurane for an adequate period of time to achieve anesthesia, we ensured that the mice were anesthetized by applying additional physical methods.Subsequently, the mice were secured in a stereotaxic apparatus, and a midline incision was made to open the chest cavity and sternum, thereby exposing the heart. After cardiac perfusion, the brain tissue surrounding the model was removed and washed with cold physiological saline. Then, the sample was stored in a -80 °C freezer for subsequent analysis.The euthanasia and anesthesia methods were carried out in accordance with the guidelines of the 2020 AVMA Animal Euthanasia guidelines.
Western blotting
The total protein of brain tissue in each group was extracted according to the instructions of the protein extraction kit, and the protein concentration was detected by BCA protein content test kit. Equal amounts of extracted proteins was added to 5X loading buffer, heated to 95 ° C for denaturation, and the proteins were separated by electrophoresis in 10% polyacrylamide gel.After transferring the proteins to PVDF membrane by wet transfer method, they were blocked in 5% bovine serum protein for 1 h, then the following primary antibodies were added and incubated overnight at 4 ° C: BDNF (1:4000, 25699-1-AP, Proteintech), AKT (1:4000, 10176-2-AP, Proteintech), phospho-AKT (1:2000, 66444-1-Ig, Proteintech), PI3K (1:1000, 20584-1-AP, Proteintech), TrkB (1:4000, 13129-1-AP, Proteintech), GAPDH (1:4000, GB12002-100, Servicebio).The following day, the primary antibodies were removed, and the PVDF membranes were washed three times with TBST, each wash lasting 5 min. Secondary antibodies (anti-mouse or anti-rabbit, 1:5000, GB15002-100/GB15004-100, Servicebio) were then added and incubated at room temperature for 1 h. After washing the membranes another three times, chemiluminescent reagents were applied to detect the proteins. Band intensities were analyzed using ImageJ software.
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mouse brain tissues using a total RNA extraction kit (Seven, Beijing, China). The isolated RNA was reverse-transcribed into complementary DNA (cDNA). PCR amplification was performed using a Bio-Rad thermal cycler, with GAPDH serving as an internal reference for quantifying mRNA levels. The primer sequences are listed below (Table 1). For real-time quantitative PCR amplification, cDNA was used as the template in combination with specific primers and MAX SYBR Green fluorescent dye. The thermal cycling conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 45 cycles of denaturation at 94 °C for 30 s and annealing at 60 °C for 30 s. The results were analyzed using the 2-ΔΔCt method.
Transmission electron microscopy for mitophagy detection
Brain tissues from each group of mice were harvested, and 1 mm2 samples were fixed in 2.5% glutaraldehyde. After fixation, the samples were rinsed with deionized water and dehydrated using a gradient series of ethanol (50%, 70%, 80%, 90%, and 100%), with each dehydration step lasting 20 min. The samples were then placed on embedding plates and incubated overnight at 37 °C. Following this, the samples were polymerized at 60 °C for 48 h. Ultrathin sections of the brain tissue were prepared and sent to the electron microscopy laboratory for further processing and TEM observation to identify autophagic vesicles.Early-stage mitophagosomes were identified by their characteristic double membranes and cristae, while later stages of mitophagy, following fusion with lysosomes, were recognized by single membranes or residual material indicative of digestion.
TUNEL staining for apoptosis detection
To quantitatively analyze apoptotic neurons, the brain tissues of mice in each experimental group were collected and fixed, and the apoptosis level was detected by TUNEL method in situ. The operation instructions of TUNEL apoptosis detection kit were strictly followed, and the double staining of NeuN (blue) and TUNEL(green) was performed.After treatment with pepsin K for 25 min, the cells were infiltrated with 0.1% Triton at 37 ° C for 20 min, rinsed thoroughly with double steamed water, and counterstained with hematoxylin for 1 min. The cover glass was sealed with a neutral glue sealant. The number of TUNEL-positive neurons per field was counted at x20x magnification using Image J. Apoptosis rate (%) = number of apoptotic cells/total number of cells ×100%.
