Abstract
Body composition abnormalities are prognostic markers in several types of cancer, including colorectal cancer (CRC). Using our data distribution on body composition assessments and classifications could improve clinical evaluations and support population-specific opportune interventions. This study aimed to evaluate the distribution of body composition from computed tomography and assess the associations with overall survival among patients with CRC. In this multicenter cohort study, patients (N = 635) aged 18 years and older with CRC were observed for 12 to 36 months to assess outcomes. Skeletal muscle area (SM) and index (SMI), skeletal muscle radiodensity (SMD), intermuscular adipose tissue (IMAT), subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT) were evaluated, and classified based on tertile distributions. Low muscle mass (SMI) and poor muscle composition (SMD) were independent predictors of mortality regardless of follow-up period. This risk of mortality increased to more than 3-fold when combining both low SMI and low SMD (HRadjusted 3.1, 95% CI 1.8 to 5.4, respectively). Our study indicates that body composition characteristics may vary across countries, highlighting the need for developing sex- and population-specific cutoff values for computed tomography assessments in patients with different types of cancer.
Similar content being viewed by others
Introduction
The increase in cancer incidence and prevalence is a consequence of several factors, including aging demographics, population growth, and changes in risk factors related to socioeconomic development1. In Brazil, a middle to upper income country, an estimated 704,000 new cancer cases are expected between 2023 and 20252. Among these, colorectal cancer (CRC) is ranked as the third most commonly diagnosed type, with approximately 46,000 new cases in 20232. Therefore, the investigation of reliable and potentially modifiable factors associated with both short- and long-term cancer outcomes becomes pivotal.
Patients with CRC often face abnormalities in body composition, including conditions like sarcopenia, malnutrition, frailty and cachexia3,4,5. Low muscle mass is a common prevalent feature in many of these syndromes, occurring irrespective of changes in overall body weight5,6,7. Well-established evidence has demonstrated that variations in body weight could potentially mask the detection of low muscle mass when weight is evaluated in isolation6,7,8. Consequently, there is a growing need to enhance body composition assessment in this population. To address this, computed tomography (CT) has emerged as an opportunistic valuable tool for accurate measurement of muscle mass and adiposity, given its routine integration into oncology care9,10,11.
Muscle abnormalities can be assessed using CT scans, which can measure both muscle quantity and composition (such as radiodensity)6,12,13. Additionally, CT scans can detect alterations in adiposity depots, including excess visceral adiposity6,12,13. These body composition abnormalities are relevant prognostic markers in various cancer types, including CRC, as demonstrated by our previous research and others’14,15,16,17,18. Conditions such as impaired muscle mass or composition are linked to chemotherapy toxicities and poor survival19,20,21,22, reinforcing the importance of adequate body composition assessment, as it can aid clinicians in tailoring strategies to mitigate these associated adverse effects. However, the analysis and classification of body composition often depend on cutoff values that necessitate population-specific validation.
Few validated cutoff values have been proposed for classifying body composition in patients with CRC and these values are typically derived from North American, European or Asian populations14,23,24,25,26. Brazil is a diverse country with a blended population, characterized by a significant proportion of mixed-race individuals from varied ethnic backgrounds27. In this context, our hypothesis posits that body composition in our population may be distinct from others. It is possible that utilizing our own data distribution may potentially lead to improved clinical evaluations and timely interventions for our specific population. Therefore, this study aimed to describe CT-derived body composition and its association with mortality among Brazilian patients diagnosed with CRC. Our secondary aim was to assess the prognostic value of combining muscle-health-related abnormalities that are independently associated with survival.
Methods
Design and subjects
This multicenter cohort study with retrospective data collection was conducted in six oncology reference centers across different Brazilian regions: Liga Norteriograndense Contra o Cancer (LIGA), Natal-RN; Onofre Lopes University Hospital (HUOL), Natal-RN; Ceará Cancer Institute (ICC), Fortaleza-CE; National Cancer Institute (INCA), Rio de Janeiro-RJ, State University of Campinas (Unicamp), Campinas-SP; and Federal University of Minas Gerais (UFMG), Belo Horizonte-MG. Each center used its own system to identify the number of patients diagnosed with CRC and conducted data collection from individual medical records. This process followed standardized inclusion and exclusion criteria to ensure consistency across all centers. Data collection occurred over a two-year period, from June 2021 to July 2023.
