Abstract
To date, no prospective study has been conducted to compare the safety and effectiveness of endoscopic snare resection with an elastic band (ESR-EB) and endoscopic snare resection with a transparent cap (ESR-C) for treating gastric muscularis propria lesions. We aimed to compare the safety and effectiveness of ESR-EB with those of ESR-C for gastric muscularis propria lesions less than 10 mm in diameter. A total of 64 patients were enrolled prospectively from May 2023 to November 2023 at Shenzhen Hospital of Southern Medical University, the First Affiliated Hospital of Shantou University, and the People’s Hospital of Zhongshan City. The study compared clinical characteristics, tumour features, and surgical outcomes between the two groups. Of 64 patients, 29 underwent ESR-C, and 35 underwent ESR-EB. There were no differences in age, gender, location, tumour size, growth pattern, resection time, histology diagnosis, or follow-up time (P > 0.05). Complete resection was achieved in all the patients. The operation time was significantly greater in the ESR-C group than in the ESR-EB group (41.31 ± 9.87 min vs. 26.26 ± 10.32 min, P = 0.000). In the ESR-C cohort, 21 patients (72.41%) had perforation, and 1 patient (3.45%) had bleeding. In the ESR-EB group, 7 patients (20.00%) had perforation. The complication rate varied significantly between the two groups (P = 0.000). No recurrence or metastasis was observed in either group during the follow-up period. Both ESR-C and ESR-EB achieved a 100% complete resection rate for gastric muscularis propria lesions less than 10 mm in diameter.ESR-EB had the potential to reduce the operation time and lower the occurrence of complications. Chinese Clinical Trial Registry Identifier ChiCTR2300072856.
Similar content being viewed by others
Introduction
For gastric muscularis propria lesions less than 20 mm in diameter, the use of current treatments is controversial. The National Comprehensive Cancer Network (NCCN) guidelines suggest that endoscopic surveillance may be considered for patients without high-risk endoscopic ultrasonography (EUS) features at an interval of 6–12 months1. In contrast, the European guidelines state that endoscopic resection is an option for avoiding unnecessary follow-up if a difficult preoperative histological diagnosis is unavailable2. The Asian consensus indicates that gastric muscularis propria lesions less than 20 mm in length have a low risk of malignancy, and preoperative diagnosis is difficult; however, malignant risk cannot be ruled out3, so close monitoring is particularly important. However, the long-term follow-up of patients with gastric muscularis propria lesions have some problems: ① Patients have poor compliance and loss to follow-up; ② Patients have high psychological burden and reduced quality of life; ③ Adverse events due to gastroscopy or ultrasound gastroscopy increase during follow-up. Therefore, to avoid unnecessary follow-up, histological diagnosis after endoscopic resection is a less invasive option2.
There are many endoscopic resection methods for treating gastric muscularis propria lesions, but there is no uniform standard for which method to choose4. The choice mostly depends on the operator’s experience, tumour size, location, depth, etc., and each endoscopic treatment has its own advantages and disadvantages. For gastric muscularis propria lesions less than 10 mm, it is difficult to use the conventional endoscopic resection method because these lesions are very small. The results of our previous study [5]suggested that for these small muscularis propria lesions, the complete resection rate can reach 100% by using endoscopic snare resection with elastic band (ESR-EB) and endoscopic snare resection with a transparent cap (ESR-C). However, according to the results of our previous study5and the recently reported literature6,7,8,9,10,11,12,13, the complication rate of ESR-C was greater than that of ESR-EB. To further validate the safety and effectiveness of ESR-C and ESR-EB for treating gastric muscularis propria lesions, a multicentre prospective cohort study was conducted to provide evidence-based medical evidence for endoscopic treatment options for patients with gastric muscularis propria lesions less than 10 mm in length.
