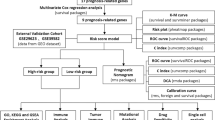
Abstract
Necroptosis, a type of programmed cell death, has been increasingly linked to cardiovascular disease development, yet its role in dilated cardiomyopathy (DCM) remains unclear. In this study, we analyzed the GSE5406 dataset from the GEO database to explore necroptosis-related prognostic signatures in DCM using LASSO regression. We identified five necroptosis-related genes (BID, CAMK2B, GLUL, HSP90AB1, CHMP5) that define a necroptosis-related signature with strong predictive value, evidenced by ROC curve areas of 0.852 and 0.957 in training and test sets, respectively. Our analyses, including GO and GSEA enrichment, focused on pathways associated with high necroptosis-related scores (NRS) and revealed significant immune cell infiltration. Notably, nTreg and iTreg cells were enriched in the high NRS group, while CD8 naive T cells and CD8 T cells positively correlated with NRS. Small molecule drugs fenofibrate, procyclidine, and tienilic acid emerged as potential therapeutic agents for high-risk patients, with fenofibrate showing efficacy in inhibiting DCM progression in an inflammatory animal model. These findings underscore the clinical relevance of necroptosis-related genes in assessing DCM progression and prognosis and highlight their potential for targeted therapeutic development.
Similar content being viewed by others
Introduction
Dilated cardiomyopathy (DCM) activates networks that maintain physiological function, presenting a multifactorial systemic disease affecting approximately 1–2% of the adult population1. A large proportion of patients with DCM have an underlying genetic, inflammatory or autoimmune disease basis2. The molecular basis of irregular cardiac structure in DCM is reactive or genetic alterations in cardiomyocyte structure and composition, and leads to remodeling of the myocardium3. The underlying molecular mechanisms of remodeling reveal a complex network of cellular signaling pathways which have not been fully understood. Techniques involving genetics, imaging, or cardiovascular are currently used in the diagnosis and prognostic assessment of DCM4,5. Novel and precise genes and biomarkers are needed for early identification and customization of personalized patient management programs.
Current research suggests that regulation of necroptosis, a form of programmed necrosis, plays an important role in the progressive decline of myocardial contractility in dilated cardiomyopathy6,7. Changes in the expression of necroptosis proteins may underlie decreased cardiac function in failing hearts, which was demonstrated in a recent study showing that the expression of the necroptosis protein pThr 357-MLKL is increased in the myocardial tissue of patients with DCM. As mentioned previously, the molecular mechanisms of these necroptosis-related biomarkers in DCM progression remain ambiguous8,9. Our understanding of the role of necroptosis in the pathological process of DCM and its prognostic value is far from complete10.
This study focuses on a specific set of genes in the necroptosis pathway that are associated with DCM and explores their role in disease progression and prognostic assessment. We investigated the variations between patients with elevated and reduced necroptosis-related scores (NRS) in pathway enrichment and immune cell infiltration, and identified specific drugs for those with high NRS. Our study offers a comprehensive understanding of necroptosis-associated signaling pathways and molecularly targeted therapies in the pathology of DCM.
Materials and methods
Data acquisition and differential gene analysis
The mRNA expression profile of GSE540611 which includes 86 systolic heart failure myocardium due to idiopathic dilated cardiomyopathy and 16 normally functioning myocardium from unused non-failing (NF) donor heart. The “limma” package was employed to measure genes with differential expression between DCM and NF, using an adjusted p-value threshold of less than 0.05. Necroptosis-related genes were obtained from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database12.
Construction of necroptosis-related signatures
Least absolute shrinkage and selection operator (LASSO) estimates the regression coefficients to construct feature genes related to necroptosis. Dilated cardiomyopathy (DCM) patients were randomly assigned to the training cohort or the test cohort in a 7:3 ratios by a hierarchical system approach. The observed value of patients with left ventricular (LV) ejection fraction (EF) < 15% was set as 1, and the observed value of patients with LVEF > 15% was set as 0. The differential genes related to necroptosis were used as the dependent variable. The model’s predictive performance was assessed using the receiver operating characteristic (ROC) curve and the area under the curve (AUC)13. According to the median NRS, patients were divided into low and high NRS subgroups.
Functional enrichment analysis
According to the median NRS, DCM patients were divided into low NRS subgroup (n = 43) and high NRS subgroup (n = 43).
