Abstract
A dual-stage model for classifying Parkinson’s disease severity, through a detailed analysis of Gait signals using force sensors and machine learning approaches, is proposed in this study. Parkinson’s disease is the primary neurodegenerative disorder that results in a gradual reduction in motor function. Early detection and monitoring of the disease progression is highly challenging due to the gradual progression of symptoms and the inadequacy of conventional methods in identifying subtle changes in mobility. The proposed dual-stage model utilized a hypertuned Random Forest Tree (RFT) to classify the subjects into PD and non-PD classes at Stage 1 and a hypertuned Ensemble Regressor (ER) to predict the severity of illness at Stage 2. Further, we have implemented the proposed model on the data signals gathered from both feet of 166 participants using Vertical Ground Reaction Force Sensors (VGRF). The dataset comprised 93 persons with Parkinson’s disease and 73 healthy controls. The dataset (imbalance) collected from both feet is passed to the preprocessing phase (for balancing data using the SMOTE method), followed by the feature extraction phase to extract features related to time, frequency, spatial, and temporal features domains that are highly effective for detecting and assigning severity levels of PD. A Recursive Feature Elimination method is also used to select the optimal set of features to improve the model performance. It is acknowledged that the early detection of Parkinson’s disease is contingent upon critical parameters, including stride length, stance duration, swing interval, double limb support, step time, and step length. The crucial evaluation metrics used for evaluating model performance include accuracy, mean absolute error, and root mean square error. The findings indicate that the suggested model significantly surpasses current methodologies. It attained an accuracy of 97.5 ± 2.1%, Sensitivity of 97% ± 2.5%, and average Specificity of 95% ± 2.2% in differentiating between PD and non-PD participants utilizing RFT and evaluated disease severity with an average accuracy of 96.4 ± 2.3%, an average mean absolute error of 0.065 ± 0.024, and a root mean square error of 0.080 ± 0.06. The results indicate that the proposed dual-stage model is exceptionally successful in the early detection and severity assessment of Parkinson’s disease and demonstrates better efficacy than alternative models.
Similar content being viewed by others
Introduction
Background
Parkinson’s is a widespread neurodegenerative disease that causes various life-changing symptoms, most notably tremors in the upper extremities. A sudden reduction in motor functions in patients suffering from mild to severe PD is frequently observed, resulting in bent upper body posture, shuffling, short steps, condensed walking pace, more considerable gait variation, and gait freezing1.
Parkinson’s Foundation report states that around ten million people are suffering from PD worldwide2. Along with that, findings from different studies show that men suffer 1.5 times more than women from PD3. In most cases, symptoms of PD are not visible at early stages, i.e., before 5–10 years; up to that, around half of the dopamine cells/neurons are destroyed. Mostly, symptoms of PD appear at age 40 and progress with time4. Significant motor symptoms typically seen in PD patients include tremors, bradykinesia(slowness in movement), trembling, postural imbalance, speech impairments, etc5,6.
Medical professionals assess all these motor symptoms to detect PD. Additionally, different rating scales were designed by healthcare associations to determine the severity of the deterioration of motor symptoms in PD patients. Hoehn and Yahr (H & Y) and UPDRS (Unified Rating Scale) are the two most commonly used rating scales designed to distinguish postural instability in patients with mild to severe PD7. However, these rating scales are influenced by individual observations and experiences and can cause doubtful results when identifying the onset of the disease. Therefore, to find practical solutions for early and effective diagnosis of the disease and its progression, regular monitoring of significant parameters related to PD must be required as it varies from person to person.
Challenges
In the past decades, due to the unavailability of smart health monitoring devices, patients had to visit hospitals to monitor their health parameters regularly. However, due to the absence of physicians or irregular visits by patients to hospitals, valuable recordings that are highly effective in diagnosing PD may be missed. Additionally, people above the age of 60 who are more prone to PD face lots of medical and social issues, including physical disabilities, living alone, less knowledge, reliance on foot or bicycle transportation, longer distances to public transportation, less number of hospitals available in their areas, inability to drive, etc. for regular monitoring of their health parameters. So, effective solutions must be proposed to monitor patient health symptoms without visiting the hospitals. Many researchers worked in this domain and found smart sensor-based technologies, including plantar, acoustic, Inertial Measurement Units (IMU), VGRF, accelerometers, etc., that will reduce the need for costly laboratory setups for monitoring significant PD-related parameters and avoid regular visits to hospitals8,9,10. Among all these sensors, VGRF sensors are the most commonly used sensors for monitoring significant gait patterns highly related to PD, with the capability to capture movements among joints and muscle activities with extreme precision10,11.
However, analyzing gait patterns using traditional methods is very challenging because it is influenced not only by physical fitness and age factors but also by other significant factors, including mood, personality, and socio-cultural factors. In the last few years, ML techniques have come with the potential to effectively and automatically analyze gait patterns, thus reducing the time and effort wasted in analyzing gait patterns with traditional approaches. Additionally, such techniques can resolve the problems of identifying PD severity. Therefore, the collaboration of ML techniques with wearable sensors can provide a fast and proficient way to diagnose the disease at an early stage12. Due to the unavailability of effective treatment for PD, early diagnosis can assist physicians in considerably reducing the progression of this disease and enhancing the patient’s life quality13. In the past, many of the researchers have worked with different ML techniques, including SVM, Bayes Classifier (BC), Decision Tree (DT), Random Frest Tree (RFT), Ensemble Classifier (EC), K-Nearest Neighbor (K-NN), and deep learning techniques, including Artificial Neural Network (ANN) and Convolution Neural Network (CNN) for the diagnosis of Parkinson’s disease on sensors based dataset. However, the current work emphasizes the utilization of a Random Forest Tree (RFT) in place of other complex approaches such as ensemble deep learning or semi-supervised methods due to the following reasons:
-
1.
RFT is an ensemble of decision trees that inherently balance bias and variance through bootstrapping and random feature selection. It also provides better insights into feature importance and relationships within the dataset better than deep learning models.
-
2.
RFT can works efficiently on all types of data including tabular, unstructed or structured datasets even with fewer dimensions. In contrast, deep learning models work efficiently for data, like images, text, or audio, where complex feature hierarchies can be effectively learned through layers of abstraction.
-
3.
Additionally, the basic behavior of RFT makes it more robust than other learning models.
-
4.
RFT is computationally less intensive and more scalable for small to medium-sized datasets. Therefore, they can be trained quickly and scaled horizontally with distributed computing if needed in comparison to other learning models.
-
5.
Though it is less interpretable but it contains many other supporting features that help analysis to be more efficient and faster in comparison to other learning models.
Therefore in the current study we have selcetd RFT for the diagnosis of Parkinson’s disease.
Objectives
This study aims to propose a dual-stage Parkinson’s disease diagnosis model utilizing data from wearable sensors with effective ML techniques. The key objectives of this manuscript are summarized as follows:
-
1.
This study proposes and implements a dual-stage PD Diagnosis model based on data collected from VGRF, smart wearable sensors, which capture gait signals from both feet related to an individual’s joint movements.
-
2.
At Stage 1, a Random Forest Tree classifies subjects into PD and non-PD classes. At Stage 2, an Ensemble Regressor assigns the severity level of PD to the patients using the extracted features.
-
3.
The performance of the proposed model is evaluated using different performance evaluation measures.
-
4.
Finally, the results are compared with the existing PD detection and severity classification models to verify and validate the proposed model’s performance.