Materials and methods
Study design and ethics
This was a multicentre prospective cohort study performed in three cities: Southern Medical University Shenzhen Hospital (Shenzhen), the First Affiliated Hospital of Shantou University (Shantou), and Zhongshan People’s Hospital (Zhongshan). The study was conducted in accordance with the Declaration of Helsinki (revised in 2008) and approved by the Institutional Review Board and Ethics Committee of Shenzhen Second People’s Hospital (2023-091-01PJ). The three centres enrolled patients beginning in May 2023 and ending in November 2023. Informed consent for endoscopic resection was obtained from each patient. The endoscopists decided on the procedure to be performed on each patient according to their own experience.
Sample size calculation
According to previous literature6,7,8,9,10,11,12,13, ESR-C had a complication rate of 54.8%, and that of ESR-EB was 15.13%. The main observation index of this study was the complication rate, for which the sample size was calculated as \(\alpha\:= 0.05,\:\beta\:= 0.1,\:\kappa\:= \frac{{n}_{A}}{{n}_{B}}\) =1; the calculation formula is as follows:
Patient data were calculated based on the complication rate =\(\:\left({p}_{A}\left(1-{p}_{A}\right)+{p}_{B}\left(1-{p}_{B}\right)\right){\left(\frac{{{z}}_{1-\alpha/2}+{{z}}_{1-\beta\:}}{{p}_{A}-{p}_{B}}\right)}^{2}\)= \(\left(0.15\times\:\left(1-0.15\right)+0.55\times\:\left(1-0.55\right)\right)\times\:{\left(\frac{1.96+1.28}{0.15-0.55}\right)}^{2} = 25\: (\text {patients}).\)
Based on the incidence of complications, more than 25 patients were included in each of the ESR-C and ESR-EB groups.
Indications for endoscopic resection
The following conditions are suitable for ESR-C and ESR-EB resection: ① lesions originating from the gastric muscularis propria; ② EUS reveals lesions less than 10 mm in diameter; ③ no lymph node involvement or distant metastases, which was confirmed by CT; and ④ generally stable conditions without severe cardiopulmonary insufficiency and the ability to tolerate endoscopic surgery. Two different types of endoscopic resection procedures were chosen according to each surgeon’s habits and personal experience5.
Instruments
The following instruments were used in this study: an endoscopic image processor (Olympus, Japan, CLV-290SL); an operating gastroscope (Olympus, Japan, HQ260J); an endoscopic ligation device (Boston Scientific Corporation, USA, M00542251); a high-frequency electric generator (ERBE, Germany, VIO300D); a snare (Boston Scientific Corporation, USA, M00561231; Monofilament, 20 mm); a rotatable and repeated opening and closing of a soft tissue clip (Nanwei Medical Technology Co., Ltd., China, POCC-D-26–195); and an endoscopic transparent cap (Olympus, Japan, D-201).
Procedure for ESR-C
A transparent cap was installed at the front end of the endoscope, and the lesion was found in the gastric cavity (Fig. 1A). The snare at the cap slot was opened to fit the snare perfectly into the cap. The transparent cap resisted the lesion, and the lesion was placed in the centre of the field of view (Fig. 1B). The attraction was applied to ensure that the tumour body was completely attracted to the transparent cap. The snare was tightened, and the lesion was resected (Fig. 1C). We observed whether the wound surface was bleeding, perforated, etc. (Fig. 1D). If there was active bleeding or a suspected vessel stump, haemostasis was performed. The wound was sutured by using clips (Fig. 1E). The specimen was recovered, and its size was measured in vitro (Fig. 1F). The specimen was subsequently sent for pathological examination.
Procedure of endoscopic snare resection with a transparent cap (ESR-C) A: The lesion was found by white-light endoscopy; B: The snare was opened at the transparent cap slot. The transparent cap resists the lesion. C: The lesion is attached to the transparent cap. D: The wound surface is observed. E: The wound is sutured using clips. F: The specimen is recovered, and the size is measured in vitro.