The DEGs between patients with elevated and reduced NRS were examined using the “limma” package14, with a threshold of an adjusted p-value of less than 0.05. The Metascape platform (accessible at www.metascape.org) was utilized to conduct gene ontology (GO) enrichment analysis on DEGs, using an FDR of less than 0.05 as the threshold. GSEA analysis was performed on KEGG target gene sets in patients with high NRS and patients with low NRS by the “clusterProfiler” package. Pathways with a false discovery rate below 0.05 were deemed to be statistically significant.
Analysis of gene regulatory networks
Gene interactions were predicted using Network Analyst v3.0 network tool (www.networkanalyst.ca), a comprehensive web-based platform designed for the analysis of gene networks, pathways, and regulatory interactions. The JASPAR database is used to predict the interaction between transcription factors and genes, which is a well-established open-access database of transcription factor binding profiles. The miRTarBase database is used to predict the interaction between miRNAs and genes. The analyzed network results were imported into Cytoscape15. We screened for interaction pairs with interaction scores greater than 0.4 and the MCC algorithm of Cytoscape’s Cytohubba plugin was used to calculate the connectivity between nodes, and the top 30 nodes were retained.
Immune infiltration analysis
ImmuCellAI tool16 (bioinfo.life.hust.edu.cn/ImmuCellAI) was utilized to perform immune infiltration analysis. This tool is capable of accurately predicting the abundance of 24 immune cell types in a sample based on RNA-Seq or microarray transcription data, providing insights into the immune landscape associated with different conditions.
We employed the Wilcoxon rank sum test to compare immune cell infiltration levels between the high and low NRS groups, enabling us to determine statistically significant differences in immune cell populations across these groups. Furthermore, Spearman correlation analysis was utilized to explore the relationships between key regulators identified in our previous analyses and the various immune cell types, providing insights into the potential interactions between regulatory elements and the immune response.
The connectivity map (CMap) analyses
CMap17 analyzed the DEGs from patients with varying NRS levels, yielding a correlation score derived from the enrichment of DEGs within the reference gene expression profile. CMap assesses gene expression profiles to identify potential correlations between DEGs and small molecule compounds, yielding a correlation score that indicates the similarity of the DEGs to the reference gene expression profiles within the dataset. Small molecule compounds with enrichment scores exceeding 90 were deemed promising candidates, and an analysis was conducted to predict their mechanisms of action.
Establishment of a nomogram
To establish a nomogram that integrates NRS with clinical interventions, the “rms” package in R was employed. This package allows for the development of predictive models based on regression analysis, providing a graphical representation of the predicted probabilities of an event occurring (in this case, the onset or progression of disease).
The precision of the nomogram was evaluated using calibration curves, which assess how well the predicted probabilities align with actual outcomes. Additionally, decision curve analysis was utilized to evaluate the practical value of the nomogram, considering the potential benefits and harms of using the model for clinical decision-making.
Human samples
Left ventricular tissue was obtained from six patients undergoing heart transplantation at the Union Hospital. All patients with an EF of less than 40% were diagnosed with DCM at least three months prior to receiving heart transplantation. Non-heart failure tissue was provided by three organ donors whose hearts could not be transplanted due to size problems, ABO mismatch, or other factors. The study conformed with the Helsinki Declaration (revised 2013).
Real-time polymerase chain reaction (PCR)
RNA was isolated from cardiac tissue of human using TRIzol reagent, and the PrimeScript RT kit (TaKaRa) was employed to convert the RNA into cDNA. SYBR green (TaKaRa) was utilized for conducting real-time fluorescent quantitative PCR. The 2−ΔΔCt technique was employed to determine relative gene expression. GAPDH served as the control gene. Table 1 provides the primer sequences.
Animals
Male BALB/c mice (6–8 weeks old) were housed in specific pathogenfree conditions at the experimental animal center, Henan Medical College of Zhengzhou University. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were randomly divided into three groups: control group, DCM group, DCM + fenofibrate group. For the control group, BALB/c mice were not administrated with anything. The other two groups of mice were injected with α-myosin heavy chain at days 0, days 7, and days 14, and oral administration with saline or fenofibrate (30 mg/kg per mouse), respectively. All mice were anesthetized with 2% pentobarbital and euthanized by cervical disloaction at days 45.
Masson staining
Adjacent paraffin-embedded heart sections were cut and stained with Masson. The fibrosis was scored blindly by two independent observers. Areas of fibrosis relative to total left ventricular area were measured and expressed as percentages by computerized planimetry using Image Pro Plus software.
Echocardiography
Transthoracic echocardiography was performed on mice for 45 days using a Vevo 1100 instrument and transducer MS400. To induce sedation and immobility before echocardiography, mice were anaesthetized with ketamine. Two-dimensional parasternal short-axis images of the left ventricle in diastole were obtained at the level of the papillary muscle followed by two-dimensional guided M-mode images.
