Abstract
Previous observational studies have suggested at a potential link between migraine, particularly migraine with aura, and the susceptibility to early-onset ischemic stroke. We aimed to investigate the causal effects of genetically determined migraine and its subtypes on the risk of early-onset ischemic stroke using the two-sample Mendelian randomization method. Genetic instrumental variables associated with migraine and its subtypes were acquired from two sources with the largest sample sizes available. Summary data for early-onset ischemic stroke was acquired from a study encompassing individuals aged 18–59 years, comprising 16,730 cases and 599,237 non-stroke controls. The random-effects inverse variance weighted method was used as the primary analysis approach. Additionally, linkage disequilibrium score regression analysis was used to evaluate the genetic correlation. The Mendelian randomization analysis revealed no association between overall migraine and migraine without aura with the risk of early-onset ischemic stroke. However, migraine with aura showed a suggestive association with an elevated risk of early-onset ischemic stroke, with odds ratios of 1.114 (95% confidence interval = 1.005 to 1.236, p-value = 0.040) and 1.062 (95% confidence interval = 1.002 to 1.126, p-value = 0.042) based on instruments from two independent sources. The odds ratio was 1.074 (95% confidence interval = 1.022 to 1.130, p-value = 0.005) based on instruments from both two sources. No evidence of heterogeneity or horizontal pleiotropy was found. By contrast, migraine with aura was not related to ischemic stroke in all adults. Furthermore, a significant positive genetic correlation was found between migraine with aura and early-onset ischemic stroke (genetic correlation = 0.208, 95% confidence interval = 0.038 to 0.377, p-value = 0.016). This study provides evidence of a causal relationship between migraine with aura and the risk of early-onset ischemic stroke, as well as a positive genetic correlation between them.
Similar content being viewed by others
Introduction
Migraine, a prevalent neurological disorder, is characterized by recurrent and often severe headaches, frequently accompanied by a range of associated symptoms. It has a global age-standardized prevalence of 14.4%1. Many observational studies have consistently highlighted an elevated risk of cardiovascular diseases, including coronary heart disease, stroke, and venous thromboembolism, in individuals with migraine2,3,4,5,6,7,8. This risk may be particularly heightened in cases featuring aura,[3; 4; 9; 10] as well as in female cases11,12.
Mendelian randomization (MR), an emerging research methodology, provides a novel approach to investigating causal relationships between different phenotypes13. By simulating randomized controlled trials, MR studies offer promise for mitigating reverse causality and addressing confounding factors more effectively than traditional observational studies13. Despite this, prior MR studies have yet to confirm causal effect of migraine and its subtypes on increased risk of ischemic stroke14,15. A potentially protective effect of genetically liability to migraine on ischemic stroke and coronary heart disease risk was even identified by MR studies16,17. As for venous thromboembolism, our recently MR study suggested a potential association between migraine and the risk of venous thromboembolism, accompanied by a significant positive genetic correlation between migraine and venous thromboembolism18.
The lack of conclusive MR findings linking migraine and its subtypes with ischemic stroke may be attributed to a potential association of migraine with early-onset stroke rather than late-onset stroke11,12. Notably, previous genome-wide association studies (GWASs) of ischemic stroke have not distinguished between early and late onset19,20, possibly contributing to subsequent inconclusive MR outcomes. The recent release of GWAS summary data pertaining to early-onset ischemic stroke provides an opportunity to explore the relationship between migraine and early-onset ischemic stroke21.
In this study, we employed the two-sample MR method to assess the correlation between migraine and its subtypes and early-onset ischemic stroke (Fig. 1), complemented by linkage disequilibrium score regression (LDSC) analysis to ascertain their genetic correlation.
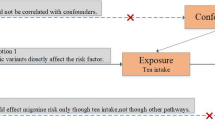
Design of two-sample Mendelian randomization in the present study. In MR study, three key assumptions are crucial: the relevance assumption, stating that the genetic variant strongly associates with the exposure; the independence assumption, ensuring the genetic variant’s independence from confounders; and the exclusion restriction assumption, which dictates that the genetic variant solely affects the outcome through the exposure factor. For instrumental SNPs used in both data sources, we first combined un-clumped SNPs from the two sources and then conducted the clumping procedure. Abbreviations: Any, any migraine; MA, migraine with aura; MO, migraine without aura; SNP, single nucleotide polymorphism; IHGC, International Headache Genetics Consortium.