Procedure for ESR-EB
A ligation device was installed at the front of the endoscope, and the lesion was found in the gastric cavity (Fig. 2A). The lesion was attracted to the transparent cap, and the elastic band was released for ligation (Fig. 2B). The snare was placed under the elastic band, and the lesion was resected (Fig. 2C). We observed whether the wound surface was bleeding, perforated, etc. (Fig. 1D). If there was active bleeding or a suspected vessel stump, haemostasis was performed. The wound was sutured by using clips (Fig. 1E). The specimen was recovered, and its size was measured in vitro (Fig. 1F). The specimen was subsequently sent for pathological examination.
The procedure of endoscopic snare resection with an elastic band (ESR-EB). A:The lesion is found by white-light endoscopy; B: Ligation of the lesion by using an elastic band; C: The snare is placed under the elastic band to resect the lesion; D: The wound surface is observed; E: The wound is sutured using clips; F: The specimen is recovered, and the size is measured in vitro.
Specimen processing
All specimens were processed and fixed in 10% formalin solution for 6–24 h. The sections were then stained with haematoxylin and eosin (H&E), and all the specimens were subjected to immunohistochemistry using antibodies against CD117, DOG 1, CD34, SDHB, and Ki67. Pathology was performed by a gastrointestinal pathologist at our hospital, who evaluated the tumour size, risk grade, resection margin, and vascular invasion5.
Clinical outcomes
We analysed the endoscopic complete resection rate, operation time, resection time, and complication rate (including intraoperative bleeding and intraoperative perforation). The patients were followed to observe whether recurrence and/or metastasis occurred. Complete resection was defined as one-time resection of the lesion, no macroscopic residual tissue remained, and the histological diagnosis was margin negative. The operation time was defined as the time from endoscopic entry into the oesophagus to endoscopic exit from the oesophagus. The resection time was defined as the time from the start of contact between the transparent cap and the lesion to the completion of the wound being sutured by clips. Intraoperative bleeding was defined as active bleeding that required endoscopic haemostasis. Intraoperative perforation was defined as the visualisation of intraabdominal organ tissue or the omentum. Recurrence was defined as the presence of new bulging masses at the original location during follow-up. Metastasis was defined as lymphatic metastasis or organ metastasis detected during follow-up imaging. Each patient was instructed to undergo an endoscopy or CT scan 1 year after surgery.
Statistical analysis
All the data were statistically analyzed using SPSS28 statistical software. The study was a prospective cohort study, and the sample size was calculated based on the data from published articles. The number of patients in both groups was greater than or equal to the calculated sample size. The count data (e.g., gender, growth pattern, location, complication rate, histology diagnosis, etc.) were analyzed by the χ2 test. For continuous variables (e.g., age, tumour size, operation time, resection time, follow-up time, etc.), the data were fitted to a normal distribution using the t-test for two independent samples. The Mann‒Whitney U test was used for nonnormally distributed data. P < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of patients who underwent ESR-C and ESR-EB
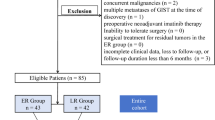
A total of 64 patients were enrolled; 29 patients underwent ESR-C, and 35 patients underwent ESR-EB. There were no differences in age, gender, location, tumour size, or growth pattern (P > 0.05); therefore, it was not necessary to match the two groups (Table 1).
Comparison of treatment results between ESR-C and ESR-EB
There were no significant differences in resection time, histology diagnosis, or follow-up time between the two groups (P > 0.05). Complete resection was achieved in all the patients. The operation time was significantly greater in the ESR-C group than in the ESR-EB group (41.31 ± 9.87 min vs. 26.26 ± 10.32 min, P = 0.000). The resection time of ESR-C was shorter than that of ESR-EB, and there was no significant difference between the two groups (9.55 ± 4.99 min vs. 10.00 ± 4.36 min, P = 0.703). In the ESR-C cohort, 21 patients (72.41%) had perforation, and 1 patient (3.45%) had bleeding. In the ESR-EB group, 7 patients (20.00%) had perforation, and no patients had bleeding. The complication rate varied significantly between the two groups (P = 0.000). All perforations were successfully sutured endoscopically using tissue clips. In one case of bleeding, hemostasis was achieved using endoscopic electrocoagulation. No recurrence or metastasis was observed in either group during the follow-up period (Table 2).