MR analysis of mitophagy and inflammation-related targets
To investigate the causal relationship between mitophagy-related targets located in the caudate nucleus of the basal ganglia and ICH, we conducted a two-sample MR analysis. Additionally, we explored the causal relationship between RvD1 and 91 circulating inflammatory factors. We obtained SNPs related to mitophagy targets from the GTEx database (https://www.gtexportal.org) and SNPs related to ICH, RvD1, and inflammatory factors from the GWAS Catalog database (https://www.ebi.ac.uk/gwas/).We performed MR analysis using the “TwoSampleMR” package and adopted the inverse variance weighted (IVW) method as the primary approach to assess the relationship between the expression levels of core targets and relevant outcomes. To test the robustness of the results, we also used several other methods, including Cochran’s Q statistic to test for heterogeneity, where p < 0.05 indicates heterogeneity in the IVW results. We used MR-Egger regression and MR-Presso to evaluate potential horizontal pleiotropy, with p < 0.05 in the IVW results indicating the presence of horizontal pleiotropy.To better explore the efficacy of drug targets in MR analysis, we established the following criteria:①The selected SNPs should have a strong association with the exposure factor, with corresponding p-values < 5 × 10^-5 or 3 × 10^-5.②During the linkage disequilibrium clumping process, we set the r2 threshold to 0.3.③For clustering analysis, we defined a window size of 500k base pairs.④The F-statistic of SNPs associated with the exposure factor should be greater than 10.
Identification and molecular docking of RvD1 structures
To evaluate the binding interactions and affinity of RvD1 with its related targets, we performed molecular docking simulations using AutoDock Vina 1.2.2. The three-dimensional structure of RvD1 was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and checked using Chem3D v21.0.0. The three-dimensional structures of the proteins identified as positive results from the MR analysis were obtained from the PDB database (http://www.rcsb.org/). For proteins not found in the PDB database, we used ALPHAFold to predict their three-dimensional structures.The preparation for docking included converting the protein and ligand files to PDBQT format, removing water molecules, and adding polar hydrogen atoms. The grid box was centered on the binding site of each protein and sized to encompass the entire protein. After performing the docking simulations using AutoDock Vina 1.2.2, we used the PLIP tool (https://plip-tool.biotec.tu-dresden.de/plip-web/plip/index) to analyze the results and visualized them using PyMol.
Data analysis
Data processing and statistical analyses were conducted using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA). Results are presented asmean ± standard error of mean (SEM) for each experimental group. For comparisons among multiple groups, one-way analysis of variance (ANOVA) was utilized. When analyzing data across different time points, two-way ANOVA was employed to assess trends and effects over time. In this study, the sample size (n) refers to the number of experimental animals used. Statistical significance was defined as p < 0.05.
Data availability
All data related to this study can be obtained on request, while all the analyzed data are included in this published article and its supplementary information files.Correspondence and requests for materials should be addressed to B.Z.
References
Poon, M. T. C., Fonville, A. F. & Al-Shahi Salman, R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 85, 336–344 (2014).
Takamatsu, Y. et al. Potential effect of physical exercise on the downregulation of BDNF mRNA expression in rat hippocampus following intracerebral hemorrhage. Neurosci. Lett. 824, 137670 (2024).
Chen, S., Yang, Q., Chen, G. & Zhang, J. H. An update on inflammation in the acute phase of intracerebral hemorrhage. Translational stroke Res. 6, 4–8 (2015).
Szczuko, M. et al. Lipoxins, RevD1 and 9, 13 HODE as the most important derivatives after an early incident of ischemic stroke. Sci. Rep. 10, 12849 (2020).
Bathina, S. et al. Resolvin D1 ameliorates nicotinamide-streptozotocin-induced type 2 diabetes mellitus by its anti-inflammatory action and modulating PI3K/Akt/mTOR pathway in the brain. Arch. Med. Res. 51, 492–503 (2020).
Jun-Sub, J. et al. Changes in plasma lipoxin A4, resolvins and CD59 levels after ischemic and traumatic brain injuries in rats. Korean J. Physiol. Pharmacol. 24, 165 (2020).
Dalia, G., Huda, H. & M. & Resolvin D1 prevents the impairment in the retention memory and hippocampal damage in rats fed a corn oil–based high fat diet by upregulation of Nrf2 and downregulation and inactivation of p66Shc. Neurochem Res. 45, 1576–1591 (2020).
Krashia, P. et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 10, 3945 (2019).
Anzell, A. R., Maizy, R., Przyklenk, K. & Sanderson, T. H. Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol. Neurobiol. 55, 2547–2564 (2018).
Atsushi, H. et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 4, 2308 (2013).
Zhang, X., Zheng, Y. & Chen, Z. Autophagy and mitochondrial encephalomyopathies. Adv. Exp. Med. Biol. 1207, 103–110 (2020).
Patergnani, S. et al. Mitochondria in multiple sclerosis: molecular mechanisms of pathogenesis. Int. Rev. cell. Mol. biology. 328, 336–344 (2017).