The organization of this manuscript is as follows: section Related study describes the earlier research conducted by several researchers in this area. Section “Materials and methods” illustrates the novel model proposed in the current work, the dataset used, the preprocessing stage, the analysis stage, and different performance evaluation measures. Section “Result and discussion” discusses the results obtained after the implementation of the proposed model. Section “Conclusions and future works” discusses the conclusion and future work. Lastly, section “Limitations” describes the limitations of the proposed work.
Related study
Numerous studies have been conducted to find an effective solution for diagnosing Parkinson’s disease by adopting different methods and tools. In the past, researchers have used different ML and DL techniques and worked with various sensors to analyze both motor and non-motor symptoms. However, rapid advancements have been observed among sensors-based technologies for analyzing motion symptoms. Among all, VGRF sensors and wearable inertial sensors were observed as the most prominent sensors for analyzing gait patterns with their unique capabilities and challenges. So, we conduct this literature to cover all these sections to get an in- depth knowledge about the past research with these sensors for the purpose of early detection and severity assessment of PD.
In this direction, Khoury et al.11 developed an ML-based methodology for detecting patients suffering from PD using gait cycles from force sensors. The proposed methodology was evaluated by using different supervised learning methods, including, NB, K-NN, DT, SVM, RF, and Gaussian Mixture Model (GMM) and K-means as unsupervised classification methods. The performance of developed models was estimated using accuracy, recall, F1 score, and precision using LOOCV (Look cross-validation method). The developed methodology is highly effective in differentiating subjects into PD and HC with an accuracy rate of 90% using K-NN. However, the model was limited only to the classification of subjects in PD and healthy controls, but this model still does not identify the severity level of PD. However, the accuracy can be further enhanced by using ensemble and deep learning approaches.
Balaji et al.12 suggested a method for evaluating the effectiveness of several supervised machine-learning algorithms using the PD and Gait datasets. In the proposed work, first of all, both temporal and spatial-temporal features were extracted from gait patterns, and a correlation-based feature selection was performed before being passed to any learning algorithms. The author analyzed the most commonly used ML algorithms, SVM, Bayes Classifier (BC), Decision Tree (DT), and Ensemble Classifier (EC), to predict the severity of PD. Their performance was analyzed using different performance evaluators, including Accuracy, Specificity, and Sensitivity with leave one out, bootstrap, and P-fold CV methods. However, the proposed approach was limited to supervised learning methods and worked on small data sizes.
Trabassi et al.14 developed a novel model to identify people suffering from PD using IMU sensors. Initially, 22 features were derived from the sensors and passed to three levels of the feature selection process: where at the first level, a T-test; at the second level, a Correlation Coefficient; and at the third level Sequential Backward Selection (SBS) with Random Forest was used, which results into seven most significant features that were passed to five ML models (SVM, ANN, DT, RF, and K-NN). The performance of all these models was evaluated in terms of accuracy, recall, precision, and F1 score using the k-fold CV method. Computed results revealed that all ML models give reliable classification performance. Additionally, the model reduces the risk of over-representing multi-collinear gait features and overfitting in the test performances. The proposed work achieved a low accuracy score of 86% with a less generalized error of 2.95%. However, the developed model was implemented only on the dataset collected from a single lumbar-mounted IMU sensor. Further, to check the efficiency of the proposed model, it must be applied to another gait dataset, which will be collected from multiple sensors.
Maachi et al.15 an automatic feature learning approach was used to classify PD and Healthy Controls (HC) and assign UPDRS severity levels to PD patients to avoid hand-crafted gait feature limitations. The novel model was developed by integrating 18 parallel 1-D CNNs and implemented on the VGRF dataset, which scored the highest accuracy of 98.7% for PD identification but led to a low severity assessment accuracy of 85.3% with high computational costs under the 10-fold CV method. However, due to automatic feature extraction, a deep understanding of Parkinson’s Gait and its features was not known. Additionally, a more efficient approach must be proposed for better severity-level assessment.
Seitwan et al.16 introduced a PD detection and severity classification model comprising preprocessing, feature transformation, and classification processes. The developed model focused on time and frequency domain features modified using Convolution Wavelet Transformation (CWT) to generate a spectrogram for better visualization and Principal Component Analysis (PCA to enhance performance of model. The model was implemented on the VGRF dataset using deep learning algorithms with a 10-fold CV method. CNN was used for classification and attained a low accuracy score of 97.42%. In the same way, ResNet was used for severity assessment and achieved a low accuracy score of 96.52%. Along with poor performance, the proposed model had some major drawbacks that could be improved in the future. Firstly, the developed model worked only with time and frequency domain features; therefore, more sophisticated features must be considered to get more efficient results. Secondly, the collected data contains a small number of PD patients. Thus, the proposed model should be implemented on a large number of PD patients. Further, hybrid ML models and better feature enhancement methods should be adopted to improve the model’s performance.
Aşuroğlu et al.17 suggested a novel model for assigning the severity to PD patients based on the UPDRS. The proposed model was implemented on a gait dataset collected from wearable force sensors. The author proposed a hybrid model by combining “Convolutional Neural Networks and Locally Weighted Random Forest (LWRF)” to assign severity to PD patients. The proposed model scored 0.897 on the correlation coefficient, 3.009 of MAE, and 4.556 of RMSE. Huntington’s disease and Alzheimer’s datasets were used to validate the generalization capability of the proposed model. However, the proposed model outperformed other ML models on the small data sample, but its performance must be further validated on a large data sample. Additionally, some future optimization approaches must be followed to improve model performance.
Khera et al.18 designed an age-gender-specific PD classification and severity assessment model. The author used different ML techniques, including K-NN, SVM, DT, and RF, with a recursive feature selection technique to design and implement the model on the gait dataset using the 10-fold CV method. Implemented results found that the age-gender specific model outperformed another generalized model by using SVM as the best classifier and attained an accuracy score of 98.5% in the classification of PD and a low accuracy of 94.02% in the assessment of PD severity. However, the proposed model works only with time-domain features and achieves a low accuracy score in assigning severity to PD; therefore, some effective data approaches must be applied to improve model performance.
Nair et al.19 developed a PD classification model that classifies subjects into PD and HC. The author used the DWT (Dynamic Wavelet Transform) technique to detect minute variations among distinguish gait cycles and applied the K-means approach to extract the significant features fed to logistic regression to classify PD. It was implemented on a gait dataset collected from subjects, including 83 subjects at an early stage of PD, 10 subjects at a moderate stage of PD, and 70 as health controls. The model’s performance was validated using the leave one out and k-fold CV method on VGRF dataset, and computed results showed an average accuracy score of 98%. However, the model achieved a better accuracy score in classifying subjects into PD, and healthy controls were unable to assign the severity level of PD. Therefore, the proposed model must be extended further by applying some significant approaches to assigning the severity level of PD.
Erdaş et al.20 introduced “an artificial intelligence-based disease detection and severity prediction system for different neurodegenerative diseases including PD, HD (Huntington’s disease), ALS (Amyotrophic Lateral Sclerosis) using gait features.” It was implemented on the gait dataset captured through force sensors by adopting different machine learning methods (i.e., K-NN, RF, XT, MLP) and the one-dimensional CNN method. The voting and stacking methods were also followed to enhance model performance. The novel model achieved a poor accuracy of 92.7 using limited datasets of three different samples. However, neurodegenerative diseases are not limited; therefore, the same model could be applied to other diseases, and more sample datasets must be used to check the efficiency of the models.