Discussion
The most common gastric muscularis propria lesion is a gastrointestinal stromal tumour (GIST)14,15. Among the 64 patients enrolled in this study, 38 had GISTs, 22 had leiomyomas, 2 had neurofibromas, 1 had a schwannoma and 1 had anangioma. Among these, GISTs were the most common, followed by leiomyomas, which accounted for 93.75% of all tumours. EUS is limited in distinguishing GISTs from leiomyomas. For leiomyomas, resection or endoscopic monitoring are not needed. However, because GISTs are malignant tumours and have a risk of distant metastasis, timely resection is recommended by most experts and guidelines (The European Society of Medical Oncology, The Japanese Society of Clinical Oncology, The Chinese Society of Clinical Oncology)3,16.
There is still controversy over small gastric muscularis propria lesions. The European Oncology Society recommends that small lesions require resection if the histological diagnosis is a GIST17,18. The problem is that preoperative diagnosis is difficult for small gastric muscularis propria lesions. Therefore, according to the European guidelines, due to the difficulty of obtaining a preoperative histological diagnosis, endoscopic resection can be used to avoid unnecessary follow-up2.
There are many endoscopic resection methods for treating gastric muscularis propria lesions, including endoscopic snare resection19, endoscopic submucosal dissection (ESD)20, endoscopic submucosal excavation (ESE)21, submucosal tunnelling endoscopic resection (STER)22, endoscopic full-thickness resection (EFTR)23, and laparoscopic endoscopic cooperative surgery (LECS)24. The Chinese Consensus on Endoscopic Diagnosis and Management of Gastrointestinal Submucosal Tumours recommended ESE and EFTR for resection methods of gastric muscularis propria lesions25. Endoscopic snare resection and the associated assisted snare resection technique are suitable only for resection of the mucosal muscularis and submucosal lesions26.
However, regarding gastric muscularis propria lesions (≤ 10 mm) in clinical practice, it is difficult to find tumours when using guideline-recommended resection methods; sometimes, the tumour cannot be found. The reasons may be as follows: ① The tumour growth pattern was mixed growth or extraluminal growth. Therefore, even if the knife cuts to the muscularis propria, the tumour body still fails to be exposed. ②: Bleeding occurs during the mucosal incision process, and electrocoagulation and haemostasis lead to unclear submucosal layers and poor visual field. ③: Although the position is marked before the incision, the position of the mark point shifts after submucosal injection. In the present study, in terms of growth pattern, only 6 patients had an intraluminal growth type, only 1 had an extraluminal growth type, and the remaining 57 had mixed growth types. This means that it is more difficult to find tumours via the conventional endoscopic resection method after the incision of the mucosa. ESR-C and ESR-EB can be used to circumvent situations where tumours cannot be found. ESR-C and ESR-EB can be used to effectively and safely resect gastric muscularis propria lesions less than 10 mm in diameter, and these two endoscopic resection methods are simple to perform and easy to popularise6,7,8,9,10,11,12,13. The results of this study also confirm that each of these two endoscopic resection methods can result in 100% resection of gastric muscularis propria lesions, and the therapeutic effect is positive.