Inclusion criteria consisted of consecutive male and female patients over 18 years of age, newly diagnosed with CRC, with no prior treatment, who had undergone abdominal CT scans. The CT images taken closest to the diagnosis date were selected to capture the patient’s initial clinical condition. Exclusion criteria were patients without data on body weight (kg) and height (m), or those with conditions such as ascites, subcutaneous edema, and anatomical variations that could impede the interpretation of CT images. Body weight measurements were included within a ± 4-month interval from the CT scan date. In this retrospective study, patients were observed for a minimum of 12 months and up to a maximum of 36 months to assess outcomes, or until the date of death, whichever occurred first.
This study adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of the Onofre Lopes University Hospital (CAAE 73316117.8.0000.5292) and other participating centers. Due to its retrospective nature and the use of secondary data from medical records, the need for obtaining informed consent was waived. Following approval, each participating center forwarded patients’ clinical, anthropometric information, and CT scans to the research coordinating center in Natal, RN. This center was responsible for conducting CT scans and performing data analysis.
Covariates and outcomes
Data on sex, age, ethnicity, education level, marital status, smoking, alcohol exposure, date of diagnosis (considered the date of the first CT image or histopathological examination), tumor site, Eastern Cooperative Oncology Group – Performance Status (ECOG-PS scale) and staging (using the Tumor Nodes Metastasis – TNM classification) were recorded. Additional information on antineoplastic treatment was collected, including chemotherapy (type and regimen), radiotherapy (type), and surgery.
Charlson comorbidity index (CCI) was utilized to classify comorbid burden, an index that includes 19 conditions such as cardiovascular disease, diabetes mellitus, liver disease, and pulmonary disease. Each condition is assigned a score, contributing to patient’s total score and resulting in a combined age-comorbidity score. A higher score indicates a higher comorbidity burden. A free online calculator (https://www.mdcalc.com/calc/3917/charlson-comorbidity-index-cci) was used to compute the CCI. The primary outcome of interest was overall mortality incidence, measured from date of diagnosis and assessed at three time points: 12, 24, and 36 months. Body weight (kg) and height (m) were recorded to calculate body mass index (BMI) – kg/m2, classified according to the World Health Organization guidelines.
Body composition from computed tomography scans
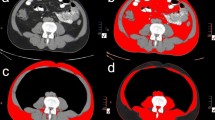
Single cross-sectional images at the third lumbar vertebra (L3) were examined using Slice-O-Matic software (v5.0, Tomovision®, Montreal, Canada). All contrast-enhanced CT scans were analyzed. Tissues were delineated using specific Hounsfield Units (HU) thresholds: -29 to + 150 for skeletal muscle, -150 to -50 for visceral adipose tissue (VAT), and − 190 to -30 for subcutaneous (SAT) and intermuscular adipose tissue (IMAT)28. Subsequently, cross-sectional areas (cm2) of these tissues were computed for each image. SM values (cm2) were normalized by dividing total muscle cross-sectional area by height2 (SMI in cm2/m2). Skeletal muscle radiodensity (SMD) was obtained by averaging the HU of SM from cross-sectional images.
Two trained researchers with anatomical expertise selected and analyzed the images. To assess test-retest reliability, the interclass correlation coefficient (ICC) and the 95% confidence of interval (95% CI) were evaluated using 30 images based on single measurement, absolute-agreement, 2-way mixed-effects model. The ICC and CI for repeated measurements were 1.00 (1.00–1.00).
Statistical analysis
Data were analyzed using the R Studio version 4.3.2, and SPSS version 20.0 (IBM®, Chicago, IL, USA). Data normality was assessed using the Shapiro-Wilk test. Normally distributed data were described as mean ± standard deviation (SD) and compared through the independent Student’s t-test. Non-normally distributed data were described as medians and interquartile (IQ) ranges, compared through the Mann-Whitney U test. Categorical variables were presented as absolute (N) and relative (%) frequencies, and association tests were performed using Pearson’s χ2 test.