Methods
This study is reported as per the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guideline for the reporting of MR studies22. Ethics approval and consent to participate were obtained for the original studies. For the present study, they were not required since only summary-level data were used.
Instrumental variable selection
Supplementary Fig. 1 shows the flow diagram of instrumental variables selection. There are three assumptions for MR study (Fig. 1)13,23,24. Firstly, the genetic variant must be strongly associated with the exposure. Secondly, the genetic variant must be independent of confounders. Thirdly, the genetic variant can only affect the outcome through the exposure of interest.
The exposures being studied include any migraine, as well as the subtypes of migraine with aura and migraine without aura. In order to satisfy the first assumption, significant single nucleotide polymorphisms (SNPs) at the genome-wide level (with a p-value < 5 × 10–8) were utilized as instrumental variables for any migraine. However, due to a limited number of SNPs with a p-value < 5 × 10–8 for migraine with aura and migraine without aura, the threshold for instrumental variables was relaxed to < 1 × 10–5. This approach has been commonly employed in previous MR studies, including those utilizing migraine subtypes as exposures14,25,26. In addition, we also excluded SNPs with evidence of heterogeneity (with I2 larger than 50% and a p-value of the Q statistic smaller than 0.05) before conducting the clumping procedure.
To fulfill the second assumption, for SNPs associated with exposures with a p-value less than 5 × 10–8, we excluded SNPs associated with potential confounders with a p-value less than 5 × 10–8 in the IEU OpenGWAS database13. Similarly, for SNPs associated with exposures with a p-value less than 1 × 10–5, we excluded SNPs associated with potential confounders with a p-value less than 1 × 10–5 in the IEU OpenGWAS database. In total, 836 GWAS summary datasets for phenotypes primarily encompassing cardiometabolic risk factors were selected (Supplementary Table 1).
Summary data for migraine
For each of the three migraine phenotypes, three sets of instrumental SNPs were used.
The first set of instrumental SNPs were chosen from the GWAS performed by Bjornsdottir et al. recently (Supplementary Table 2)27. The authors conducted GWAS meta-analyses of clinically defined any migraine (79,495 cases and 1,259,808 controls), migraine with aura (16,603 cases and 1,336,517 controls) and migraine without aura (11,718 cases and 1,330,747 controls) using datasets from six study cohorts of European descent. At least age, sex and leading principal components were included as covariates in the models to test for genome-wide associations between sequence variants and migraine phenotypes.
The second set of instrumental SNPs were chosen from two GWASs performed by the International Headache Genetics Consortium (IHGC) (Supplementary Table 3)28,29. The latest GWAS conducted by them included an additional 42,410 new migraine cases from four study cohorts of European descent, added to their previous GWAS meta-analysis. The total sample comprised 102,084 cases and 771,257 controls. Covariates included at least sex and ≥ four leading principal components in the models, with age included as a covariate when available.
For instrumental SNPs potentially to be included, we excluded those SNPs that were not present in the 1000G v3 reference panel of European population, those with evidence of heterogeneity, those with palindromic alleles (as the frequency of the effect allele was absent in the outcome data), and those associated with confounders prior to carrying out the clumping procedure (Supplementary Fig. 1 and Supplementary Table 4). The clumping procedure was then carried out by employing an r2 value of 0.01 and a physical window of 10 MB18.
Finally, the first set of instrumental SNPs was composed of 37 SNPs associated with any migraine, 43 SNPs associated with migraine with aura, and 30 SNPs associated with migraine without aura. The second set of instrumental SNPs encompassed 82 SNPs associated with any migraine, 35 SNPs associated with migraine with aura, and 28 SNPs associated with migraine without aura. When combining the SNPs for each exposure together to carry out the clumping procedure, there were 107 SNPs associated with any migraine, 78 SNPs associated with migraine with aura, and 57 SNPs associated with migraine without aura (Fig. 1 and Supplementary Fig. 1). All instrumental SNPs exhibit an F statistics value greater than 10, and this was arrived at by computing beta2 divided by se2. If instrumental SNPs are not found in the outcome data, proxy SNPs (with an r2 > 0.8) will be used if available. This was achieved using the “query_gwas” function in the R package “gwasvcf”.
