Abstract
Entomopathogenic fungi engineered to express insect-specific neurotoxins have demonstrated potential as microbial control agents against malaria mosquitoes. Currently, the primary application method is via direct contact of spores with indoor resting mosquitoes. However, many malaria-transmitting mosquitoes feed and rest outdoors. To target these, we have developed an alternative application method that exploits the lethality of transgenic fungi as a sexually transmitted mosquito disease. This approach has both a wider interdisciplinary significance and important implications for preventing mosquito-borne diseases.
Similar content being viewed by others
Mosquito resting behavior is highly variable, even within species, with individuals displaying either endophilic (resting indoors) or exophilic (resting outdoors) tendencies after a blood meal1. Current interventions targeting indoor mosquitoes, such as long-lasting insecticidal nets or newly developed transgenic fungi, are less effective against exophilic mosquitoes, and their sustainability is threatened by shifts in mosquito behavior toward outdoor feeding2. Horizontal sexual transmission of Metarhizium spp. could enhance the impact of both wild-type and modified fungus on exophilic and endophilic mosquitoes, and could be compatible with other promising vector control tools, like the Sterile Insect Technique (SIT) and Wolbachia-based technologies. Previous studies indicate that Metarhizium can be transmitted sexually, but inoculum loads (~ 25 conidia /female mosquito) and resultant mortality rates are low3. However, unlike the wild-type fungus, even a single transgenic Metarhizium spore can be lethal4, potentially mitigating the impact of low inoculum. While autodissemination of fungal spores by female Anopheles gambiae s.s. has been reported5, data on transfer from fungus-infected male mosquitoes to uninfected females is lacking3. In addition, no data exist on Metarhizium spp. as a sexually transmitted infection under field-like conditions.
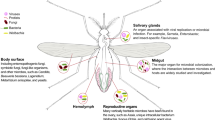
To potentially combine transgenic entomopathogenic fungi with SIT biotechnologies, we evaluated the duration post-treatment with Metarhizium during which males could transmit a lethal infection of conidia to mating partners. To this end, we analyzed the complex relationships between dose, virulence, and sexual transmission of fungi under field conditions. Ethical permissions were obtained according to Burkina Faso National Biosecurity Agency regulations from the Institutional Review of Institut de Recherche en Science de la Santé (IRSS) and Centre Muraz Ethics Committee for importing and using Metarhizium transgenic fungi in semi-field and laboratory work. We have previously reported semi-field results using the same Metarhizium pingshaense strains6, used in this study, which were transformed to constitutively express red or green fluorescent protein (RFP or GFP). The GFP construct additionally included expression of Hybrid-toxin under the hemolymph-specific Mcl1 promoter4. The RFP-expressing strain demonstrates wild-type virulence and growth and was used as a proxy for the wild type6. Both strains were maintained on potato dextrose agar at 27 °C with 70% relative humidity. For sexual transmission bioassays, adult Anopheles coluzzii mosquitoes were reared from larval collections at Vallée du Kou (11°23′N, 4°24′W), where mosquitoes are resistant to multiple insecticides4 but vulnerable to pathogenic fungi4. Three experiments conducted in the laboratory and semi-field contained facility6 studied the sexual transmission of fungal infections in mosquitoes, as determined from the inoculation rate (percentage of mosquitoes with spores on their cuticle), inoculum load (the number of spores on each mosquito), and mortality curves/LT50 values (median lethal time after exposure to fungal infections). The first experiment evaluated female survival after mating with males infected with a transgenic or wild-type fungus. The second experiment assessed female survival after mating with males treated with no fungus (controls), wild-type fungus, or transgenic fungus at various intervals. The third experiment evaluated mating rates and survival of mosquitoes released into semi-field compartments.