Ethics approval and consent to participate
The study involving animal subjects was conducted under the approval of the Zhengzhou University Committee for the Management and Use of Experimental Animals. The study involving human data was approved by The Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China; approval number: UHCT-IEC-SOP-016-03-01). All patients or their families provided their written informed consent. All experiments were performed in accordance with relevant named guidelines and regulations, and this study is reported in accordance with the ARRIVE guidelines.
Statistical analysis
All statistical tests were performed using R statistical software 4.2.1. Student’s t-test was used for differences between paired sample groups. One-way ANOVA and Tukey’s post hoc multiple comparison test were used for multiple group comparisons. Correlations between variables were tested using Spearman’s correlation test. All statistical p-values were two-sided and p < 0.05 was considered statistically significant.
Results
Identification of necroptosis-related differentially expressed genes (NRDEGs)
In our analysis, we began by identifying differentially expressed genes (DEGs) associated with necroptosis within the dataset GSE5406. By overlapping these necroptosis pathway-related genes with the DEGs, we successfully identified 27 overlapping NRDEGs for further investigation (Fig. 1A). The volcano plot of DEGs and expression heatmap of NRDEGs are presented in Fig. 1B,C. The up-regulated and down-regulated NRDEGs are listed in Table 2.
Identification of key NRDEGs to construct DCM disease-related progression and prognosis models
Ejection fraction is often used as an important indicator to assess the progression and prognosis of DCM and defines the patient’s phenotype. When patients with left ventricular ejection fraction < 15%, the mortality rate is greatly increased18. The patient was then divided into two groups based on LVEF cutoff of 15%. One group was defined at EF < = 15%, while another group was defined at EF > 15%. This stratification allowed us to focus on the most clinically relevant populations. DCM patients were randomly assigned to the training cohort or the validation cohort in a 7:3 ratios by a hierarchical system approach. Higher scores represent more severe progression and worse prognosis of DCM patients. Our algorithm identifies a model consisting of 5 genes which has high ROC values in the training and test sets. Five genes, including BID, CAMK2B, GLUL, HSP90AB1, CHMP5 with coefficients of 1.486, −0.963, 0.92, −0.466 and 1.812 were identified to construct the NRDEGs-related progressive and prognostic signature (Fig. 2A,B). In training set, the AUC of the ROC curve was 0.873 (Fig. 2C). In test set, the AUC value of ROC curve was 0.957 (Fig. 2D). In the overall set, the model achieved an ROC of 0.835 (Fig. 2E). It is demonstrated that NRDEGs-related features have excellent diagnostic performance in predicting the progression and prognosis of DCM patients.
Identification of key NRDEGs to construct DCM disease-related progression and prognosis models. (A,B) Least absolute shrinkage and selection operator (LASSO) logistic regression algorithm to screen key genes. (C–E) Receiver operating characteristic (ROC) curves analysis of training set (C), testing set (D) and total dataset (E). AUC, area under the curve.
Signaling pathway enrichment analysis in relation to NRS
To clarify the dysregulated gene expression between patients in the high and low NRS groups, we identified over 1573 differentially expressed genes involved in necroptosis between high and low NRS groups. GO analysis indicated that enrichment of circulatory system process, mitotic cell cycle process, neural nucleus development, regulation of axon extension involved in axon guidance of ion transport were identified down-regulated in high-NRS group (Fig. 3A). Meanwhile, cellular response to biotic stimulus, regulation of kinase activity and negative regulation of anokis were enriched in high-NRS group (Fig. 3B). GSEA analysis showed that cytokine -cytokine receptor interaction, autophagy-animal, spliceosome, ubiquitin mediated proteolysis was enriched in high-NRS group. But, cytokine -cytokine receptor interaction was negatively correlated with high-NRS (Fig. 3C), suggesting complex interactions between these signaling pathways and DCM progression.
Gene regulatory networks identify transcription factors and miRNAs involved with signature genes
To delve deeper into the regulatory mechanisms of the identified NRDEGs, we predicted the interaction between transcription factors and genes. Circles in the figure represent transcription factors and squares represent genes. The color of the nodes reflects the degree of connectivity. Nodes with higher connectivity are considered to be important hubs of the network. We found CREB1 and RELA to be the major transcription factors (Fig. 4A). Meanwhile, we identified a network of miRNA-NRDEGs interactions, and we found has-mir-155-5p to be a major regulator of the signature genes (Fig. 4B), highlighting the intricate regulatory landscape influencing DCM.