Summary data for early-onset ischemic stroke
Jaworek et al. conducted a GWAS meta-analysis on early-onset stroke, with individuals ranging in age from 18 to 59 years21. They utilized either individual-level data or summary statistics obtained from 16,730 cases and 599,237 non-stroke controls across 48 distinct studies. All the patients underwent brain imaging in order to rule out diagnoses other than ischemic stroke. In most of the studies, additional screening was carried out to eliminate cases that were considered to be attributed to a known monogenic cause (such as sickle cell disease) or a non-genetic cause (such as drug use or complications resulting from procedures). The covariates mainly encompassed sex as well as up to 10 principal components. We employed summary data specific to Europeans, which encompassed 11,114 cases and 435,540 controls, to carry out the MR analysis.
For the purpose of comparison, we also conducted MR analysis using summary data of ischemic stroke involving all adults aged 18 and older, which consisted of 34,217 cases and 406,111 controls of European descent19.
Mendelian randomization analysis
We employed the random-effects inverse variance weighted method as the primary analysis approach. This method assumes that all SNPs are valid instrumental variables with no horizontal pleiotropy, and it provides a comprehensive estimation of the causal association by combining the effects derived from each SNP30. When SNPs exhibit horizontal pleiotropy, and this pleiotropy is balanced, the effect estimation tends to be asymptotically unbiased, despite the standard error being overly precise. We also utilized MR-Egger, weighted median, and mode-based MR methods to account for the impact of horizontal pleiotropy in MR studies. These methods are able to provide unbiased estimates when all or some of the instrumental variables exhibit horizontal pleiotropy. We used MR-Egger regression to identify horizontal pleiotropy. To evaluate the potential heterogeneity among individual SNPs, we adopted the Cochrane’s Q test for the inverse variance weighted method and the Rücker’s Q test for the MR-Egger method. Additionally, we employed the MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) approach to identify potential outliers. In case outliers were detected, the MR-PRESSO method would provide an estimate after excluding those outliers31.
We performed the Steiger test for each SNP to determine if the SNP-exposure effect is larger than the SNP-outcome effect32. Sensitivity analysis was then conducted by excluding SNPs with a SNP-exposure effect smaller than the SNP-outcome effect.
We carried out MR analysis by utilizing the TwoSampleMR R package within R (version 3.6.1). We deemed a Bonferroni-corrected p-value less than 0.016 (0.05 divided by 3; taking into account 3 migraine exposures) as signifying statistical significance. A p-value ranging between 0.016 and 0.05 was considered as having suggestive association.
Linkage disequilibrium score regression
We estimated the genetic correlation between migraine and ischemic stroke using the LD score software (LDSC v1.0.1). We did not include variants located within the major histocompatibility complex region. We also eliminated SNPs for which the Chi-square values exceeded 80, as the regression might be unduly affected by extreme values33.
Results
Migraine on early-onset ischemic stroke
The SNP-outcome associations showed p-values smaller than 1 × 10–5 for all instrumental SNPs (Supplementary Table 5). The MR analysis demonstrated that overall migraine was not related to the risk of early-onset ischemic stroke. The odds ratios (OR) based on the three sets of instrumental SNPs from Bjornsdottir et al., IHGC, and both these two sources were 0.921 (95% CI = 0.777 to 1.091, p-value = 0.340), 0.945 (95% CI = 0.846 to 1.056, p-value = 0.317), and 0.941 (95% CI = 0.843 to 1.050, p-value = 0.277), respectively (Fig. 2 and Supplementary Table 6). Although evidence of heterogeneity was detected, there was no evidence of horizontal pleiotropy. One outlier (rs6690217) was detected when using SNPs from both sources. The outlier-corrected odds ratio was 0.959 (95% CI = 0.863 to 1.064, p-value = 0.431).
Inverse variance weighted estimates of migraine on risk of early-onset ischemic stroke. Het_p indicates p-value of heterogeneity test. Ple_p indicates p-value of pleiotropy test. Abbreviations: SNP, single nucleotide polymorphism; IHGC, International Headache Genetics Consortium; OR, odds ratio; CI, confidence interval.
Regarding migraine subtypes, migraine with aura was suggestively or significantly associated with an increased risk of early-onset ischemic stroke (Figs. 2, 3, Supplementary Fig. 2, and Supplementary Table 6). The odds ratios based on instrumental SNPs from Bjornsdottir et al., IHGC, and both sources were 1.114 (95% CI = 1.005 to 1.236, p-value = 0.040), 1.062 (95% CI = 1.002 to 1.126, p-value = 0.042), and 1.074 (95% CI = 1.022 to 1.130, p-value = 0.005), respectively. Although the estimates were not significant based on most other MR methods, all estimates showed a consistent direction of effect (ORs > 1). There was no evidence of heterogeneity or horizontal pleiotropy. The MR-PRESSO method did not detect any outliers. Leave-one-out analysis demonstrated that the results were not driven by any single SNP (Supplementary Fig. 3). Lastly, the Steiger test did not find reverse causal SNPs for instrumental SNPs for migraine with aura.