Both ESR-C and ESR-EB can be used to effectively resect gastric muscularis propria lesions, but which method is most suitable has not been reported in the literature. Our previous retrospective study showed that the incidence of adverse events in patients who underwent ESR-C was greater than that in patients who underwent ESR-EB5. To further confirm the safety of these two endoscopic resection methods, in the present study, a multicentre prospective cohort study was conducted in three cities in southern China. This is the first study to compare the safety and effectiveness of these two endoscopic methods for the resection of gastric muscularis propria lesions. The results showed that the adverse event rate of the ESR-C group was much greater than that of the ESR-EB group. The reason for this result is still unclear, and we speculate that the cause may be that ESR-EB uses ligation with an elastic band. Due to the flexibility of the elastic band, even if the full gastric wall is ligated, the lamina propria will slowly break away from the elastic band during peristalsis or expansion, thus reducing full-thickness resection of the gastric wall. ESR-C tightens the root of the lesion with a snare. Because the snare is inelastic and the time from tightening to resection is short, the muscularis propria is not able to easily break free of the snare, resulting in full-thickness resection of the gastric wall. Although the incidence of perforation in ESR-C patients is high, the perforation is small, and all the perforations can be stitched by clips. None of the patients developed peritonitis, which may be related to the short perforation exposure time.
Another advantage of ESR-C and ESR-EB over endoscopic resection techniques such as ESE and EFTR is the short resection time. Yan Meng et al.8 used ESR-EB to resect 72 gastric GISTs, and the resection time was 17.11 ± 4.97 min, which was significantly less than that of ESD. Jinping Yang et al.13 showed that the resection time of ESR-C was significantly shorter than that of EFTR. The difference in resection time between ESR-C and ESR-EB has not been reported in the literature. In the present study, the operation time in the ESR-C group was significantly greater than that in the ESR-EB group. This is because the ESR-C requires opening of the snare at the front of the transparent cap. Because this procedure is performed inside the gastric cavity, it is difficult to access the front end of the snare when the snare is stuck into the transparent cap slot, which requires repeated attempts and sometimes needs to be positioned against the gastric wall. This results in a significant amount of time being spent in the preresection preparatory phase in ESR-C, resulting in a prolonged operation time. Unlike in ESR-C, when ESR-EB is performed, the ligation device is installed in vitro and has a simple installation operation that saves overall operation time. Although ESR-C resection time was shorter than ESR-EB resection time, the difference was not significant.
Our study has several limitations. First, due to the limitations of the transparent cap and ligation device size, these two endoscopic procedures are suitable only for resection of gastric muscularis propria lesions less than 10 mm in diameter. Second, the mean follow-up time of this study was short, which prevents us from evaluating postoperative recurrence and metastasis. It is necessary to extend the follow-up time in future studies. Three endoscopists performed endoscopic resection in this study. We cannot avoid the difference in the ability of our staff to perform endoscopic evaluation and treatment; however, each of the endoscopists had more than 5 years of working experience, and all the staff members had completed more than 100 cases of ESR-EB and more than 100 cases of ESR-C. Fourth, this study compared only ESR-C and ESR-EB and not common methods such as ESE or EFTR. Large-scale randomised controlled studies are needed to further explore the advantages and disadvantages of these endoscopic procedures, including ESE and EFTR, and our team is currently working on these studies.
In conclusion, both ESR-C and ESR-EB are effective and safe for the resection of gastric muscularis propria lesions. Overall, ESR-EB, which is a safer and time-saving endoscopic operation technique, can significantly reduce the incidence of adverse events and shorten the operation time.
Data availability
All data generated or analysed during this study are included in this published article.
References
Demetri, G. D. Mehren MV,Antonescu CR (2012) Update on the management of patients with gastrointestinal stromal tumors. J. Natl. Compr. Canc. Netw. 8:S1–S41 .
Pierre, H., Vieth, I., Borbath, T. G. & Moreels Els Nieveen van Dijkum,Jean-Yves Blay, Jeanin E. van Hooft (2022) Endoscopic management of subepithelial lesions including neuroendocrine neoplasms European Society of Gastrointestinal Endoscopy (ESGE) Guideline.Endoscopy 54: 412–429.
Qin, J. L. Y. Y. J. W. B. Z. S. Yingqiang Shi, Yulong He, Xiaobo Liang, Xiufeng Liu, Ye Zhou, Xin Wu, Xinhua Zhang, Ming Wang, Zhidong Gao, Tianlong Lin, Hui Cao, Lin Shen (2017) Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin. J. Cancer Res. https://doi.org/10.21147/j.issn.1000-9604
Yu Zhang, Li-Ping Ye, Xin-Li Mao (2015) Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J. Gastroenterol. 21:9503–9511.