The transcription factors and miRNAs involved with signature genes. (A) Transcription factor-necroptosis-related differentially expressed genes (NRDEGs) regulatory network in DCM. Red boxes represent NRDEGs and blue dots represent transcription factors. The colors of the border lines in this network represent correlations. (B) MiRNA-NRDEGs regulatory network in DCM. Red boxes represent NRDEGs, green boxes represent miRNAs. Green squares represent miRNAs. The colors of the edge lines in this network represent correlations.
Immune cell infiltration profiling in relation to NRS levels
To further understand the immune landscape associated with DCM, we calculated the immune cell abundance in myocardial tissue of DCM patients. First, we performed Pearson’s correlation coefficient analysis on different types of immune cells in myocardial tissue to assess the correlation between immune cells (Fig. 5A). Subsequently, we compared the proportions of different types of immune cells in the low-risk and high-risk groups. Among the 24 immune cells analyzed, 8 immune cells were significantly different between high and low groups. CD4 T cell, Th1, Th2, Th17, immune cells were enriched in low-score group. Whereas nTreg, iTreg and central -memory was noticeably enriched in the high-score group (Fig. 5A,B). Correlation analysis shows CD8 naïve T cell, CD8 T cell, Gamma-delta and exhaust of cells had statistically positive correlation with NRS (Fig. 5C). This correlation may reflect changes in the immune microenvironment in the myocardium of patients with DCM. Infiltration of CD8 T cells, which is usually associated with cytotoxic responses, may play a key role in the course of myocarditis and tissue damage. In contrast, Gamma-delta T cells also play an important role in regulating the immune response and participating in the inflammatory response. Increased depleted cells, on the other hand, may be associated with long-term inflammatory responses and immune escape mechanisms, which are common in chronic heart disease. The GLUL was predominantly favorably correlated with CD8 naïve T cell, CD8 T cell, central memory and Gamma-delta infiltration and CHMP5 was primarily positively correlated with CD8 naïve T cell and Gamma -delta cell infiltration, meanwhile, CHMP5 was negatively correlated with DC cells. HSP90AB1 was negatively correlated with Tfh cells (Fig. 5D, Supplementary table 1). These findings suggest that immune cell composition may play a significant role in the disease context.
Immune cell infiltration profiling in relation to NRS levels. (A) Heat map depicting the correlation between different immune cell compositions. (B) Violin plot showing the difference in myocardial immune infiltration scores between the high and low NRS groups. (C) Correlation analysis of immune cell infiltration with NRS. (D) Correlation analysis of immune cell infiltration with characteristic genes.
Identification of potential therapeutic agents for high-risk DCM patients
To identify potential therapeutic agents for patients at high risk for DCM, we predicted potential anti-DCM small molecule compounds by CMap analysis in order to find targeted drugs for high-risk patients. We found fenofibrate, procyclidine, and tienilic-acid to be Top3 ranked drugs with drug susceptibility scores greater than 99 (Fig. 6A). Fenofibrate, a peroxisome proliferators-activated receptor (PPAR) agonist, was associated with downregulation of inflammation and necrosis-related pathways. Procyclidine, an acetylcholine receptor antagonist, may act by reducing inflammation associated with myocardial hyperexcitability. Tienilic-acid, a sodium/potassium/chloride transport inhibitor, was linked to the modulation of cardiomyocyte energy metabolism. Research suggested that these small molecule complexes may interfere with metabolism and ion channel transport in dilated cardiomyopathy.
Identification of potential therapeutic agents for high-risk DCM patients and development of a nomogram for predicting disease progression and prognosis in DCM. (A) Prediction of potential anti-DCM small molecule compounds by CMap analysis. (B) Establishment of an integrated nomogram of signature genes for DCM prediction. In the nomogram, each variable corresponds to a score, and the total score can be calculated by adding the scores of all variables. (C) Calibration curves estimate the predictive accuracy of the nomogram. (D) Decision curve analysis shows the clinical benefit of the nomogram.
Development of a nomogram for predicting disease progression and prognosis in DCM
To create a clinically useful tool for assessing DCM progression and prognosis, we developed a nomogram incorporating both clinical features and NRS. In the constructed model, factors such as gender, age, coronary artery bypass grafting (CABG), inotropes, angiotensin converting enzyme inhibitors (ACEI), beta -block (BB), intra-aortic balloon counter pulsation (IABP), NRS are included, and each subvariable is quantified to a specific score, and then the cumulative scores for all variables are matched against the outcome scale to obtain predicted probabilities (Fig. 6B). We constructed decision analysis curves for assessing clinical usefulness. The farther the model curve is from the X and Y axes, the stronger its clinical practicability (Fig. 6C,D). The nomogram constructed in this study can be well applied to the prognosis evaluation of patients with heart failure.