Scatter plots of SNP effect on migraine with aura and SNP effect on early-onset ischemic stroke. (A) instrumental SNPs based on study by Bjornsdottir et al. 2023; (B) instrumental SNPs based on study by IHGC; C: instrumental SNPs based on both two sources. Abbreviations: SNP, single nucleotide polymorphism; IHGC, International Headache Genetics Consortium.
All three sets of instrumental SNPs indicated that migraine without aura was not related to the risk of early-onset ischemic stroke, and there was no evidence of heterogeneity or horizontal pleiotropy.
We conducted sensitivity analysis by exclusively relying on SNPs that exhibited low evidence of heterogeneity across different stroke cohorts (with I2 less than 50% and a p-value of the Q statistic greater than 0.05). The estimates remained significant when using instrumental SNPs from both sources (OR = 1.069, 95% CI = 1.014 to 1.127, p-value = 0.013, 74 SNPs) (Fig. 4 and Supplementary Table 7). Additionally, we conducted sensitivity analysis by using SNPs without consideration of whether they were associated with potential confounders. The estimates also remained significant when using instrumental SNPs from both sources (OR = 1.064, 95% CI = 1.017 to 1.114, p-value = 0.008) (Fig. 4 and Supplementary Table 8).
Sensitivity analyses of migraine with aura on early-onset ischemic stroke. (A) Sensitivity analysis was conducted by exclusively relying on SNPs that exhibited low evidence of heterogeneity across different stroke cohorts (with I2 less than 50% and a p-value of the Q statistic greater than 0.05). (B) Sensitivity analysis was conducted using SNPs, without consideration of whether they were associated with potential confounders. Het_p indicates p-value of heterogeneity test. Ple_p indicates p-value of pleiotropy test. Abbreviations: SNP, single nucleotide polymorphism; IHGC, International Headache Genetics Consortium; OR, odds ratio; CI, confidence interval; MA, migraine with aura.
To make a comparison, we also conducted MR analyses using the summary data of all adult ischemic strokes (Supplementary Fig. 4). The findings indicated that migraine with aura was not related to the risk of all adult ischemic strokes, with ORs of 1.033 (p-value = 0.388), 1.002 (p-value = 0.934), and 1.009 (p-value = 0.609). Interestingly, we observed that any migraine was potentially associated with decreased ischemic stroke risk, with ORs of 0.947 (p-value = 0.341), 0.895 (p-value = 0.005), and 0.899 (p-value = 0.006).
Migraine on early-onset ischemic stroke subtypes
Due to the limited sample size of stroke subtypes, MR analysis did not find consistent associations among the three migraine data sources (Supplementary Fig. 5 and Supplementary Table 6).
Any migraine and migraine without aura were found to be suggestively associated with a decreased risk of other causes of stroke based on the SNPs from Bjornsdottir et al. However, these associations were non-significant based on the other two sets of SNPs. Migraine with aura was found to be suggestively associated with an increased risk of undetermined cause of stroke based on the SNPs from IHGC. However, the results based on the other two sets of SNPs did not confirm this association.
Reverse Mendelian randomization analysis
We performed reverse MR analysis to assess the causal effects of early-onset ischemic stroke on migraine (Supplementary Fig. 6). The results showed that early-onset ischemic stroke was not associated with the risk of any migraine (OR = 0.992, p-value = 0.587), migraine with aura (OR = 1.007, p-value = 0.700) and migraine without aura (OR = 0.962, p-value = 0.212).
Linkage disequilibrium score regression
LDSC analysis showed a significant positive genetic correlation between any migraine and early-onset ischemic stroke (rg = 0.190, 95% CI = 0.078 to 0.303, p-value = 9.26 × 10–4), as well as a significant positive genetic correlation between migraine with aura and early-onset ischemic stroke (rg = 0.208, 95% CI = 0.038 to 0.377, p-value = 0.016) (Fig. 5). However, there was a significant negative genetic correlation between migraine without aura and early-onset ischemic stroke (rg = -0.197, 95% CI = -0.391 to -0.002, p-value = 0.047). Due to limited sample size, we were unable to perform LDSC for ischemic stroke subtypes.