Zhang et al.21 proposed a deep learning model for the identification of PD and non-PD patients by monitoring their gait symptoms using IMU sensors and achieved an accuracy score of 86% with 5-fold CV method. However, the sensors are highly effective in capturing the resting termors in PD patients, but the results reveals a low accuracy score. Additionally, the efficiency of the propsed model shoud be checked with large number of subjects with different termor’s. Moreover, the collected dataset is imbalanced in terms of age, gender and class which need to be balanced for better results.
Trabassi et al.14 developed a model using different supervised machine learning approaches including SVM, ANN, DT, RF, and K-NN and implemented it on IMU sensors dataset. The proposed model with RF showed the best accuracy score higher than 80%. However, the proposed model reduces the risk of overfitting and overpresenting multicollinear gait features, but the accuracy score achieved by the model is very low. Therefore, more advanced ML approaches can be applied on same dataset for effective classification of the gait signals.
Mirelman et al.22 proposed a model to identify the severity of PD from the most sensitive mobility features using machine learning algorithms. The developed model was implemented on a dataset collected from inertial sensors placed at the participant’s lower back, wrists, and bilateral ankles. The developed model identified high discrimination among the motor features at different stages of PD and scored a low average sensitivity in the range of 72–83%, low Specificity in the range of 69–80% range, and low area under the curve (AUC) score in the range of 0.76–0.90% that could be gradually improved. Regularity and stride timing were the most discriminative features in advanced stages, and the best-discriminated controls were observed from lower limb sensors. The model achieved poor performance in terms of AUC in identifying the severity of PD based on discriminated features, which must be further improved.
Hssayeni et al.23 introduced a new way to monitor PD patients at home and reduce clinical visits. The author developed a novel hybrid deep-learning model combining CNN and Long Short-Term Memory(LSTM) to estimate the UPDRS III rating. The proposed model combined the knowledge of the clinical expert by manually extracting the features with the deep-driven features extracted using a deep learning approach. Two inertial sensors at the ankle and wrist were used to monitor the patient’s health parameters at home. The developed model achieved an MAE of 5.92 in assigning severity to PD patients using the LOOCV method. However, the dataset was collected while performing only daily activities that can be extended to other activities. Additionally, the dataset was collected from only 24PD patients, which was very small in size. Therefore, more training data must be considered to analyze and improve the model’s performance.
Rastegari et al.24 developed a Parkinson’s disease detection model using a wrist-based accelerometer dataset. The author focused on the idea that the wrist is a popular and appropriate body part for wearing an accelerometer for regular monitoring. The model was implemented on a wrist-worn accelerometer dataset collected from Healthy and PD subjects by continuous monitoring for seven days. “Epoch-based statistical feature engineering and the document-of-words” methods were used to improve performance. SVM came out as the best classifier but scored a low accuracy of 85% across 100 runs with the 10-fold CV method. The computed results found that the “document of words” significantly enhances model performance in comparison to the statistical feature engineering approach. However, the achieved accuracy score of the model was unsatisfactory, and it must be improved by using more sophisticated approaches in the future. It must also be analyzed using a larger sample dataset.
Gao et al.25 developed a predictive model to discriminate falls and non-falls in PD patients and implemented it on two separate datasets, including “Michigan (VGRF sensors) + TelAviv(Inertial Sensors).” The developed model focused on four major challenges: data compliance, feature selection, fall prediction, and prediction about “tremor dominance (TD) vs. posture instability” and gait difficulty (PIGD) to discriminate falls and non-falls in PD patients. LR, RF, SVM, and XGBoost were employed as machine-learning approaches to develop the model. Further, the forecasted results’ reliability was assessed using the 5-fold CV method as internal statistical and out-of-bag validation as external statistical. The computed results showed a poor accuracy score of 70–80%. However, the developed model is the first to identify falls among PD patients, but the frequency or severity of falls was not examined in this study due to the unavailability of sufficient data that can be examined in the future.
Indu et al.26 developed a novel K-NN approach to detect PD patients effectively, which used the concept of δ-neighborhood and weights in place of voting and neighboring points to make predictions about unknown test samples. In such an approach, computed weights were preferred over voting to efficiently classify subjects into healthy and PD patients. The proposed technique was implemented on three datasets: Gait, handwriting, and voice. The proposed method achieved a satisfactory accuracy score for all three datasets. However, the major drawback of the current work was the increasing time complexity of classification with increasing sample size, which was highly sensitive toward highly overlapped data that must be considered in the future.
Abedinzadeh Torghabeh et al.27 presented an intelligent model using a hybrid deep learning approach to identify and assess the severity of PD. Time domain and fuzzy features were extracted and passed to the Bi-LSTM model for further classification. Bayesian optimization approach was also followed to get more optimized results. The proposed approach was employed on CVGRF(Cumulative VGRF), which contains signals of both the foot. However, the proposed model has yielded promising outcomes without including the preprocessing steps, emphasizing only its simplicity. The proposed model relied solely on gait sensor data; its performance could be further improved by considering multi-sensor data. Moreover, the model worked only with limited features; thus, more crucial information must be considered for better PD diagnosis.
From the past study, it can be concluded that advanced sensors based technologies including VGRF, inertial sensors like gyroscopes, accelerometers, and magnetometers have been adopted for the effective analysis of motion among PD patients. However, both of these sensors have their own significance in monitoring and analyzing the movements among people. Most of the researchers have worked with VGRF sensors, due to their ability in measuring the force sensors while the feet are in direct contact with the ground. On the other hand, wearable inertial sensors, offer a more flexible and portable way of analyzing gait patterns in real-time. But the VGRF sensors yields high precision for measurements in comparison to the wearable inertial sensors due to direct contact with the ground. Additionally, as per the conclusion derived from the literature study, the VGRF sensors achieve high accuracy in comparison to other wearable inertial sensors. Therefore, the current study focuses on the utilization of VGRF sensor dataset in comparison to inertial sensors dataset.
The major limitations found in the existing literature are related to poor performance, a small sample size of the considered dataset, and limited work on severity assessment, which must be considered in the future. Additionally, the following conclusions have been drawn after reviewing and analyzing the extensive literature related to PD.
-
1.
Most of the existing research has been conducted on a smaller sample size; therefore, more training data should be considered.
-
2.
Different types of sensors can be used to capture the gait signals for diagnosing PD because they can efficiently capture changes in motor activities.
-
3.
Among all sensors, the gait patterns captured from VGRF sensors contain more valuable knowledge about movements. Their utilization is highly cost-effective and non-invasive, making them more appropriate for monitoring significant PD symptoms.
-
4.
It is noticed that most of the researchers have considered only time and frequency domain features that are insufficient for early and effective diagnosis and severity assessment of PD. However, different spatial and temporal features and time and frequency domain features could be highly effective for early and effective PD diagnosis and severity assessment.
-
5.
Additionally, limited work was done for the severity assessment of PD, leading to poor performance; therefore, more sophisticated approaches must be applied in this direction to get more efficient results.
Therefore, these limitations motivated us to propose a PD diagnosis and severity assessment model by focusing on more significant features that will be highly effective for diagnosing PD and assigning severity to PD patients more effectively. Further, ML techniques can be applied for effective analysis to extract and select features to achieve significant results for effective diagnosis and severity assessment of PD that could be clinically applicable in diagnosing and monitoring PD.