Low CT-muscle parameters were defined by sex-stratified tertiles (< 33rd percentile), as follows: (i) SMD < 32.4 for males, < 26.7 for females; (ii) SM < 133.8 for males, < 91.3 for females; (iii) SMI < 48.3 for males, < 38.6 for females. Abnormal CT-adiposity parameters were defined as the highest sex-stratified tertile (> 66th percentile), as follows: (i) SAT > 122.3 for males, > 200.2 for females; (ii) VAT: > 168.6 for males, > 114.4 for females; (iii) IMAT: > 10.3 for males, > 11.5 for females. We chose to use lowest and highest tertiles as cutoff points because the sample size was relatively small, and similar studies also used these cutoff points24. Kaplan-Meier (KM) curves were constructed to evaluate the associations of these abnormalities in body composition with 36-month survival (Log-rank test).
A subsequent Cox regression analysis was employed, both in its crude and adjusted forms, to assess the independent associations between body composition and mortality. Adjustments were selected based on prognostic factors and BMI (no multicollinearity based on VIF values < 10: SAT: 1.40 VAT: 1.71 IMAT: 1.63 SMD: 1.74 SM: 5.83 SMI: 5.24). For all tested independent variables (SAT, VAT, IMAT, SM, SMD, SMI), based on our primary endpoint, the proportional hazards assumption was assessed using the Schoenfeld residuals test, with all P-values indicating no violation of this assumption: SAT (P = .52), VAT (P = .72), IMAT (P = .13), SM (P = .91), SMD (P = .20), and SMI (P = .96). Statistical significance was set at P < .05 for all analyses.
Results
Initially, data from 915 eligible patients were screened. After exclusions, a total of 635 patients were included in this final analysis, Fig. 1. There was a similar sex distribution (50.2%), and age distribution for individuals younger and older than 60 years (56.5%). Tumor location distribution was also relatively even, with 51% in the rectum and 49% in the colon. Most patients were diagnosed at clinical TNM stages III to IV, and had normal range BMI (48.8%) to excess weight (44.8%), only 6.5% were underweight. Mortality rates increased over time, with 13.9% at 12 months, 23.6% at 24 months, and 32.6% at 36 months. Table 1 shows detailed clinical data. Additional center- and sex-stratified body composition characteristics are shown in Supplementary Tables 1 and 2. Anthropometric and body composition assessments by sex are showed in Table 2. We did not stratify the groups according to disease stage because body composition did not differ when only the early-stage tumor group was included in the analysis (see Supplementary Table 2).
Figure 2 demonstrates the 36-month survival KM curves in relation to body composition abnormalities. Patients with both low SM and low SMI had significantly poor survival. Using continuous variables (model 1), Cox regression analysis demonstrated that higher SMI and SMD were inversely and independently associated with mortality at 12, 24, and 36 months. Additionally, higher IMAT independently increased the hazard of mortality at all-time points. Using categorical variables (model 2), Cox regression analysis demonstrated that low SMD and SMI (based on the lowest tertile) was independently associated with a higher hazard for mortality at all time points. No associations were observed for adipose tissue compartments (Table 3). Supplementary Table 4 presents sex-specific associations between body composition and survival at the three time points, using continuous variables. The findings consistently show inverse associations between muscle-related parameters and mortality at 12 and 24 months.
Kaplan-Meier curves of 36-month survival probability in patients with colorectal cancer by tertiles of CT-derived body composition abnormalities. CT, computed tomography; SMD, skeletal muscle radiodensity; MM, muscle mass; SM, skeletal muscle; SMI, skeletal muscle index; SAT, subcutaneous adipose tissue; VAT, visceral adipose; IMAT, intramuscular adipose tissue. Shaded areas mean 95% confidence intervals for survival probabilities.
Based on our survival-related results, we categorized muscle abnormalities into the following phenotypes: normal SMI + normal SMD, normal SMI + low SMD, low SMI + normal SMD, and low SMI + low SMD. Cox regression analysis demonstrated that patients with low SMI and normal SMD had more than 2 times higher risk for 12-month mortality. This risk increased to more than 3-fold when combining both low SMI and low SMD (HRadjusted 2.1, 95% CI 1.1 to 3.8; HRadjusted 3.1, 95% CI 1.8 to 5.4, respectively). This trend was also observed at the 24-month timepoint, but not at 36-month (Table 4).
Discussion
To our knowledge, this is the first study to demonstrate the distribution of CT-derived body composition in a multicenter cohort of patients with CRC in Brazil. Our main findings demonstrated that both muscle mass (from SM/SMI) and muscle composition (from SMD and IMAT) were independently associated with overall survival at all follow-up timepoints (12, 24, and 36 months). Combining phenotypes of muscle abnormalities (low SMI + low SMD) further enhanced prognostic value, increasing the hazard for mortality at both 12 and 24-months.