For comparison, we also conducted LDSC analysis using the summary data of ischemic stroke based on all adults (Fig. 5). However, this analysis did not find a significant genetic association between any migraine, migraine with aura, and migraine without aura with ischemic stroke in all adults.
Discussion
The MR analyses results of this study demonstrated that migraine with aura was associated with an increased risk of early-onset ischemic stroke. However, overall migraine and migraine without aura were not associated with the risk of early-onset ischemic stroke. These results were supported by the positive genetic correlation between migraine with aura and early-onset ischemic stroke.
While some studies have indicated an increased risk of stroke in both migraine without aura and migraine with aura groups5,34,35, more studies have consistently shown that the association was only significant in migraine with aura, but not in migraine without aura36. An early meta-analysis comprising 14 studies (11 case–control studies and 3 cohort studies) demonstrated that the risk of stroke associated with migraine was consistent in people who had migraine with aura (relative risk 2.27, 1.61–3.19) and migraine without aura (relative risk 1.83, 1.06–3.15)35. Subsequent meta-analyses showed that the risk of stroke associated with migraine was non-significant in people who had migraine without aura3,4,36. A recently published meta-analysis involving 18 prospective cohort studies consisting of 370,050 migraine patients and 1,387,539 controls showed migraine with aura was associated with an increased risk of ischemic stroke (relative risk 1.67, 1.26–2.22) whereas migraine without aura was not associated with risk of ischemic stroke (relative risk 1.18, 0.94–1.49)3. Our analysis confirmed the association between migraine with aura and ischemic stroke risk, consistent with previous observational studies, while no significant association was observed in migraine without aura patients.
As for age-onset of ischemic stroke, previous studies suggested that migraine, especially migraine with aura, might be associated with ischemic stroke in young adults, but not in older. A propensity score-matched cohort study with 119,017 migraine cases and an equal number of controls showed migraine was associated with increased risk of ischemic stroke in individuals aged 20–30 (hazard ratio 2.70, 1.12–6.56), 30–40 (hazard ratio 2.02, 1.25–3.27), and 40–50 years (hazard ratio 2.08, 1.51 to 2.87), but not among older patients (age > 50 years) (with hazard ratios ≤ 1.21)12. A nationwide population-based cohort study involving 220437 migraine cases and matched controls with 1:5 ratio showed migraine was associated with increased risk of early-onset (age ≤ 60 years) ischemic stroke in both women (hazard ratio 1.21, 1.13 to 1.30) and men (hazard ratio 1.23, 1.10–1.38)11.
The age-related differential risk might be explained by the fact that, migraine and age-related risk factors such as hypertension might have shared underlying mechanisms. In older individuals, age-related risk factors condition may saturate the biological mechanisms that ultimately lead to ischemic stroke, so that there are few open pathways for migraine to affect ischemic stroke. This speculation is supported by the finding that when stratified by Framingham risk score, the association between migraine with aura and risk of ischemic stroke was strongest in the lowest risk score group37. In addition, this speculation is also supported by a recently published population-based cohort study among women who had delivered at least one child38. The study found women with migraine alone (hazard ratio 1.85, 1.61–2.13) and women with pregnancy-induced hypertension alone (hazard ratio 2.56, 2.25–2.92) were both associated with increased risk of early-onset ischemic stroke compared with women with neither migraine nor pregnancy-induced hypertension. However, women with both migraine and pregnancy-induced hypertension showed a similar increased ischemic stroke risk (hazard ratio 2.95, 1.82–4.79) compared with women with pregnancy-induced hypertension only38.
There were several possible underlying mechanisms for the association between migraine and ischemic stroke. First, previous studies employing the LDSC method have revealed that migraine shares a genetic basis with cardiometabolic risk factors such as type 2 diabetes, lipid levels, and blood pressure39,40. Individuals with migraine, even in the absence of cardiometabolic risk factors initially, may be more susceptible to developing these factors over time, consequently heightening the risk of ischemic stroke. Second, previous studies suggested individuals with migraine with aura exhibit a heightened susceptibility to thrombosis12. Our recently LDSC analysis demonstrated a significant positive genetic correlation between venous thrombosis and both migraine with aura (genetic correlation = 0.267, se = 0.053, p-value = 4.30 × 10–7) and migraine without aura (genetic correlation = 0.262, se = 0.048, p-value = 5.37 × 10–8)18. In addition, higher genetic propensity for venous thrombosis are more strongly associated with early-onset ischemic stroke than with late-onset ischemic stroke21. On the other hand, migraine with aura shows a higher prevalence of patent foramen ovale compared to migraine without aura, potentially elevating the risk of paradoxical embolism41. Migraine with aura also shows a higher prevalence of atrial fibrillation42. Third, spreading depolarizations likely play a pivotal role in the increased risk of ischemic stroke in individuals with migraine, especially those with migraine accompanied by aura43. Last, additional pathophysiological mechanisms might involve inflammatory markers, endothelial and vascular indicators, as well as sex hormones44.