Materials and methods
This section contains a complete explanation of the materials and methods utilized for implementing the proposed model. Materials and methodology mainly refer to the data sources and techniques applied in the developed model. Therefore, this section begins with a thorough description of the proposed model, followed by a description of the dataset used, preprocessing phases, feature extraction, feature selection, and finally, different performance evaluators used for evaluating mode performance.
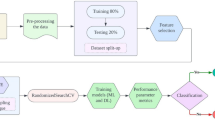
Figure 1 thoroughly represents the proposed model, which is divided into two stages. Stage 1 classifies the subjects into PD and non-PD classes using a Random Forest Tree as a classification method, and Stage 2 assigns the severity level of PD to the patients using the Ensemble Regressor based on the extracted features.
A stepwise description of the proposed model is described here:
-
1.
Initially, the VGRF dataset is first passed to the preprocessing phase, where it undergoes filtration to remove unwanted recordings (noise added during signal recording and starting and ending effects from gait patterns).
-
2.
After that, significant gait features are extracted and selected using Recursive Feature Elimination (RFE).
-
3.
A comparative analysis between different ML approaches is conducted to select the best classifier, and we find RFT to be the best classifier.
-
4.
The preprocessed data and the extracted features are passed to Stage 1: RFT (Random Forest Tree) to classify subjects into PD and non-PD classes.
-
5.
Based on the derived results, the subjects suffering from PD are passed to Stage 2 of the proposed model to assign the severity level of PD.
-
6.
At Stage 2, the previously extracted gait features (from Stage 1) are combined with a newly extracted gait feature named DLS (Double Limb Support) that is highly related to PD severity and then passed to ER (Ensemble Regressor) for assigning PD severity.
-
7.
As a result, PD patients are assigned three severity levels: 2(Initial), 2.5(Mild), and 3(Advanced Stage).
-
8.
Finally, some preventive measures are also suggested for PD patients based on their assigned severity level under the supervision of physicians.
Data set
Due to the unavailability of smart technologies, real-time data collection is nearly impossible. Hence, the current work focuses on a secondary dataset but can be extended to newly collected data. The data set used to implement the suggested model is accessed from Psyionet28 and collected by a group of three scientists at the “Movement Disorder unit of the Tel-Aviv Sourasky Medical Center, Israel” and was named after them. Yogev et al.29 collected the data of persons while moving on level ground, Frenkel-Toledo et al.30, who obtained data of people while using a treadmill, and Hausdorff et al.31, who recorded data of people while walking in a relaxed environment while wearing RAS (Rhythmic Auditory Simulation). For small and straightforward representation, these datasets are named Ga29, Si30, and Ju31. The existing dataset consists of 306 recordings (with 19,320 gait cycles) collected from 73 healthy (including 33 females and 40 males) and 93 PD patients (including 35 females and 58 males)32. The dataset al.so contains qualitative measures related to PD severity, including the H&Y (Hoehn & Yahr) staging for individuals suffering from PD. To quantify the level of disability among patients, H & Y rating scale is utilized. It is the most important scale, followed by most of the researchers to assign different levels of severity to PD patients with range from 0 to 5. The recording from each patient consists of VGRF signals captured by fixing eight sensors under each foot, with 16 sensors under the left and right foot, as shown in Fig. 2.
These force sensors identify gait patterns that vary with time, which is also helpful in analyzing the risk of falls, postural disability, impaired balancing, poor mobility issues, etc. that would be effective for PD diagnosis. To better understand the dataset, Tables 1 and 2, and 3 provide a complete description of all the features considered for the present work.
Table 1 describes the demographic and clinical features of subjects who participated in the current study. The considered dataset consists of recordings for healthy and PD patients, categorized on the basis of their Gender, Weight, Height, and Age.
Further, Table 2 describes the severity rating assigned to PD subjects of the considered datasets. Further, the severity level is classified into three categories: Severity 2, 2.5, and 3.
Table 3 describes the dataset, which contains 19 columns: one column denotes the timestamp, two columns signify the total force value on both the foot, and 16 columns denote individual recording of each force sensor applied on each foot. The level of disability among patients is quantified using an H & Y rating scale, which assigns the severity between 2 and 3 (Average = 2.26 ± 0.34)28.
Figure 3 compares the total force patterns of both the foot for HC and PD patients, respectively, over a series of gait cycles. Here, the blue line indicates the HC, and the red line indicates the PD patients. From the figure, it can be observed that force patterns of healthy controls are relatively consistent across each step because the force peaks are regularly spaced and reach similar heights with each cycle. Additionally, the trimming for each cycle is regular. In the case of PD, a significant variability among force patterns can be observed due to lower and inconsistent force peaks at each step.
Additionally, the timing for each cycle is irregular. Thus, from Fig. 3, it is concluded that PD patients has significant variability in swing and stance duration compared to healthy controls. Additionally, depending on disease-essential conditions, PD patients have impaired postural control, high asymmetry, and variable gait patterns compared to healthy controls. In this direction, this study aims to compute the stride, swing, and stance duration of the individual gait cycle to identify variations among different groups of subjects. The stride time is compared with the sum of swing and stance timings to ensure high accuracy.
Data preprocessing
Initially, the collected data set is filtered by removing the 20-second data from the starting and ending of each gait cycle to exclude the starting and ending effects33. Because the starting and ending patterns typically involve complex patterns that can vary significantly in healthy subjects, they can introduce noise and affect the analysis. Therefore, starting and ending effects from gait patterns are removed to obtain significant and effective results. Further, when data is recorded from sensors or other digital devices, noise disturbances can be added that may skew the results. Therefore, before passing the data for analysis, such a noise effect must be removed to increase the model performance. Depending on the results of different filtering approaches applied to gait datasets by other researchers, a 10-point median filter was the best approach to remove noise from the collected gait signals34. Therefore, a 10-point median filter approach is adopted to reduce the noise effect among collected gait signals, improving the robustness of the proposed model against the noise effect. It works on the principle of sliding the window over the data and replacing each value with the median of values in the window. Here, a 10-point indicates that the window size is set to 10. To further improve the performance of the proposed model, all collected gait signals are rescaled using a min-max scaling approach.
Moreover, we found that the collected sensor data is highly imbalanced, containing a total of 166 subjects, as listed in Table 1, out of which 93 are Parkinson’s patients and 73 are healthy controls. An imbalanced dataset can cause biased results towards the majority class. Therefore, it is highly necessary to balance the dataset by using different data-balancing approaches before analysis. A large number of data balancing approaches exist, like under sampling, SMOTE, bootstrapping, Generative Adversarial Network (GAN), and Conditional Tabular Generative Adversarial Network (ctGAN)35,36. Among all the above-mentioned balancing approaches, SMOTE (Synthetic Minority Oversampling Techniques) is popularly used due to its simplicity and low computability37. More specifically, SMOTE is always applied only on the training set and not on the complete dataset. Because, when SMOTE is applied to the testing dataset, it could result in oversampling the testing set, leading to an overestimation of the model’s performance. Therefore, the current work applies SMOTE only to the training dataset. Following is the description of the SMOTE method used for balancing the dataset:
-
1.
Initially, minority class samples are selected from the original dataset.
-
2.
After that, the k-nearest neighbor (using Euclidean distance or other distance metrics) is selected for each minority class identified.
-
3.