Ma, Y. et al. FGF21 attenuates neuroinflammation following subarachnoid hemorrhage through promoting mitophagy and inhibiting the cGAS-STING pathway. J. Translational Med. 22, 436 (2024).
Penna, L., Pinheiro, J., Ramalho, S. & Ribeiro, C. Effects of aerobic physical exercise on neuroplasticity after stroke: systematic review. Arq. Neuropsiquiatr. 79, 832–843 (2021).
Santos, M. V., Pagnussat, A. S., Mestriner, R. G. & Netto, C. A. Motor skill training promotes sensorimotor recovery and increases microtubule-associated protein-2 (MAP-2) immunoreactivity in the motor cortex after intracerebral hemorrhage in the rat. ISRN Neurol. 2013, 159184 (2013).
Chen, J. C. & Shaw, F. Z. Progress in sensorimotor rehabilitative physical therapy programs for stroke patients. World J. Clin. cases. 2, 316–326 (2014).
Kinoshita, K. et al. Mature adult mice with exercise-preconditioning show better recovery after intracerebral hemorrhage. Stroke 52, 1861–1865 (2021).
Mang, C., Campbell, K. L., Ross, C. J. D. & Boyd, L. A. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys. Ther. 93, 1707–1716 (2013).
Ted Kheng Siang, N., Hui, C. S., Wilson Wai San, H., Ee Heok, T. & Roger Chun-Man, H. Decreased serum brain-derived neurotrophic factor (BDNF) levels in patients with Alzheimer’s disease (AZD): a systematic review and meta-analysis. Int. J. Mol. Sci. 20, 257 (2019).
Alomari, M. A., Khabour, O. F., Alzoubi, K. H. & Alzubi, M. A. Forced and voluntary exercises equally improve spatial learning and memory and hippocampal BDNF levels. Behav. Brain. Res. 247, 34–39 (2013).
Zhao, S. et al. BIO alleviates inflammation through inhibition of GSK-3β in a rat model of intracerebral hemorrhage. J. Neurosurg. 133, 383–391 (2019).
Lee, H. J., Lim, I. J., Lee, M. C. & Kim, S. U. Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J. Neurosci. Res. 88, 159–171 (2010).
Kaminari, A., Giannakas, N., Tzinia, A. & Tsilibary, E. C. Overexpression of matrix metalloproteinase-9 (MMP-9) rescues insulin-mediated impairment in the 5XFAD model of Alzheimer’s disease. Sci. Rep. 7, 683 (2017).
Devi, L. & Ohno, M. TrkB reduction exacerbates Alzheimer’s disease-like signaling aberrations and memory deficits without affecting β-amyloidosis in 5XFAD mice. Translational psychiatry. 5, e562 (2015).
Wei, Y. et al. Salidroside inhibits inflammation through PI3K/Akt/HIF signaling after focal cerebral ischemia in rats. Inflammation 40, 1297–1309 (2017).
Yang, J., Yan, H., Li, S., Zhang, M. & Berberine Ameliorates, M. C. A. O. Induced cerebral ischemia/reperfusion injury via activation of the BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem. Res. 43, 702–710 (2018).
Xu, H. et al. Methylene blue attenuates neuroinflammation after subarachnoid hemorrhage in rats through the Akt/GSK-3β/MEF2D signaling pathway. Brain. Behav. Immun. 65, 125–139 (2017).
Murata, H. et al. A new cytosolic pathway from a Parkinson disease-associated kinase, BRPK/PINK1: activation of AKT via mTORC2. J. Biol. Chem. 286, 7182–7189 (2011).
Chen, S. et al. Activation of melanocortin receptor 4 with RO27-3225 attenuates neuroinflammation through AMPK/JNK/p38 MAPK pathway after intracerebral hemorrhage in mice. J. Neuroinflamm. 15, 106 (2018).
Liu, Z. et al. The neuroprotective effect of lithium chloride on cognitive impairment through glycogen synthase kinase-3β inhibition in intracerebral hemorrhage rats. Eur. J. Pharmacol. 840, 50–59 (2018).
Shi, L. et al. Chronic inflammation, cognitive impairment, and distal brain region alteration following intracerebral hemorrhage. FASEB journal: official publication Federation Am. Soc. Experimental Biology. 33, 9616–9626 (2019).
Wang, K. W. et al. Simvastatin-ezetimibe enhances growth factor expression and attenuates neuron loss in the hippocampus in a model of intracerebral hemorrhage. Fundam. Clin. Pharmacol. 35, 634–644 (2021).