The present study has several limitations. First, as females exhibit a higher susceptibility to develop migraine and the augmented risk of ischemic stroke might be more pronounced among female migraine sufferers, conducting a sex-stratified analysis becomes imperative. Regrettably, due to the unavailability of sex-specific summary data, we were unable to carry out such an analysis. Second, since only a few SNPs associated with migraine with aura reach genome-wide significance (p-value < 5 × 10–8), we employed a more lenient threshold for instrumental variables (p-value < 1 × 10–5). Third, due to the limited sample size of stroke subtypes, MR analysis did not identify consistent associations among the three migraine data sources. Additionally, LDSC analysis using data for stroke subtypes was not feasible. Lastly, the conclusions drawn from this study rely on summary data from European populations, potentially limiting their generalizability to other ethnic groups.
Conclusion
In conclusion, this study provides evidence of a causal relationship between migraine with aura and early-onset ischemic stroke using a Mendelian randomization approach and a positive genetic correlation between migraine with aura and early-onset ischemic stroke. The findings have implications for the understanding of the pathophysiological mechanisms underlying the association between migraine and ischemic stroke, and may have potential implications for the prevention and treatment of early-onset ischemic stroke. However, further studies are needed to confirm the findings and to address the limitations of this study.
Availability of data and materials
The supporting data for this study can be found in the supplementary files of the article as well as in the referenced studies within this article. Summary data for migraine by Bjornsdottir et al. were obtained from https://www.decode.com/summarydata/. Summary data for migraine by International Headache Genetics Consortium were obtained from https://www.headachegenetics.org/datasets-cohorts. Summary data for early-onset ischemic stroke were obtained from the Cerebrovascular Disease Knowledge Portal (https://cd.hugeamp.org/).
Abbreviations
- CI:
-
Confidence interval
- GWAS:
-
Genome wide association study
- IHGC:
-
International Headache Genetics Consortium
- LDSC:
-
Linkage disequilibrium score regression
- MR:
-
Mendelian randomization
- OR:
-
Odds ratio
- SNP:
-
Single nucleotide polymorphism
References
(2018) GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 17(11):954–976. https://doi.org/10.1016/s1474-4422(18)30322-3
Bigal, M. E., Kurth, T., Hu, H., Santanello, N. & Lipton, R. B. Migraine and cardiovascular disease: Possible mechanisms of interaction. Neurology 72(21), 1864–1871. https://doi.org/10.1212/WNL.0b013e3181a71220 (2009).
Ng, C. Y. H. et al. Myocardial infarction, stroke and cardiovascular mortality among migraine patients: A systematic review and meta-analysis. J. Neurol. 269(5), 2346–2358. https://doi.org/10.1007/s00415-021-10930-x (2022).
Mahmoud, A. N. et al. Migraine and the risk of cardiovascular and cerebrovascular events: A meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open 8(3), e020498. https://doi.org/10.1136/bmjopen-2017-020498 (2018).
Kwon, M. J. et al. A higher probability of subsequent stroke and ischemic heart disease in migraine patients: A longitudinal follow-up study in Korea. J. Headache Pain 24(1), 98. https://doi.org/10.1186/s10194-023-01632-y (2023).
Adelborg, K. et al. Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. Bmj 360, k96. https://doi.org/10.1136/bmj.k96 (2018).
Kalkman, D. N. et al. Migraine and cardiovascular disease: What cardiologists should know. Eur. Heart J. 44(30), 2815–2828. https://doi.org/10.1093/eurheartj/ehad363 (2023).
Batur, P. et al. Use of combined hormonal contraception and stroke: A case-control study of the impact of migraine type and estrogen dose on ischemic stroke risk. Headache 63(6), 813–821. https://doi.org/10.1111/head.14473 (2023).
Martinez-Majander, N. et al. Association between migraine and cryptogenic ischemic stroke in young adults. Ann. Neurol. 89(2), 242–253. https://doi.org/10.1002/ana.25937 (2021).