Validation of the key genes in the heart tissues
To validate our findings, we verified the expression of these five genes in the myocardial tissue of patients with DCM. CAMK2B was expressed at a higher level in the DCM heart tissues than in the NF heart tissues. BID and GLUL were expressed at lower levels in the DCM heart tissues than in the NF heart tissues (P < 0.01), whereas HSP90AB1and CHMP5 showed no significant differences in expression level between the two groups (Fig. 7).
Animal experiments verify the therapeutic effect of fenofibrate on dilated cardiomyopathy
In order to verify the effect of fenofibrate on dilated cardiomyopathy, we established an autoimmune dilated cardiomyopathy model by injecting α-MHC peptide and treated mice with fenofibrate by gavage. Masson found that fenofibrate significantly reduced fibrosis in the hearts of DCM mice (Fig. 8A). Echocardiography showed that the use of fenofibrate restored EF and FS values and improved cardiac dilation in mice (Fig. 8B). We assessed the expression levels of myocardial necroptosis-related genes in DCM mice by RT-qPCR and found that the expression of Ripk1, Ripk3, and Mlkl was elevated in DCM mice and decreased after fenofibrate treatment (Fig. 8C). In addition, we assessed the levels of five NRDEGs and found that fenofibrate restored their changes in DCM (Fig. 8D). These findings suggest that fenofibrate may serve as a promising therapeutic agent for managing DCM.
The therapeutic effect of fenofibrate on dilated cardiomyopathy. (A) Representative images of Masson staining ventricular tissue section slices (scale bar = 200 μm) and percent values of left ventricular fibrosis (n = 7–8). (B) Representative images and mean values of echocardiographic analysis for left ventricular EF, FS, LV mass, LV Vol; d and LV Vol; s (n = 7–8). (C) The levels of Ripk1, Ripk3, and Mlkl mRNA expression in myocardium tissue from each group. (D) The levels of Bid, Chmk2b, Glul, Hsp90ab1, Chmp5 mRNA expression in myocardium tissue from each group.
Discussion
Heart failure spreads worldwide, links to poor outcomes, and remains one of the leading causes of death, reduced labor capacity, and poor quality of life18. The current classification of HF is still based on LVEF. The use of the LVEF classification as a tool for assessing disease progression and response to therapy is a common approach for selecting patients for clinical trials19. Recent studies have suggested that in addition to apoptosis, necroptosis, a pro-inflammatory form of cell death, play an important role in the pathogenesis of dilated cardiomyopathy though contributing to low self-renewal of postmitotic cardiomyocytes20. The discovery of molecular complexes such as caspase-8, receptor-interacting serine/threonine-protein kinase 1 and 3 represents a major advance in the study of core components of the necroptosis pathway6. However, the genes that control necroptosis in adult DCM patients remain elusive.
The results of our analysis indicated that the necroptosis pathway was significantly enriched and up-regulated in DCM samples. Five genes, including BID, CAMK2B, GLUL, HSP90AB1, CHMP5 were identified to construct the necroptosis-related progressive and prognostic signature. It was verified by PCR that among these necroptosis-related genes, BID, CAMK2B, GLUL have distinct differences between the myocardial tissue of the DCM patients and those of the NF control. Then, we focused on the influence of these genes on the therapeutic strategy and progression of DCM.
BH3-interacting domain death agonists (BIDs), as the intersection of apoptosis and necroptosis, play a key role in the cellular interactions between ferroptosis-induced ER stress responses and TRAIL-induced apoptosis by regulating mitochondrial outer membrane permeability21. Meanwhile, The BID inhibitor BI-6c9 mediates the protective effects of ferroptosis and mitochondrial damage, which are most commonly final execution steps in the oxidative cellular response paradigm22. Besides, Bid is an abundant pro-apoptotic protein of the Bcl-2 family that is crucial for death receptor-mediated apoptosis in many cell systems. A deficiency in pro-apoptotic protein BID leads to increased numbers of cells in the synovium through enhanced proliferation, lack of death, or increased migration of infiltrating leukocytes23. For our research, we speculated that BID may play a role in the intersection of different programmed cell death processes.
Previous studies have been consistent in their findings that inflammation is associated with cardiac remodeling and heart failure, but how it initiates myocardial inflammation in the absence of cell death in response to nonischemic intervention is unclear24. Ca2 + / calmodulin-dependent protein kinase II (CaMKII), an adrenergic activated kinase, may trigger mitochondrial dysfunction and induce necroptosis, which contributes to arrhythmias in models of heart disease, can activate inflammation in response to cardiac pressure overload25,26. The activated inflammatory response can provide signals for macrophage recruitment, fibrosis, and myocardial dysfunction in myocardial tissue, leading to adverse myocardial remodeling27. The Intervention of CAMKIIB signaling pathway which induces early inflammation may be a candidate therapeutic target in DCM.