Further, randomly select one k-nearest neighbor for each minority sample.
-
4.
A new synthetic sample is generated by interpolating between randomly selected neighbor and minority class samples.
-
5.
Repeat steps 1 to 4 until the desired number of synthetic samples is achieved.
The SMOTE method significantly improves model performance and reduces the risk of overfitting by balancing the dataset. Therefore, the dataset is balanced in the current work by increasing the minimum samples to the original data using the SMOTE method. After implementing the SMOTE method, the dataset is thoroughly balanced, containing 186 samples with 93 PD and 93 healthy subjects. After noise removal, the next important step is to extract the significant features.
Feature extraction
Given that PD significantly influences a person’s gait, the variations in gait patterns can be used to assess how the disease progresses. Gait represents an individual’s way and style of walking, as presented in Fig. 4.
Different phases of gait patterns38.
The stance and swing phases are the two significant phases of a gait cycle. In Fig. 4, both feet remain in touch with the floor for 20% of the complete gait cycle, with 10% occurring at the start of the stance phase and 10% at the end. Different features are extracted and divided into broad autonomous categories.
“Toe-of-force” and “Centre of pressure (COP_x, COP_y)” are significant features that are highly relevant to PD diagnosis. A reduced “Toe-off force” indicates bradykinesia (slowness of movement) in PD patients. “Centre of pressure” indicates posture instability and balancing problems that are highly efficient in diagnosing balancing issues in PD patients.
The dataset consists of dependent and independent features that must be preprocessed separately for efficient results. For independent features, including height (H), age (A), weight (W), gender (G), and the individual’s self-choosen walking speed (S), a “Spearman rank correlation” is calculated and represented in Table 4.
Table 4 proves a moderate correlation between height and gender (− 0.672) and weight (0.55). Therefore, height is not incorporated into a best-fitted regression model, and it is clear that age, weight, and walking speed are significant independent features.
Further, the regression model is constructed for each dependent feature by eliminating the least significant variable with p < 0.001. The exact process will continue for all the dataset’s significant dependent features. After that, each parameter is normalized using the following Eq. (2):
Here, the dependent spatiotemporal characteristics of the ith observation are denoted by Xi, Xi, j for the jth physical characteristics, such as weight, age, walking speed, and height stands for the unidentified “regression coefficient,” and “i for the residual error” seen for the ith repetition. The data dispersion is reduced on the basis of the coefficient of variation with a Standard Error (SE) and 95% Confidence Level (CL). The coefficient of variation is used to depict the reduction in data dispersion obtained through a multivariant normalization approach, which is listed in Table 5.
Table 5 shows a significant reduction in the dispersion of dependent features when using a multivariant normalization approach. After preprocessing and extracting the significant gait features, all these features are further passed to the feature section phase.
Feature selection
After the feature extraction phase, all these features are further passed to the feature selection phase. As mentioned in Table 6, a different set of features is extracted using different methods. Each feature offered a different contribution while evolving the performance of any classification technique. Selection of the best feature according to its contribution is the primary phase of any classification model. Here, the best collection of features is chosen based on their contribution to the classification accuracy using the RFE technique with Random Forest (RF). RFE is the most popular technique in bioinformatics for feature selection that will improve the model performance by reducing the dimensions of data and resolving the problem of overfitting. In addition, other researchers39 followed the RFE for the gait feature selection, which gives better results in improved model performance. Based on their results, we have chosen RFE as the feature selection technique for our model. In RFE, Information Gain is used to determine the relevance of gait features. The RFE algorithm, in its entirety, is explained below.
The procedure followed by the RFE method for optimal feature selection.

The standard Python instructions from the SciKit-learn library are used to implement RFE for machine-learning algorithms. The significant level assigned to different gait characteristics assigned by RFE is represented in Fig. 5.
As represented in Fig. 5, the most efficient features selected by RFE for detecting PD include force variation at heel strike, step length, Center of pressure, toe of force, double limb support, and swing stance ratio. These highly efficient selected gait features are passed to Stage 1 of the proposed model for further analysis.
The hyperparameters are also implemented at both stages of the proposed model to get more optimized results using the Grid Search Optimization approach.
Classification
After the feature selection phase, the selected set of features is passed to the classification phase. The proposed model follows two stages for classification. Stage 1 of the proposed model focuses on diagnosing the disease and determining whether or not the patient is suffering from it. If the patient is diagnosed with PD, then Stage 2 of the model determines the severity level of the disease, and effective treatment must be provided based on the severity level to ensure that the patient’s life can be saved and improved effectively.
-
1.
Stage 1: Detection.
At Stage 1, classification is performed through RFT. RFT was selected based on the comparative analysis of different ML approaches presented in Table 8, which is under the “Result and discussion” section. RFT is an ensemble learning method combining various classifiers to handle the most issues and thus enhance the model’s overall performance. Accordingly, the RFT is generated by combining multiple DTs. The RFT finds the average of all the results computed through separate DTs depending on their trained data subset to enhance model performance. The RFT makes a final prediction by applying the concept of majority voting on predictions conducted by each DT rather than relying solely on one DT. Better accuracy and reduced overfitting are achieved through the combinations of multiple decision trees.
RFT follows the bagging technique, which is also called bootstrap aggregation. In such a technique, random samples of features are selected from the given dataset, and different sets of models are generated from generated samples (bootstrap samples). After that, each model is trained separately, and their results are evaluated before the final decision is reached. Lastly, the final output is obtained through majority voting by integrating the outcomes of each model40. The algorithm followed by RFT is described below:

After applying RFT at Stage 1, the data samples are classified into two PD and non_PD classes. Further, the classified PD patients are passed to Stage 2 to assign PD severity levels.
-
2.
Stage 2: Severity prediction.
Only the PD patients that are detected at Stage 1 are passed to Stage 2 for assigning the severity of the disease and to suggest some preventive measures to PD patients based on their assigned severity level under the supervision of physicians. The severity level mainly refers to the degree of hardness of the disease, so appropriate treatment must be suggested to the patients. At Stage 2, a regression technique is used to assign disease progression/severity depending on extracted features into three different classes: 2, 2.5, and 3, respectively. The H& Y rating scale has been applied to assign the severity to the patients depending on their symptoms. Stage 2 of the proposed model is activated if Stage 1 results in PD patients.
At Stage 2, one highly prone severity feature named Double Limb Support (DLS), (previously extracted) is combined with a previously selected set of features (from Stage 1) and passed to the ER model to determine the severity level of PD18,41,42. ER works on the principle of merging multiple models to improve the prediction accuracy for solving several learning problems where the targeting variable is numeric. The generation, pruning, and integration phases are three essential phases followed by an ER. DLS is a more prone feature that affects the severity level of people with PD. The severity level of PD increases when DLS increases. The suggested model uses the gait patterns to deliver the precise value of severity level depending on the motor abnormalities43. Finally, the PD patients are assigned three severity levels: 2(initial), 2.5(mild), and 3(advanced stage). Finally, a few suggestions, including diet, exercise schedule, and physician consultation, are provided to assist PD patients based on their severity level.
Performance evaluation measures
This section describes the various performance evaluation measures used in this study to assess the effectiveness of our model. The efficiency of the proposed model is evaluated by utilizing Accuracy (Eq. 2), that is, the ratio of accurate predictions to the total predictions. Sensitivity or recall measures (Eq. 3) are the ratio of true positives to the total number of actual positive predictions supporting identifying a patient with a disease state. Specificity/ Precision (Eq. 4) specifies the ratio between truly negative and total negative values in identifying the individual with a PD state. F1_Score is the harmonic mean of precision and recall/Sen. (Eq. 5).