Describing population-specific body composition is pivotal for achieving more accurate interpretation of acquired measurements, as unique population characteristics can influence this assessment, potentially leading to disparities in epidemiological and nutritional contexts29. Therefore, relying on cutoffs proposed for European or North American populations may not adequately reflect the subtle yet important variations observed in our population.
The importance of population-specific cutoff points has also been highlighted in Japanese studies30,31. Our identified tertile distribution data were as follows: SMI < 48.3 cm2/m2 for males, < 38.6 cm2/m2 for females; SMD < 32.4 HU for males, < 26.7 HU for females. These values differ from those reported in North American and European studies, likely due to variations in tumor location, staging, and socioeconomic characteristics across cohorts. Previously established SMI cutoffs ranged from 43 to 54.3 cm2/m2 for males, and from 38.5 to 46.6 cm2/m2 for females, while SMD values ranged from 32.4 to 35.5 HU for males, and from 26.7 to 32.5 HU for females24,25,26. Some of these studies also proposed BMI-based cutoffs, distinguishing individuals with and without obesity. However, this distinction was not feasible in our study due to sample size constraints. These observed differences further underscore the importance of developing population-specific cutoff values.
Our additional findings align with previous studies demonstrating the influence of muscle mass on survival in patients with CRC32,33,34,35. In a larger study, Kroenke et al.14 showed that patients with CRC and low SMD exhibited higher overall mortality. Moreover, individuals with both low SMD and low SMI exhibited the highest overall mortality rates, indicating a synergistic effect. Considering that SMD serves as a proxy for myosteatosis, the combined decline in muscle area and density may be linked to an intensified systemic inflammatory response, and metabolic dysregulation, thereby indicating a poorer prognosis36,37. The lack of hazard differences at the 36-month time point could be attributed to sample constraints, as we lost nearly 10% of patients by this time. Additionally, survivor bias may have played a role, as patients who underwent treatment could have been influenced by other factors that became more relevant as competing risks over time (e.g., treatment modalities).
In regards to adiposity, our findings demonstrated that lower values of SAT and VAT were not independently associated with mortality. In contrast, muscle composition, as indicated by higher fat infiltration (marked by low SMD and higher IMAT), was independently associated with poor survival. Growing evidence has demonstrated that changes in muscle composition may occur earlier than changes in muscle mass quantity, potentially affecting force-generating capacities and linking to adverse outcomes16,38. In this sense, our study adds to the growing body of evidence highlighting the importance of assessing muscle composition alongside muscle mass to better understand and predict survival outcomes in patients with CRC.
Although we did not find associations with survival, adipose tissue wasting may occur in cancer-related cachexia as a consequence of lipolysis due to severe inflammatory response39. The accelerated breakdown of adipose tissue is frequently associated with shorter survival times during cancer progression39,40. Normal levels of fat compartments are generally physiologically beneficial; however, extremes -whether excessively high or low - might contribute to poorer prognosis40. Supporting this viewpoint, Malietzis et al.41 highlighted that elevated visceral adiposity (VAT) correlated with lower survival rates. Furthermore, an exploratory study indicated a prognostic significance of VAT in patients with metastatic cancer, where higher VAT values were predictive of worse outcomes42.
This study is not without limitations. The relatively small sample size may not fully represent the entire spectrum of patients with CRC in Brazil. Convenience sampling introduces inherent bias. Furthermore, limitations include the absence of BMI-specific categories, which was unfeasible in our cohort. Although not causal, individuals with higher body mass are expected to have greater muscle and adipose tissue, making BMI-specific cutoffs valuable for more precise assessments. Moreover, our regression models may not fully account for unmeasured factors or residual confounders, which could introduce overlooked biases in the analysis.
Contrast infusion during CT analysis can increase muscle radiodensity measurements, and this factor was not controlled in our study. Additionally, reliance on medical records poses a limitation, potentially affecting the accuracy of mortality data and other clinical variables. The retrospective design also limited access to important variables, such as malnutrition diagnostic tools, weight loss, laboratory tests, and concurrent medication use. Despite limitations, the study’s multicenter cohort design, incorporating body composition evaluation from CT scans, strengthens the robustness and novelty of our findings.