The gene Glutamate-ammonia ligase (GLUL) encoding glutamine synthetase showed a relevant association on the formation of coronary atherosclerosis. GLUL mRNA was 2.2-fold upregulated in carotid plaques in patients with stroke compared with plaques in asymptomatic patients28,29. And recent studies have shown that the single nucleotide polymorphism rs10911021 at the GLUL locus is associated with an increased risk of CHD in patients with type 2 diabetes mellitus30. Additionally, studies have shown that GLUL promotes the metabolic process of immune cells by increasing the use of glutamine within cells, such as B cells, M1 and M2 macrophages, neutrophils, and dendritic cells31. The expression level of GLUL is positively correlated with the function of these immune cells, suggesting that it may play an important role in regulating the immune microenvironment and inflammatory response.
Despite the results suggests that GLUL is a promising target to intervene, more research is still needed in order to know the role of GLUL in DCM.
The identified GO category and enriched GSEA pathway fits well with the concept that cell homeostasis mediates cardiac hypertrophy and myocardial fibrosis, leading to myocardial remodeling, which is the pathological basis of the occurrence and development of heart failure. Autophagy and ubiquitin mediated proteolysis are significantly enriched in high-NRS group. Apoptosis and autophagy are two interacting forms of programmed cell death involved in the development and prognosis of coronary heart disease32. Apoptosis and autophagy have complex interactions and exhibit opposite roles in pathological processes such as myocardial ischemia, ischemia/reperfusion (I/R) injury, and post-ischemic cardiac remodeling33. It can provide a new concept for the treatment of heart disease by balancing the conversion of the two responses. Extensive studies have shown that the UPS system is an important regulatory mechanism mediating eukaryotic cell function and is closely related to cancer, neurodegeneration, metabolic disorders, cardiovascular disorders and inflammation34,35. Intervention on necroptosis-related genes have a protective effect against myocardial injury by preserving cardiac function during the resolution of inflammation, and by regulating wound healing and tissue remodeling after myocardial injury.
Inflammation of myocardial tissue induces the recruitment of innate immune cells, such as macrophages and T cell subsets, initiates tissue repair processes. During the maturation phase (chronic phase), Th1, Th2, Th17 and Tfh cells are exhausted, and the T-cell subpopulation is predominantly Treg cells, which play an important role in repairment and anti-inflammatory36. In the case of heart failure, the number of CD8 + T cells was found to increase significantly, and their migration and state transition were characterized, which may be closely related to the inflammatory and fibrotic processes of the heart. In the pathological process of heart failure, the CD8 + T population was activated, differentiated into CD8 + T EMRA (recently activated effector memory T cells), which exhibit high proliferative capacity and are enriched in the heart muscle and CD8 + TEX (depleted T) cells37, which are typically present in chronic infection or tumor settings and exhibit low cytotoxicity and proliferation capacity, possibly associated with immune escape mechanisms.
Tregs are known to regulate immune responses and suppress excessive inflammation. In the context of DCM, Tregs may protect cardiomyocytes by reducing inflammation and oxidative stress, both of which contribute to myocardial damage and fibrosis38. Tregs could also play a role in neovascularization and repair of the heart, promoting functional recovery after myocardial injury.
Fenofibrate, a PPAR agonist, is used to treat high cholesterol and high triglyceride levels. Heart failure is marked by a shift toward a fetal-like metabolism, where glucose metabolism dominates, and fatty acid oxidation is impaired due to dysfunctional mitochondria. Cardiomyocyte restricted PPARγ knockout mice develop a dilated cardiomyopathy that is felt to be a consequence of reduced superoxide dismutase 2. Past studies have found that activation of PPARγ reduces pro-inflammatory cytokines, apoptosis and necrosis in the myocardium and prevents septic myocardial dysfunction39. Besides, researchers demonstrated that short-term PPARα activation after pressure overload could maintain cardiac fatty acid oxidation, improve myocardial energetics, and partially prevent cardiac remodeling in heart failure40. These results suggest that early activation of PPAR could help preserve energy production in the failing heart and protect against some of the structural changes induced by pressure overload41.