Tn: True Negative Values, Tp: True Positive Values, Fp: False Positive, and Fn: False Negative value. An additional parameter named MAE (Mean Absolute Error) is used to assess the gap between the actual and the predicted rates. Equation (6) presents the MAE formula.
The total number of strides, the model projected value, and the actual value are represented by variables m, Xpredj, and Xj, respectively. A model whose outputs match the observed value is one in which Xj is the average of [X1, X2, …, Xn]. SSR (sum of squared residuals) is equal to zero, and R2 is equal to one. However, the total sum of squares determines the variance of the data (SST). The model performance is also evaluated using RMSE (Root Mean Square Error), which is computed using Eq. 7.
Equation 7 presents the RMSE formula. Here, Ti denotes the truly predicted value of ith observation, Pi denotes the observed value, and the total number of observations is represented by N.
Result and discussion
The proposed model is implemented on a Laptop with an Intel Core i3, 8GB RAM, and a CPU@ 3.30 GHz processor using a Jupyter Notebook and evaluated using different evaluation measures. Different inbuilt libraries are available in Python programming, including pandas for importing, analyzing, and cleaning data, NumPy for numerical computation of data, sci-kit for importing different ML techniques and evaluation metrics, matplotlib, and seaborn for results plotting and visualization, TensorFlow, Keras, and Pytorch for implementing the deep learning models, are used for the implementation of the proposed work.
The robustness of the proposed model is checked by implementing it on the PD dataset accessed from the Psyionet28 repository, which consists of recordings from 166 subjects, out of which 93 are PD patients, and 73 are healthy controls. For the robust evaluation of the model, the dataset is portioned into two subsets: 70% for training and 30% for testing and validation. This strategic portioning of data supports a comprehensive evaluation of the proposed model. Intrinsic imbalancing in the dataset is observed due to the large number of PD patients compared to healthy controls. In the current work, this issue is addressed by implementing the SMOTE method, which will ensure the balanced distribution of data and, thus, improve the model generalizability. This study creates opportunities for early diagnosis and better patient outcomes by utilizing state-of-the-art algorithms and resolving data discrepancies.
Moreover, the current research also incorporates a grid search optimization approach to find the optimal set of hyperparameters for our model. A 10-fold CV method is applied for validation, in which the overall training dataset is partitioned into ten equivalent parts. Each time, one part is kept for validation, and the other nine parts are applied to train the proposed model. This process is repeated until each part is utilized for validation. Finally, the model performance is calculated by averaging the scores achieved in each fold. Each machine-learning technique is tuned with hyperparameters selected through a grid search optimization approach, as listed in Table 7.
Further, different ML techniques tuned with hyperparameters are compared to select the best classifier on the balanced (After applying SMOTE) and original (Before applying SMOTE) dataset. The results are evaluated in terms of Accuracy, Sensitivity, Specificity, and F1-Score, as listed in Table 8 and shown in Figs. 6 and 7.
It is clearly evidenced from Table 8 that all considered ML techniques provide efficient results when applied to a balanced dataset(after SMOTE) as compared to the original dataset (Before SMOTE). Further, from the comparative results from Table 8; Figs. 6, and 7, we find RFT to be the best classification technique used at Stage 1 of the proposed model for classifying subjects into PD and non-PD classes. At this stage, the model achieved an average Accuracy of 97.5% ± 2.1% (Acc. ±Std), an average Sensitivity of 97% ± 2.5% (Sen. ± Std), and an average Specificity of 95% ± 2.2%(Spec. ±Std). Moreover, the average computational time for training the model is 14.57 s, which is computationally effective in comparison to other models.
Additionally, to verify the statistical significance of the proposed model at Stage 1, a 95% Confidence Interval (CI) and a hypothetical t-test are computed and calculated for all evaluation measures. The computed value of 95% of CI for Accuracy is [96.25%, 98.85%], for Sensitivity is [94.5%, 99.5%], and for Specificity is [93.93%, 96.07%]. Moreover, the resulting p-value computed through the t-test for all these measures is less than 0.01, indicating our model’s improved performance over the baseline model.
In the same way, a comparative analysis is also carried out between different regressor techniques, tuned with hyperparameters, on balanced (After applying SMOTE) and original (Before applying SMOTE), to select the best technique for Stage 2 of the proposed model. Each regressor technique is tuned with hyperparameters through grid search optimization, which is listed in Table 9. The results achieved through the comparative analysis are listed in Table 10 and shown in Figs. 8 and 9.
The comparative results in Table 10; Figs. 8 and 9 prove that ER is the best regressor technique. This technique is further used in Stage 2 of the proposed model to assign severity levels to PD patients. The result revealed that the ER achieved an accuracy of 96.4%± 2.3% (Acc.± std), MAE of 0.065 ± 0.024, and an RMSE of 0.080 ± 0.06. Additionally, to verify the statistical significance of the proposed model at Stage 2, a 95% Confidence Interval (CI) and a hypothetical t-test are computed and calculated for all evaluation measures. The computed value of 95% of CI for Accuracy is [96.01%, 96.79%].
Table 11 represents the results achieved after implementing the proposed model by incorporating the hyperparameter-tuned RFT and ER techniques at both stages of the proposed model, respectively. The computed results show that the proposed model performs exceptionally well for PD patient classification and severity assessment. Further, a comparative analysis between the proposed and existing models is carried out to validate the results, represented in Table 12.
Comparative results shown in Table 12 indicate that our proposed model outperformed other existing models. Though some existing models achieve higher accuracy than our proposed model, they have not used other performance evaluators like MAE and RMSE to validate the results. Thus, it can be concluded that our proposed model outperforms all other existing models significantly in diagnosing PD and assigning severity to PD patients.
Conclusions and future works
PD is a widespread neurodegenerative disease that causes various movement disorders among subjects. However, the practical and accurate diagnosis of PD requires regular monitoring of PD symptoms. In the past decades, before the introduction of intelligent sensors-based technology, physicians had to examine patient’s neurological and physiological behavior under their supervision in hospitals to diagnose diseases effectively. Such a process of PD diagnosis is very time-consuming and requires regular visits of patients to hospitals. Moreover, introducing smart sensors-based devices offers a new way of monitoring patient’s health symptoms without visiting hospitals. However, sensor-recorded data must be effectively analyzed to get more significant results about patient health. Therefore, effective machine-learning techniques must be applied to these recorded data to get more significant results about patient health.
In this direction, this study emphasizes the use of sensor-based technology along with machine learning techniques to monitor PD patients, which reduces regular visits to hospitals and causes the diagnosis of PD at its early stage. Therefore, this study proposes a dual-stage PD diagnosis and severity assessment model using machine learning techniques. Before analysis during the preprocessing stage, the SMOTE technique is used to balance the considered dataset to get the optimized results, followed by future extraction and selection. Highly effective spatiotemporal features have been extracted from the recorded dataset to get more significant results. Further, at Stage 1 of the proposed model classifies the patients into PD and non-PD classes using the hypertuned RFT technique. At Stage 2 of the proposed model, the severity level of PD is assigned using the hypertuned Ensemble Regressor technique. The proposed model has been implemented and evaluated on a sensor-based data set (“Gait in Parkinson’s Disease”) collected from 16 force sensors fitted into the insoles of shoes that capture the subjects walking patterns while wearing them. The computed results prove that the proposed model is significant in classifying subjects into PD and non-PD classes by attaining an average accuracy of 97.5% ± 2.1%, average Sensitivity of 97% ± 2.5%, and average Specificity of 95% ±2.2% at the Stage 1 with the 10-fold CV method. The 95% of Confidence Interval(CI )computed for accuracy is [96.25%, 98.85%], for Sensitivity is [94.5%, 99.5%], and for Specificity is [93.93%, 96.07%]. The results evaluated through statistical analysis demonstrate that the proposed model is highly effective and reliable.