This study utilized CT-derived body composition measurements to investigate how different tertile distributions were associated with survival among Brazilian patients with CRC. Low muscle mass and poor muscle composition emerged as variables independent associated with mortality regardless of follow-up period. Based on our initial findings, clinicians and researchers in Brazil and similar regions could utilize our tertile cutoffs among patients with CRC to validate our hypothesis and for comparison analysis.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to ethical and privacy restrictions but are available from the corresponding author on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
INCA. Estimativa 2023: incidência de câncer no Brasil (2022).
Sousa, I. M., De, Silva, F. M., Virgens, I. P. A., das, Costa, E. C. & Fayh, A. P. T. Independent and joint association of sarcopenia and frailty with mortality in older patients with gastrointestinal cancer: A cohort study with prospective data collection. Support Care Cancer. 32, 1–8 (2024).
da Silva Nascimento, M. L. et al. Examining variations in body composition among patients with colorectal cancer according to site and disease stage. Sci. Rep. 14, 1–9 (2024).
da Rocha, I. M. G. et al. Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J. Cachexia Sarcopenia Muscle 10, (2019).
Prado, C. M. M. & Heymsfield, S. B. Lean tissue imaging. J. Parenter. Enter. Nutr. 38, 940–953 (2014).
Roeland, E. J. et al. Weight loss versus muscle loss: Re-evaluating inclusion criteria for future cancer cachexia interventional trials. Support Care Cancer 25, 365–369 (2017).
Brown, J. C. et al. Weight stability masks changes in body composition in colorectal cancer: A retrospective cohort study. Am. J. Clin. Nutr. 113, 1482–1489 (2021).
Prado, C. M., Cushen, S. J., Orsso, C. E. & Ryan, A. M. Sarcopenia and cachexia in the era of obesity: Clinical and nutritional impact. Proc. Nutr. Soc. 75, 188–198 (2016).
de Liguori, A., Fayh, A. P. T. & A. L. & Computed tomography: An efficient, opportunistic method for assessing body composition and predicting adverse outcomes in cancer patients. Radiol. Bras. 56, VIII–IX (2023).
Heymsfield, S. B., Gonzalez, M. C., Lu, J., Jia, G. & Zheng, J. Skeletal muscle mass and quality: Evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc. 74, 355–366 (2015).
Fayh, A. P. T., de Sousa, I. M. & Gonzalez, M. C. New insights on how and where to measure muscle mass. Curr. Opin. Support Palliat. Care 14, 316–323 (2020).
Curcio, F. et al. Biomarkers in sarcopenia: A multifactorial approach. Exp. Gerontol. 85, 1–8 (2016).
Kroenke, C. H. et al. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 124, 3008–3015 (2018).
Ebadi, M. et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br. J. Cancer 117, 148–155 (2017).
Costa Pereira, J. et al. Strength-to-muscle radiodensity: A potential new index for muscle quality. Clin. Nutr. 43, 1667–1674 (2024).
Carvalho, A. L. M. et al. Low skeletal muscle radiodensity is the best predictor for short-term major surgical complications in gastrointestinal surgical cancer: A cohort study. PLoS One 16, e0247322 (2021).
Barbalho, E. R. et al. Is skeletal muscle radiodensity able to indicate physical function impairment in older adults with gastrointestinal cancer ? Exp. Gerontol. 125, 110688 (2019).
Hopkins, J. J. et al. Change in skeletal muscle following resection of stage I–III colorectal cancer is predictive of poor survival: A cohort study. World J. Surg. 43, 2518–2526 (2019).
Malietzis, G. et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br. J. Surg. 103, 572–580 (2016).
Liu, J., Yu, X., Huang, X., Lai, Q. & Chen, J. Associations of muscle and adipose tissue parameters with long-term outcomes in middle and low rectal cancer: A retrospective cohort study. Cancer Imaging 23, 1–12 (2023).
Rocha, I. M. G. et al. Is cachexia associated with chemotherapy toxicities in gastrointestinal cancer patients? A prospective study. J. Cachexia Sarcopenia Muscle 10, 445–454 (2019).
Feliciano, E. M. C., Avrutin, E., Caan, B. J., Boroian, A. & Mourtzakis, M. Screening for low muscularity in colorectal cancer patients: A valid, clinic-friendly approach that predicts mortality. 898–908. https://doi.org/10.1002/jcsm.12317 (2018).