There are no in-depth studies on the use of fenofibrate in dilated cardiomyopathy. Our animal experiments suggest that fenofibrate can inhibit the progression of inflammatory dilated cardiomyopathy, which may be related to the anti-inflammatory and anti-oxidative stress effects of fenofibrate. Therapeutic strategies aimed at intervention in necroptosis-related pathways, perhaps through modulation of immune cells or glucose metabolism, could open new avenues for treating dilated cardiomyopathy and improve heart function.
Human DCM is a heterogeneous condition with diverse underlying causes, including genetic mutations, viral infections, and metabolic disorders42. In contrast, our autoimmune model primarily focuses on immune-mediated mechanisms, which may overlook critical factors that contribute to DCM in humans. For instance, the interplay between genetic predispositions and environmental triggers is complex and is not fully captured in the current model. Moreover, differences in immune responses between species can lead to variations in disease progression and symptomatology. This disparity highlights the necessity for caution when extrapolating findings from our model to human conditions. To address these limitations, we emphasize the need for further studies that utilize a range of animal models, including genetic models and those induced by toxins. Additionally, integrating data from human clinical studies could enhance our understanding of necroptosis and its implications in various forms of heart disease.
Conclusion
In conclusion, we describe the characteristic genes associated with necroptosis that can be used as an effective noninvasive tool for prognostic assessment in patients with DCM, and identified pathways, immune cells and targeted drugs in high-risk patient groups. However, some important limitations remain in our study. The sample size of this study is insufficient, and the etiology of DCM is not strictly classified. A larger cohort is needed to verify the practicability and accuracy of the model in clinical practice.
Data availability
The dataset supporting the conclusions of this article are available in the GEO repository [GSE5406].
References
Reichart, D., Magnussen, C., Zeller, T. & Blankenberg, S. Dilated cardiomyopathy: from epidemiologic to genetic phenotypes: A translational review of current literature. J. Intern. Med. 286, 362–372. https://doi.org/10.1111/joim.12944 (2019).
Costa, M. C. et al. Circulating circRNA as biomarkers for dilated cardiomyopathy etiology. J. Mol. Med. (Berlin) 99, 1711–1725. https://doi.org/10.1007/s00109-021-02119-6 (2021).
Jordan, E. & Hershberger, R. E. Considering complexity in the genetic evaluation of dilated cardiomyopathy. Heart 107, 106–112. https://doi.org/10.1136/heartjnl-2020-316658 (2021).
Hershberger, R. E., Morales, A. & Siegfried, J. D. Clinical and genetic issues in dilated cardiomyopathy: a review for genetics professionals. Genet. Med. 12, 655–667. https://doi.org/10.1097/GIM.0b013e3181f2481f (2010).
Japp, A. G., Gulati, A., Cook, S. A., Cowie, M. R. & Prasad, S. K. The diagnosis and evaluation of dilated cardiomyopathy. J. Am. Coll. Cardiol. 67, 2996–3010. https://doi.org/10.1016/j.jacc.2016.03.590 (2016).
Yin, H. et al. TAB2 deficiency induces dilated cardiomyopathy by promoting RIPK1-dependent apoptosis and necroptosis. J. Clin. Invest. https://doi.org/10.1172/jci152297 (2022).
Liu, J. et al. TEAD1 protects against necroptosis in postmitotic cardiomyocytes through regulation of nuclear DNA-encoded mitochondrial genes. Cell Death Differ. 28, 2045–2059. https://doi.org/10.1038/s41418-020-00732-5 (2021).
Szobi, A. et al. Analysis of necroptotic proteins in failing human hearts. J. Transl. Med. 15, 86. https://doi.org/10.1186/s12967-017-1189-5 (2017).
Fujita, Y. et al. Enhanced nuclear localization of phosphorylated MLKL predicts adverse events in patients with dilated cardiomyopathy. ESC Heart Fail. 9, 3435–3451. https://doi.org/10.1002/ehf2.14059 (2022).
Khoury, M. K., Gupta, K., Franco, S. R. & Liu, B. Necroptosis in the pathophysiology of disease. Am. J. Pathol. 190, 272–285. https://doi.org/10.1016/j.ajpath.2019.10.012 (2020).
Hannenhalli, S. et al. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation 114, 1269–1276. https://doi.org/10.1161/circulationaha.106.632430 (2006).
Kanehisa, M., Sato, Y., Kawashima, M., Furumichi, M. & Tanabe, M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 44, D457-462. https://doi.org/10.1093/nar/gkv1070 (2016).
Robin, X. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 12, 77. https://doi.org/10.1186/1471-2105-12-77 (2011).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47. https://doi.org/10.1093/nar/gkv007 (2015).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504. https://doi.org/10.1101/gr.1239303 (2003).