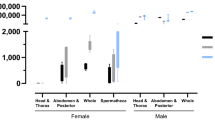
Results from artificial forced mating showed no difference in the sexually transmitted inoculum loads between the wild-type and transgenic strains (Supplementary Fig. S1), with 68 ± 4% and 89.33 ± 1.33% of the females dying within 14 days of mating with males treated with wild-type and transgenic fungi, respectively (Supplementary Fig. S2). Thus, the transgenic fungus was more effective at killing mosquitoes than the wild-type fungus, and males treated with either fungus remained similarly infectious to females for up to 24 h post-treatment (Fig. 1). Successive cohabitation experiments indicated that females did not die from contact with surfaces where treated males had landed. These findings demonstrate that both wild-type and transgenic mosquito-pathogenic fungi can cause lethal sexually transmitted infections (STI). The greater effectiveness of the transgenic fungus, coupled with treated males remaining infectious for up to 24 h, supports the potential use of transgenic fungi in combination with SIT7, whether as a classical7 or Wolbachia-based approach8, to enhance mosquito control. However, Fig. 1 suggests that fewer females died after exposure to males infected for 48 h with a transgenic fungus compared to those exposed to males infected with a wild-type fungus, likely due to the onset of symptoms. Forty-eight hours marks the beginning of hybrid toxin expression in males, reducing their ability to infect females4.
We observed that the number of couples formed in each semi-field compartment was significantly influenced by their position relative to the sunset, with a higher number observed in specific locations (Supplementary Fig. S3). Using either control or transgenic fungi did not significantly affect mating rates within 24 h post fungal application (Supplementary Fig. S4). Notably, overall mortality in female mosquitoes treated with transgenic fungus (26.1%) closely matched estimated mating rates (24.4%), indicating that mating almost inevitably led to female mortality. In contrast, the survival rate among uninfected mosquitoes was approximately 96%, despite having the same percentage of mated females (Fig. 2). For future field deployment of transgenic fungi as an STI alone or combined with other male release strategies (i.e., SIT + STI), preliminary studies on swarm characteristics will be necessary. In this regard, the reliable transfer of fungal spores from males to females during mating may serve as a tool for basic investigation of mosquito mating behavior and SIT effectiveness.
Data availability
Supplementary Materials and Methods, data and codes are available at: https://github.com/Lovett-Lab/Mosquito-STD-EPF. Raw data used for the analysis are in the supplementary materials accompanying the article.
References
Gatton, M. L. et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. https://doi.org/10.1111/evo.12063. (2013). Epub 2013 Feb 15. PMID: 23550770; PMCID: PMC3655544.
Debebe, Y. et al. Shady business: Understanding the spatial ecology of exophilic Anopheles mosquitoes. Malar. J. https://doi.org/10.1186/s12936-018-2499-7 (2018).
Ernst-Jan Scholte, E. J. et al. Autodissemination of the entomopathogenic fungus Metarhizium anisopliae amongst adults of the malaria vector. Anopheles gambiae s s. https://doi.org/10.1186/1475-2875-3-45 (2004).
Bilgo, E. et al. Improved efficacy of an arthropod toxin expressing fungus against insecticide-resistant malaria-vector mosquitoes. Sci. Rep. 7, 3433. https://doi.org/10.1038/s41598-017-03399-0 (2017).
Mascarin, G. M. et al. Current status and perspectives of fungal entomopathogens used for microbial control of arthropod pests in Brazil. J. Invertebrate Pathol. Rev. https://doi.org/10.1016/j.jip.2018.01.001 (2019).
Lovett, B. et al. Transgenic Metarhizium rapidly kills mosquitoes in a malaria-endemic region of Burkina Faso. Science 364, 894–897 (2019).
Helinski, M. E. H. et al. The Sterile Insect Technique for malaria control: A systematic review. Malar. J. 19 (1), 1–23. https://doi.org/10.1186/s12936-020-03402-5 (2020).
Hoffmann, A. A. et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476 (7361), 454–457. https://doi.org/10.1038/nature10356 (2011).
Acknowledgements
This work was supported by NIH-NIAID under award RO1-AI106998 to R.J.S. and A.D. E.B. was supported by the Wellcome Trust fellowship for the data analysis and manuscript writing Ref: 218771/Z/19/Z.
Author information
Authors and Affiliations
Contributions
E.B. B.L., R.J.S.L. and A.D. designed studies.E.B., B.L., A.S.M., I.S. and E.J.G performed the experiments, analyzed data.E.B., B.L., R.J.S.L. and A.D. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bilgo, E., Lovett, B., Millogo, A.S. et al. Transmission of transgenic mosquito-killing fungi during copulation. Sci Rep 15, 2181 (2025). https://doi.org/10.1038/s41598-024-83242-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83242-5