Zhaohui, L. Runhua Lin, Ruinuan Wu, Rui Li (2023) Comparison analysis of two different types of endoscopic resection procedures in small gastric subepithelial tumours originating frommuscularis propria. Scand. J. Gastroenterol. 59:213–217.
He, S. Guohua Li, Guoping Du, Quju You, Haoyue Ouyang (2016) Discussion of the clinical efficacy of endoscopic mucosal resection for gastric stromal tumor. Mod. Digestion Intervention 21:755–757.
Guo, J. Zhijun Liu, Siyu Sun, Sheng Wang, Nan Ge, Xiang Liu, Guoxin Wang, Xianghong Yang (2013) Ligation-assisted endoscopic enucleation for the diagnosis and resection of small gastrointestinal tumors originating from the muscularis propria: a preliminary study. BMC Gastroenterol. 13:88.
Meng, Y. Chunli Cao,Shujie Song,Yue Li,Side Liu(2016)Endoscopic band ligation versus endoscopic submucosal dissection and laparoscopic resection for small gastric stromal tumors. Surg. Endosc. 30:2873–2878.
Weijin Pan, Ding Shi. Band-assisted endoscopic mucosal resection for small (≤ 1.5 cm) submucosal tumors originating from the muscularis propria in the gastric fundus: a prospective study.Surgical Endoscopy. https://doi.org/10.1007/s00464-022-09688-8 (2022). October 13,2022.
Ko, E. J. Endoscopic Enucleation Is Effective and Relatively Safe in Small Gastric Subepithelial Tumors Originating from Muscularis Propria. Dig. Dis. Sci. 64, 524–531 (2019). Byoung Wook Bang,Kye Sook Kwon,Yong Woon Shin,Hyung KilKim.
Luo, Y. Bitao Lin,Yue Zhang, Weiguang Qiao, Qiang Zhang, Fachao Zhi, Yue Li, Side Liu (2021) Safety analysis of transparent cap-assisted endoscopic resection for ≤ 10 mm gastric submucosal tumors[J]. Mod. Digestion Intervention 26:426–431.
Weiguang & Qiao Yutang Ren, Wei Gong, Bo Jiang, Side Liu, Dan Zhou, Jing Li, Tongyin Xing, Yang Bai, Fachao Zhi (2015) Cap-aspiration lumpectomy for small submucosal tumors originating from the muscularis propria of the gastric fundus: a preliminary study (with videos). J. Dig. Dis. 16:642–648.
Jinping, Y. Muhan Ni, Jingwei Jiang, Ximei Ren, Tingting Zhu, Shouli Cao, Shahzeb Hassan, Ying Lv, Xiaoqi Zhang, Yongyue Wei, Lei Wang, Guifang. Xu(2022)Comparison of endoscopic full-thickness resection and cap-assisted endoscopic full-thickness resection in the treatment of small (≤ 1.5 cm) gastric GI stromal tumors ScienceDirect Gastrointest. Endoscopy 95:660–670.
Menon, L. & Buscaglia JM (2014) Endoscopic approach to subepithelial lesions[J]. Ther. Adv. Gastroenterol. 7:123–130.
Dumonceau, J. M. et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 49, 695–714 (2017).
ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 25, iii21–26 (2014).
Expert Committee of gastrointestinal stromal tumor of the Chinese Clinical Oncology Society. The Chinese consensus on the diagnosis and treatment of gastrointestinal GISTs. Electron. J. Compr. cancer therapy. 4, 31–43 (2017).