Caan, B. J. et al. Explaining the obesity paradox: The association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol. Biomarkers Prev. 26, 1008–1015 (2017).
Martin, L. et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 31, 1539–1547 (2013).
Prado, C. M. et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 9, 629–635 (2008).
Calvo-González, E. & Santos, R. V. Problematizing miscegenation: The fact/fiction of race in contemporary Brazil. J. Anthropol. Sci. 96, 247–254 (2018).
Mourtzakis, M. et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 33, 997–1006 (2008).
Nazare, J. A. et al. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: The international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-. Am. J. Clin. Nutr. 96, 714–726 (2012).
Yokoi, K. et al. Skeletal muscle changes assessed by preoperative computed tomography images can predict the long-term prognosis of stage III colorectal cancer. Ann. Gastroenterol. Surg. 6, 386–395 (2022).
Taguchi, S. et al. The importance of ethnic-specific cut-offs of low muscle mass for survival prediction in oncology. Clin. Nutr. 43, 134–141 (2024).
Kotti, A., Holmqvist, A., Woisetschläger, M. & Sun, X. F. Computed tomography-measured body composition and survival in rectal cancer patients: A Swedish cohort study. Cancer Metab. 10, 1–12 (2022).
Brown, J. C. et al. The deterioration of muscle mass and radiodensity is prognostic of poor survival in stage I–III colorectal cancer: A population-based cohort study (C-SCANS). J. Cachexia Sarcopenia Muscle 9, 664–672 (2018).
Dolan, R. D. et al. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J. Cachexia Sarcopenia Muscle 10, 111–122 (2019).
de Oliveira Bezerra, M. R. et al. Age-adjusted Charlson comorbidity index and its association with body composition and overall survival in patients with colorectal cancer. Support Care Cancer 32, 517 (2024).
McSorley, S. T., Black, D. H., Horgan, P. G. & McMillan, D. C. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin. Nutr. https://doi.org/10.1016/j.clnu.2017.05.017 (2016).
Malietzis, G. et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann. Surg. 263, 320–325 (2016).
Goodpaster, B. H. et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J. Appl. Physiol. 90, 2157–2165 (2001).
Ebadi, M. & Mazurak, V. Evidence and mechanisms of fat depletion in cancer. Nutrients 6, 5280–5297 (2014).
Ebadi, M. & Mazurak, V. C. Potential Biomarkers of Fat Loss as a Feature of Cancer Cachexia. Mediators Inflamm. 1–8 (2015).
Malietzis, G. et al. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: A systematic review. Eur. J. Surg. Oncol. 41, 186–196 (2015).
Basile, D. et al. Prognostic role of visceral fat for overall survival in metastatic colorectal cancer: A pilot study. Clin. Nutr. 40, 286–294 (2021).
Acknowledgements
The authors acknowledge Dr. Kleyton Medeiros and Dr. Rossana Karenina for providing support in acquiring computed tomography images of patients from the Liga Norteriograndense Contra o Câncer, Natal/RN.
Funding
This study was partially funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil (Finance Code 001) and the Brazilian National Council for Scientific and Technological Development (CNPq). APTF and MCG received a productivity scholarship from CNPq. CMP is partially funded through the Canada Research Chairs Program. The supporting sources have no involvement or restrictions on this publication.
Author information
Authors and Affiliations
Contributions
ALM and APTF contributed to the conception and design of the research. IMS, GMCF, MROB, GVC, FFM, LBM, SMMLV, SFM, JBCC, MCM and ALM conducted the acquisition of the data; JPCP conducted data analyses. ALM and JPCP wrote the manuscript. APTF, GVC, LBM, MCG and CMP critically revised the manuscript. All the authors critically reviewed, interpreted, and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
CMP has previously received honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, Nestlé Health Science, Pfizer, and AMRA Medical. MCG has received honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, and Nestlé Health Science Brazil. APTF reports receiving a grant for research from Prodiet Medical Nutrition. The other authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Miranda, A.L., da Costa Pereira, J.P., de Sousa, I.M. et al. Impact of body composition and muscle health phenotypes on survival outcomes in colorectal cancer: a multicenter cohort. Sci Rep 14, 31816 (2024). https://doi.org/10.1038/s41598-024-83082-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83082-3