Finally, the proposed model assigns severity ratings to the PD patients based on the H&Y rating scale, with an accuracy of 96.4%± 2.3% %, MAE of 065 ± 0.02, and RMSE of 0.080 ± 0.06 at Stage 2. Additionally, the statistical significance of the proposed model at Stage 2 is also evaluated using 95% CI for Accuracy, which is [96.01%, 96.79%]. The results show that the proposed model is highly efficient in classifying and assigning patient severity ratings. Advanced research in this field could improve the reliability and Accuracy of PD diagnosis and its treatment, ultimately improving patient health and quality of life. However, the proposed model is limited only to a specific age group of people due to the unavailability of the dataset, which can be extended to other age groups. Along with that, our model worked with limited features (like stance time, stride length, swing interval double limb support, step time, and step length) that can be further extended with other features such as tremor signal, cognition, and muscle strength that may increase the efficiency and applicability of the model. Additionally, other factors, including Floating Point Operations (FLOPs), number of parameters (Params), latency time, etc., can be considered in the future. Moreover, an advanced data balancing approach could be used to handle data imbalance problems to get more synthetic and generalized results. Further, the proposed model can be extended to deep learning techniques by incorporating additional sensor data, including accelerometers, smartwatches, etc. Additionally, the efficiency of the proposed model can be further improved by incorporating larger and diversified datasets collected in real clinical settings for training and testing the model.
Limitations
Although the proposed model yielded promising results, yet, it suffers from some limitations. One limitation of this work is the absence of an interpretative analysis of the results, particularly due to the use of black-box models, which inherently lack transparency in decision-making. In clinical practice, interpretability is critical because clinicians need to understand the reasoning behind predictions to make informed decisions, especially in high-stakes settings. Without interpretability, even a high-performing model may lack the trust and transparency required for real-world implementation. Therefore, future work may include more interpretability techniques like SHAP or LIME to enhance transparency and trustworthiness to make the model clinically actionable and acceptable. Another limitation of this work is the assignment of a limited severity rating of only 2,2.5 and 3 using H & Y rating scale which should be further extended to assign a severity rating in the range of 0 to 5 as per the H& Y rating scale.
Data availability
Data Availability: This research utilizes online public dataset, “Gait in Parkinson’s Disease” online available at https://physionet.org/content/gaitpdb/1.0.0/.
References
Pardoel, S., Kofman, J., Nantel, J. & Lemaire, D. Wearable-sensor-based detection and prediction of freezing of gait in parkinson’s disease: a review. Sensors 19(23), 5141. https://doi.org/10.3390/s19235141 (2019).
Statistics|Parkinson’s Foundation (2022, accessed 12 Dec 2022). https://www.parkinson.org/understanding-parkinsons/statistics.
World Health Organization. Neurological disorders: public health challenges 218 (2006, accessed 28 Nov 2022). https://apps.who.int/iris/handle/10665/43605.
Eskofier, B. M. et al. In 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 655–658 (IEEE, 2016). https://doi.org/10.1109/EMBC.2016.7590787.
Váradi, C. Clinical features of parkinson’s disease: the evolution of critical symptoms. Biology 9(5), 103. https://doi.org/10.3390/biology9050103 (2020).
Gökçal, E., Gür, V. E., Selvitop, R., Babacan Yildiz, G. & Asil, T. Motor and non-motor symptoms in Parkinson’s disease: effects on quality of life. Noro Psikiyatr Ars 54(2), 143–148. https://doi.org/10.5152/npa.2016.12758 (2017).
AlMahadin, G. et al. Parkinson’s disease: current assessment methods and wearable devices for evaluation of movement disorder motor symptoms—a patient and healthcare professional perspective. BMC Neurol. 20(1), 419. https://doi.org/10.1186/s12883-020-01996-7 (2020).
Balaji, S., Nathani, K. & Santhakumar, R. IoT technology, applications and challenges: a contemporary survey. Wirel. Pers. Commun. 108(1), 363–388. https://doi.org/10.1007/s11277-019-06407-w (2019).
Peng, Y. et al. Intelligent devices for assessing essential tremor: a comprehensive review. J. Neurol. https://doi.org/10.1007/s00415-024-12354-9 (2024).
Tong, J., Zhang, J., Dong, E. & Du, S. Severity classification of Parkinson’s disease based on permutation-variable importance and persistent entropy. Appl. Sci. 11(4), 1834. https://doi.org/10.3390/app11041834 (2021).
Khoury, N., Attal, F., Amirat, Y., Oukhellou, L. & Mohammed, S. Data driven based approach to aid parkinson’s disease diagnosis. Sens. (Basel) 19(2), 242. https://doi.org/10.3390/s19020242 (2019).
Balaji, E., Brindha, D. & Balakrishnan, R. Supervised machine learning based gait classification system for early detection and stage classification of Parkinson’s disease. Appl. Soft Comput. 94, 106494. https://doi.org/10.1016/j.asoc.2020.106494 (2020).
Hristova, A. H. & Koller, W. C. Early Parkinson’s Disease: what is the best approach to treatment. Drugs Aging 17(3), 165–181. https://doi.org/10.2165/00002512-200017030-00002 (2000).
Trabassi, D. et al. Machine learning approach to support the detection of Parkinson’s disease in IMU-based gait analysis. Sensors 22(10), 3700. https://doi.org/10.3390/s22103700 (2022).
Gao, C. et al. Model-based and model-free machine learning techniques for diagnostic prediction and classification of clinical outcomes in Parkinson’s disease. Sci. Rep. 8(1), 7129. https://doi.org/10.1038/s41598-018-24783-4 (2018).
Maachi, I. E., Bilodeau, G. A. & Bouachir, W. Deep 1D-Convnet for accurate Parkinson disease detection and severity prediction from Gait. Expert Syst. Appl. 143, 113075. https://doi.org/10.1016/j.eswa.2019.113075 (2020).
Mirelman, A. et al. Detecting sensitive mobility features for parkinson’s disease stages via machine learning. Mov. Disord. 36(9), 2144–2155. https://doi.org/10.1002/mds.28631 (2021).
Setiawan, F. & Lin, C. W. Implementation of a deep learning algorithm based on vertical ground reaction force time-frequency features for the detection and severity classification of Parkinson’s Disease. Sensors 21(15), 5207. https://doi.org/10.3390/s21155207 (2021).
Hssayeni, M. D., Jimenez-Shahed, J., Burack, M. A. & Ghoraani, B. Ensemble deep model for continuous estimation of Unified Parkinson’s Disease Rating Scale III, BioMed. Eng. 20(1), 32. https://doi.org/10.1186/s12938-021-00872-w (2021).