Christopher, D. M., Fletcher,Jules, J., Lasota, B. J., Longley, M. & Miettinen,Timothy, J. Berman, Christopher Corless, Fred Gorstein, Jerzy O\Leary, Helen Remotti, Brian P. Rubin, Barry Shmookler, Leslie H. Sobin, Sharon W. Weiss (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach.Hum Pathol 33:459–465.
Bohnacker, S., Seitz, U., Seewald, S. & Brand, B., Soehendra, N. (2003) Endoscopic snare resection of benign ampullary tumor: can intraductal growth be treated endoscopically?Gastrointestinal. Endoscopy 57:AB101.
Jinlong, H. Nan Ge, Sheng Wang, Jintao Guo, Xiang Liu, Guoxin Wang, Siyu Sun (2020) Direct endoscopic full-thickness resection for submucosal tumors with an intraluminal growth pattern originating from the muscularis propria layer in the gastric fundus. BMC Gastroenterol. 20:70.
I – L, Lee, P. Y. & Lin S.– Y.Tung, C.–H.Shen, K.–L.Wei, C.–S.Wu (2006) Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis. propria layer. Endoscopy 38:1024–1028.
Shanshan Wang, Lei Shen. Efficacy of Endoscopic Submucosal Excavation for Gastrointestinal Stromal Tumors in the Cardia.Surg Laparosc. Endosc Percutan Tech. 26, 493–496 (2016).
Yuyong Tan, X. & Tang Ting Guo, Dongzi Peng, Yao Tang, Tianying Duan, Xuehong Wang, Liang Lv, Jirong Huo, Deliang Liu (2016) Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer[J]. Surgical Endoscopy, https://doi.org/10.1007/s00464-016-5350-7.November 18,2016.
Yaqi, Z. Ningli Chai, Huikai Li, Zhongsheng Lu, Xiuxue Feng, Wengang Zhang, Shengzhen Liu, Enqiang Linghu (2019) Endoscopic submucosal excavation and endoscopic full-thickness resection for gastric schwannoma: five-year experience from a large tertiary center in China. Surgical Endoscopy, https://doi.org/10.1007/s00464-019-07285-w. December 06,2019.
Lei, C. Kunming Zheng, Honglei Wang, Yongjie Zhao, Zhengduo Yang, Wen Li (2019) Laparoscopic and Endoscopic Cooperative Dissection for Small Gastric Gastrointestinal Stromal Tumor without Causing Injury to the Mucosa. Gastroenterology Research and Practice Volume,2019, https://doi.org/10.1155/2019/7376903.December 13,2019.
NOTES and Endoscopic Surgery Group, Chinese Society of Digestive Endoscopology, Chinese Medical Association. Chinese consensus on endoscopic diagnosis and managment of gastrointestinal submucosal tumors (version 2023). The Chinese. J. Practical Surg. 43, 341–351 (2023).
Funding
This work was supported by the Shenzhen Second People’s Hospital Clinical Research Fund of Shenzhen High-level Hospital Construction Project (Nos.2023yjlcyj018, 20223357019), Medical Scientific Research Foundation of Guangdong Province of China (No.A2022329), Clinical Scientific Research Foundation of Guangdong Provincial Medical Association (No.A202302031), and Teaching Reform Research Project of Shenzhen University Medical Science Center (No.YXBJG202302).
Author information
Authors and Affiliations
Contributions
Zhaohui Liu and Xiangyu Wang analyzed the data and wrote the main manuscript text. Jiefeng Li and Yongsheng Lu provided the original data. Ruinuan Wu and Dayong Sun prepared Figs. 1 and 2; Table 1, and 2. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Disclosures
Drs. Zhaohui Liu, Xiangyu Wang, Genhua Yang, Jiefeng Li, Yongsheng Lu, Dayong Sun and Ruinuan Wu have no conflicts of interest or financial ties to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, Z., Wang, X., Yang, G. et al. A multicentre, prospective cohort study comparing two endoscopic procedures for the treatment of gastric muscularis propria lesions. Sci Rep 14, 31476 (2024). https://doi.org/10.1038/s41598-024-83203-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83203-y