Tabas, I. & Glass, C. K. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Sci. (New York N Y). 339, 166–172 (2013).
Yang, Y. et al. RvD1 improves resident alveolar macrophage self-renewal via the ALX/MAPK14/S100A8/A9 pathway in acute respiratory distress syndrome. J. Adv. Res. S2090-1232, 00030–00034 (2024). S2090-1232(24).
Wei, C. et al. Resolvin D1 ameliorates inflammation-mediated blood-brain barrier disruption after subarachnoid hemorrhage in rats by modulating A20 and NLRP3 inflammasome. Front. Pharmacol. 11, 610734 (2020).
Mao, H. et al. Aerobic exercise combined with huwentoxin-I mitigates chronic cerebral ischemia injury. Neural Regeneration Res. 12, 596 (2017).
Tamakoshi, K., Hayao, K. & Takahashi, H. Early exercise after intracerebral hemorrhage inhibits inflammation and promotes neuroprotection in the sensorimotor cortex in rats. Neuroscience 438, 86–99 (2020).
Kazim, S. F. & Iqbal, K. Neurotrophic factor small-molecule mimetics mediated neuroregeneration and synaptic repair: emerging therapeutic modality for Alzheimer’s disease. Mol. neurodegeneration. 11, 50 (2016).
Nishijima, T., Kawakami, M. & Kita, I. Long-term exercise is a potent trigger for ∆FosB induction in the hippocampus along the dorso-ventral axis. PloS one. 8, e81245 (2013).
Kılınç, M. et al. The effects of Bobath-based trunk exercises on trunk control, functional capacity, balance, and gait: a pilot randomized controlled trial. Top. Stroke Rehabil. 23, 50–58 (2016).
Vignoli, B. et al. Peri-synaptic glia recycles brain-derived neurotrophic factor for LTP stabilization and memory retention. Neuron 92, 873–887 (2016).
Cheng, B. et al. Retinoic acid protects against proteasome inhibition associated cell death in SH-SY5Y cells via the AKT pathway. Neurochem. Int. 62, 31–42 (2013).
Whitley, B. N., Engelhart, E. A. & Hoppins, S. Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion 49, 269–283 (2019).
Zheng, S. et al. FUNDC1 inhibits NLRP3-mediated inflammation after intracerebral hemorrhage by promoting mitophagy in mice. Neurosci. Lett. 756, 135967 (2021).
Liu, P. et al. Scalp acupuncture attenuates brain damage after intracerebral hemorrhage through enhanced mitophagy and reduced apoptosis in rats. Front. Aging Neurosci. 13, 718631 (2021).
Acknowledgements
We thank the Department of Human Anatomy and Clinical Medical College of Dali University for providing the experimental equipment and the volunteers who participated in the experiment.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by Key project of the special fund for basic research of local undergraduate colleges and universities granted by Department of Science and Technology of Yunnan Province (202101AN070028),Scientific Research Foundation of Education Department of Yunnan Province(2024Y883),Projects of the Research and Development Fund of Dali University (FZ2023ZD028), and Key Laboratory of medical molecular diagnosis in Colleges and Universities of Yunnan Province(2019ZD036).
Author information
Authors and Affiliations
Contributions
XL: Writing–original draft. DL: Writing–review and editing. TO: Writing–review and editing. ZZ: Data curation and software.ZL: Mendelian randomization analysis and molecular docking. ZL: Writing–review and editing. ML: Data curation and software. YH: Conducting animal experiments. YZ: Conducting animal experiments. YL: Analyzed data. CS: Writing–review and editing. SW: Conducting animal experiments. TL: Conducting animal experiments. BZ: Writing–review and editing.
Corresponding author
Ethics declarations
ARRIVE quidelines statement
The study was carried out in compliance with the ARRIVE guidelines.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiaoyu, L., Dandan, L., Tianzhao, O. et al. Resolvin D1 combined with exercise rehabilitation alleviates neurological injury in mice with intracranial hemorrhage via the BDNF/TrkB/PI3K/AKT pathway. Sci Rep 14, 31447 (2024). https://doi.org/10.1038/s41598-024-83019-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83019-w
Keywords
This article is cited by
-
Resolvin D1 accelerates resolution of neuroinflammation by inhibiting microglia activation through the BDNF/TrkB signaling pathway
European Journal of Medical Research (2025)
-
Mollugin attenuates oxygen-glucose deprivation/reperfusion-induced brain microvascular endothelial cell death and permeability through activation of BDNF/TrkB-modulated Akt pathway
Journal of Bioenergetics and Biomembranes (2025)