Lantz, M. et al. Migraine and risk of stroke: A national population-based twin study. Brain 140(10), 2653–2662. https://doi.org/10.1093/brain/awx223 (2017).
Hvitfeldt Fuglsang, C. et al. Migraine and risk of premature myocardial infarction and stroke among men and women: A Danish population-based cohort study. PLoS Med. 20(6), e1004238. https://doi.org/10.1371/journal.pmed.1004238 (2023).
Peng, K. P., Chen, Y. T., Fuh, J. L., Tang, C. H. & Wang, S. J. Migraine and incidence of ischemic stroke: A nationwide population-based study. Cephalalgia 37(4), 327–335. https://doi.org/10.1177/0333102416642602 (2017).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife https://doi.org/10.7554/eLife.34408 (2018).
Lee, K. J., Lee, S. J., Bae, H. J. & Sung, J. Exploring the causal inference of migraine on stroke: A Mendelian randomization study. Eur. J. Neurol. 29(1), 335–338. https://doi.org/10.1111/ene.15101 (2022).
Shu, M. J., Li, J. R., Zhu, Y. C. & Shen, H. Migraine and ischemic stroke: A mendelian randomization study. Neurol. Ther. 11(1), 237–246. https://doi.org/10.1007/s40120-021-00310-y (2022).
Daghlas, I., Guo, Y. & Chasman, D. I. Effect of genetic liability to migraine on coronary artery disease and atrial fibrillation: A Mendelian randomization study. Eur. J. Neurol. 27(3), 550–556. https://doi.org/10.1111/ene.14111 (2020).
Duan, X. et al. Causality between migraine and cardiovascular disease: A bidirectional Mendelian randomization study. J. Headache Pain 25(1), 130. https://doi.org/10.1186/s10194-024-01836-w (2024).
Wu, X.-P., Niu, P.-P. & Liu, H. Association between migraine and venous thromboembolism: A Mendelian randomization and genetic correlation study. Front. Genet. https://doi.org/10.3389/fgene.2024.1272599 (2024).
Malik, R. et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 50(4), 524–537. https://doi.org/10.1038/s41588-018-0058-3 (2018).
Mishra, A. et al. Stroke genetics informs drug discovery and risk prediction across ancestries. Nature 611(7934), 115–123. https://doi.org/10.1038/s41586-022-05165-3 (2022).
Jaworek, T. et al. Contribution of common genetic variants to risk of early-onset ischemic stroke. Neurology 99(16), e1738–e1754. https://doi.org/10.1212/wnl.0000000000201006 (2022).
Skrivankova, V. W. et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: The STROBE-MR statement. Jama 326(16), 1614–1621. https://doi.org/10.1001/jama.2021.18236 (2021).
de Leeuw, C., Savage, J., Bucur, I. G., Heskes, T. & Posthuma, D. Understanding the assumptions underlying Mendelian randomization. Eur. J. Hum. Genet. 30(6), 653–660. https://doi.org/10.1038/s41431-022-01038-5 (2022).
Jareebi, M. A. & Alqassim, A. Y. The impact of educational attainment on mental health: A causal assessment from the UKB and FinnGen Cohorts. Medicine (Baltimore) 103(26), e38602. https://doi.org/10.1097/md.0000000000038602 (2024).
Altamura, C. et al. Shorter visual aura characterizes young and middle-aged stroke patients with migraine with aura. J. Neurol. 269(2), 897–906. https://doi.org/10.1007/s00415-021-10671-x (2022).
Chen, H., Peng, L., Wang, Z., He, Y. & Zhang, X. Exploring the causal relationship between periodontitis and gut microbiome: Unveiling the oral-gut and gut-oral axes through bidirectional Mendelian randomization. J. Clin. Periodontol. 51(4), 417–430. https://doi.org/10.1111/jcpe.13906 (2024).
Bjornsdottir, G. et al. Rare variants with large effects provide functional insights into the pathology of migraine subtypes, with and without aura. Nat. Genet. 55(11), 1843–1853. https://doi.org/10.1038/s41588-023-01538-0 (2023).
Hautakangas, H. et al. Genome-wide analysis of 102,084 migraine cases identifies 123 risk loci and subtype-specific risk alleles. Nat. Genet. 54(2), 152–160. https://doi.org/10.1038/s41588-021-00990-0 (2022).