Aşuroğlu, T. & Oğul, H. A deep learning approach for Parkinson’s disease severity assessment. Health Technol. 12(5), 943–953. https://doi.org/10.1007/s12553-022-00698-z (2022).
Khera, P. & Kumar, N. Age-gender specific prediction model for Parkinson’s severity assessment using gait biomarkers. Eng. Sci. Technol. Int. J. 27 , 101005. https://doi.org/10.1016/j.jestch.2021.05.009 (2022).
Rastegari, E., Ali, H. & Marmelat, V. Detection of Parkinson’s disease using wrist accelerometer data and passive monitoring. Sensors 22 , 9122. https://doi.org/10.3390/s22239122 (2022).
Erdaş, Ç. B., Sümer, E. & Kibaroğlu, S. Neurodegenerative diseases detection and grading using gait dynamics. Multimed Tools Appl. https://doi.org/10.1007/s11042-023-14461-7 (2023).
Nair, P., Shojaei Baghini, M., Pendharkar, G. & Chung, H. Detecting early-stage Parkinson’s disease from gait data, Proc. Inst. Mech. Eng. H 237(11), 1287–1296. https://doi.org/10.1177/09544119231197090 (2023).
Indu, R., Dimri, S. C. & Malik, P. A modified kNN algorithm to detect Parkinson’s disease. Netw. Model. Anal. Health Inf. Bioinform. 12(1), 24. https://doi.org/10.1007/s13721-023-00420-7 (2023).
Abedinzadeh Torghabeh, F., Modaresnia, Y. & Hosseini, S. A. An efficient tool for Parkinson’s disease detection and severity grading based on time-frequency and fuzzy features of cumulative gait signals through improved LSTM networks. Med. Novel Technol. Dev. 22, 100297. https://doi.org/10.1016/j.medntd.2024.100297 (2024).
Goldberger, A. L. et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 101, 23. https://doi.org/10.1161/01.CIR.101.23.e215 (2000).
Yogev, G. et al. Dual tasking, gait rhythmicity, and Parkinson’s disease: Which aspects of gait are attention demanding? Eur. J. Neurosci. 22(5), 1248–1256. https://doi.org/10.1111/j.1460-9568.2005.04298.x (2005).
Frenkel-Toledo, S. et al. Treadmill walking as an external pacemaker to improve gait rhythm and stability in Parkinson’s disease. Mov. Disord. 20(9), 1109–1114. https://doi.org/10.1002/mds.20507 (2005).
Hausdorff, J. M. et al. Rhythmic auditory stimulation modulates gait variability in Parkinson’s disease: efects of RAS on gait variability in PD. Eur. J. Neurosci. 26(8), 2369–2375. https://doi.org/10.1111/j.1460-9568.2007.05810.x (2007).
Subramaniam, S., Majumder, S., Faisal, A. I. & Deen, M. J. Insole-based systems for health monitoring: current solutions and research challenges. Sensors 22(2), 438. https://doi.org/10.3390/s22020438 (2022).
Wahid, F. et al. Multiple regression approach to normalization of spatiotemporal gait features. J. Appl. Biomech. 32(2), 128–139. https://doi.org/10.1123/jab.2015-0035 (2016).
Salarian, A. et al. Gait assessment in Parkinson’s disease: toward an ambulatory system for long-term monitoring. IEEE Trans. Biomed. Eng. 51(8), 1434–1443. https://doi.org/10.1109/TBME.2004.827933 (2004).
Peppes, N. et al. Generating realistic parkinson’s disease freezing of gait data using GANs. Sensors 23(19), 8158. https://doi.org/10.3390/s23198158 (2023).
Trabassi, D. et al. Jun., Optimizing rare disease gait classification through data balancing and generative AI: insights from hereditary cerebellar ataxia. Sensors 24(11), 3613. https://doi.org/10.3390/s24113613 (2024).
Chawla, N. V. et al. SMOTE: synthetic minority over-sampling technique. J. Artif. Int. Res. 16(1), 321–357 (2002).
Iosa, M. et al. The golden ratio of gait harmony: repetitive proportions of repetitive gait phases, BioMed Res. Int. 2013, 1–7. https://doi.org/10.1155/2013/918642 (2013).
Rehman, R. Z. U. et al. Selecting clinically relevant gait characteristics for classification of early Parkinson’s disease: a comprehensive machine learning approach. Sci. Rep. 9(1), 17269. https://doi.org/10.1038/s41598-019-53656-7 (2019).
Gaenslen, A. & Berg, D. Early diagnosis of Parkinson’s disease. Int. Rev. Neurobiol. 90, 81–92. https://doi.org/10.1016/S0074-7742(10)90006-8 (2010).
Okada, Y., Fukumoto, T., Takatori, K., Nagino, K. & Hiraoka, K. Variable initial swing side and prolonged double limb support represent abnormalities of the first three steps of gait initiation in patients with parkinson’s disease with freezing of gait. Front. Neur. 2, 45. https://doi.org/10.3389/fneur.2011.00085 (2011).
Zanardi, A. P. J. et al. Gait parameters of Parkinson’s disease compared with healthy controls: a systematic review and meta-analysis. Sci. Rep. 11(1), 752. https://doi.org/10.1038/s41598-020-80768-2 (2021).
Veeraragavan, S., Gopalai, A. A., Gouwanda, D. & Ahmad, S. A. Parkinson’s disease diagnosis and severity assessment using ground reaction forces and neural networks. Front. Physiol. 11, 587057. https://doi.org/10.3389/fphys.2020.587057 (2020).
Khera, P. & Kumar, N. Novel machine learning-based hybrid strategy for severity assessment of Parkinson’s disorders. Med. Biol. Eng. Comput. 60(3), 811–828. https://doi.org/10.1007/s11517-022-02518-y (2022).
Sotirakis, C. et al. identification of motor progression in Parkinson’s disease using wearable sensors and machine learning. npj Parkinsons Dis. 9(1), 142. https://doi.org/10.1038/s41531-023-00581-2 (2023).
Gait in Parkinson’s Disease dataset (2024, accessed 10 Apr 2024). https://physionet.org/content/gaitpdb/1.0.0/.
Author information
Authors and Affiliations
Contributions
Navita and Pooja drove conceptualization, methodology, software development, and original writing. Yogesh, Anjani, Sarita, Vimal and Umesh led the formal analysis, investigation, and resource management and played a key role in writing and oversight. Navita, Pooja, Umesh, Anjani, and Sarita, contributed to validation, formal analysis, investigation, and resource management, actively participating in writing. Anjani, Sarita, and Vimal helps with revision work and results analysis. All authors reviewed and approved the final manuscript, showcasing a collaborative effort with distinct and complementary roles. Pooja supervised this research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All methods were performed in accordance with the relevant guidelines and regulations. This study utilized publicly available open datasets, which do not require ethical approval or informed consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Navita, Mittal, P., Sharma, Y.K. et al. Gait-based Parkinson’s disease diagnosis and severity classification using force sensors and machine learning. Sci Rep 15, 328 (2025). https://doi.org/10.1038/s41598-024-83357-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83357-9
Keywords
This article is cited by
-
C3BAM-XAI: Convolutional Block Attention Module Enhanced Explainable Artificial Intelligence-Based Parkinson’s Disease Stage Classification
Cognitive Computation (2025)
-
Harnessing Machine Learning and Deep Learning for Non-Communicable Disease Diagnosis: an In-Depth analysis
Archives of Computational Methods in Engineering (2025)