Gormley, P. et al. Meta-analysis of 375,000 individuals identifies 38 susceptibility loci for migraine. Nat. Genet. 48(8), 856–866. https://doi.org/10.1038/ng.3598 (2016).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37(7), 658–665. https://doi.org/10.1002/gepi.21758 (2013).
Verbanck, M., Chen, C. Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50(5), 693–698. https://doi.org/10.1038/s41588-018-0099-7 (2018).
Hemani, G., Tilling, K. & Davey Smith, G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. 13(11), e1007081. https://doi.org/10.1371/journal.pgen.1007081 (2017).
Bulik-Sullivan, B. et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47(11), 1236–1241. https://doi.org/10.1038/ng.3406 (2015).
Siao, W. Z. et al. Risk of peripheral artery disease and stroke in migraineurs with or without aura: A nationwide population-based cohort study. Int. J. Med. Sci. 19(7), 1163–1172. https://doi.org/10.7150/ijms.72119 (2022).
Etminan, M., Takkouche, B., Isorna, F. C. & Samii, A. Risk of ischaemic stroke in people with migraine: Systematic review and meta-analysis of observational studies. Bmj 330(7482), 63. https://doi.org/10.1136/bmj.38302.504063.8F (2005).
Schürks, M. et al. Migraine and cardiovascular disease: Systematic review and meta-analysis. Bmj 339, b3914. https://doi.org/10.1136/bmj.b3914 (2009).
Kurth, T., Schürks, M., Logroscino, G., Gaziano, J. M. & Buring, J. E. Migraine, vascular risk, and cardiovascular events in women: Prospective cohort study. Bmj 337, a636. https://doi.org/10.1136/bmj.a636 (2008).
Hvitfeldt Fuglsang, C. et al. Combined impact of migraine and pregnancy-induced hypertension on long-term risk of premature myocardial infarction and stroke. Neurology 102(1), e207813. https://doi.org/10.1212/wnl.0000000000207813 (2024).
Siewert, K. M. et al. Cross-trait analyses with migraine reveal widespread pleiotropy and suggest a vascular component to migraine headache. Int. J. Epidemiol. 49(3), 1022–1031. https://doi.org/10.1093/ije/dyaa050 (2020).
Guo, Y. et al. Phenotypic and genotypic associations between migraine and lipoprotein subfractions. Neurology 97(22), e2223–e2235. https://doi.org/10.1212/wnl.0000000000012919 (2021).
Sacco, S. et al. Microembolism and other links between migraine and stroke: Clinical and pathophysiologic update. Neurology 100(15), 716–726. https://doi.org/10.1212/wnl.0000000000201699 (2023).
Chiang, C. C. et al. Migraine with aura associates with a higher artificial intelligence: ECG atrial fibrillation prediction model output compared to migraine without aura in both women and men. Headache 62(8), 939–951. https://doi.org/10.1111/head.14339 (2022).
Pelzer, N., de Boer, I., van den Maagdenberg, A. & Terwindt, G. M. Neurological and psychiatric comorbidities of migraine: Concepts and future perspectives. Cephalalgia 43(6), 3331024231180564. https://doi.org/10.1177/03331024231180564 (2023).
van Welie, F. C., Kreft, L. A., Huisman, J. M. A. & Terwindt, G. M. Sex-specific metabolic profiling to explain the increased CVD risk in women with migraine: A narrative review. J. Headache Pain 24(1), 64. https://doi.org/10.1186/s10194-023-01601-5 (2023).
Acknowledgements
We would like to acknowledge the participants and investigators of the included genome wide association studies.
Funding
None.
Author information
Authors and Affiliations
Contributions
Rui Zhang: Data curation; Writing—original draft. Peng-Peng Niu: Formal analysis; Visualization; Writing—original draft; Writing—review & editing. Shuo Li: Data curation; Writing—original draft. Yu-Sheng Li, Conceptualization; Funding acquisition; Project administration; Writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethics approval and consent to participate were obtained for the original studies.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, R., Niu, PP., Li, S. et al. Mendelian randomization analysis reveals causal effects of migraine and its subtypes on early-onset ischemic stroke risk. Sci Rep 14, 31505 (2024). https://doi.org/10.1038/s41598-024-83344-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83344-0
Keywords
This article is cited by
-
Assessing the causal effects of type 2 diabetes and obesity-related traits on COVID-19 severity
Human Genomics (2025)
-
Migraine and stroke: correlation, coexistence, dependence - a modern perspective
The Journal of Headache and Pain (2025)