Miao, Y. R. et al. ImmuCellAI: A unique method for comprehensive T-Cell subsets abundance prediction and its application in cancer immunotherapy. Adv. Sci. (Weinh) 7, 1902880. https://doi.org/10.1002/advs.201902880 (2020).
Musa, A. et al. A review of connectivity map and computational approaches in pharmacogenomics. Brief. Bioinform. 18, 903. https://doi.org/10.1093/bib/bbx023 (2017).
Halliday, B. P., Cleland, J. G. F., Goldberger, J. J. & Prasad, S. K. Personalizing risk stratification for sudden death in dilated cardiomyopathy: The past, present, and future. Circulation 136, 215–231. https://doi.org/10.1161/circulationaha.116.027134 (2017).
Pezawas, T. et al. Multiple autonomic and repolarization investigation of sudden cardiac death in dilated cardiomyopathy and controls. Circ. Arrhythm. Electrophysiol. 7, 1101–1108. https://doi.org/10.1161/circep.114.001745 (2014).
Bauer, T. M. & Murphy, E. Role of mitochondrial calcium and the permeability transition pore in regulating cell death. Circ. Res. 126, 280–293. https://doi.org/10.1161/circresaha.119.316306 (2020).
D’Orsi, B., Niewidok, N., Düssmann, H. & Prehn, J. H. M. Mitochondrial carrier homolog 2 functionally Co-operates with BH3 interacting-domain death agonist in promoting Ca (2+)-induced neuronal injury. Front. Cell Dev. Biol. 9, 750100. https://doi.org/10.3389/fcell.2021.750100 (2021).
Neitemeier, S. et al. BID links ferroptosis to mitochondrial cell death pathways. Redox Biol. 12, 558–570. https://doi.org/10.1016/j.redox.2017.03.007 (2017).
Scatizzi, J. C., Hutcheson, J., Bickel, E., Haines, G. K. 3rd. & Perlman, H. Pro-apoptotic Bid is required for the resolution of the effector phase of inflammatory arthritis. Arthritis Res. Ther. 9, R49. https://doi.org/10.1186/ar2204 (2007).
Luczak, E. D. et al. Mitochondrial CaMKII causes adverse metabolic reprogramming and dilated cardiomyopathy. Nat. Commun. 11, 4416. https://doi.org/10.1038/s41467-020-18165-6 (2020).
Beckendorf, J., van den Hoogenhof, M. M. G. & Backs, J. Physiological and unappreciated roles of CaMKII in the heart. Basic Res. Cardiol. 113, 29. https://doi.org/10.1007/s00395-018-0688-8 (2018).
Hegyi, B., Bers, D. M. & Bossuyt, J. CaMKII signaling in heart diseases: Emerging role in diabetic cardiomyopathy. J. Mol. Cell. Cardiol. 127, 246–259. https://doi.org/10.1016/j.yjmcc.2019.01.001 (2019).
Mustroph, J., Neef, S. & Maier, L. S. CaMKII as a target for arrhythmia suppression. Pharmacol. Ther. 176, 22–31. https://doi.org/10.1016/j.pharmthera.2016.10.006 (2017).
Eelen, G. et al. Role of glutamine synthetase in angiogenesis beyond glutamine synthesis. Nature 561, 63–69. https://doi.org/10.1038/s41586-018-0466-7 (2018).
Sorto, P. et al. Glutamine synthetase in human carotid plaque macrophages associates with features of plaque vulnerability: An immunohistological study. Atherosclerosis 352, 18–26. https://doi.org/10.1016/j.atherosclerosis.2022.05.008 (2022).
Prudente, S. et al. Genetic variant at the GLUL locus predicts all-cause mortality in patients with type 2 diabetes. Diabetes 64, 2658–2663. https://doi.org/10.2337/db14-1653 (2015).
Xuan, D. T. M. et al. Glutamine synthetase regulates the immune microenvironment and cancer development through the inflammatory pathway. Int. J. Med. Sci. 20, 35–49. https://doi.org/10.7150/ijms.75625 (2023).
Dong, Y. et al. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell. Cardiol. 136, 27–41. https://doi.org/10.1016/j.yjmcc.2019.09.001 (2019).
Chien, S. C. et al. Association of low serum albumin concentration and adverse cardiovascular events in stable coronary heart disease. Int. J. Cardiol. 241, 1–5. https://doi.org/10.1016/j.ijcard.2017.04.003 (2017).
Cao, C. & Xue, C. More than just cleaning: Ubiquitin-mediated proteolysis in fungal pathogenesis. Front. Cell Infect. Microbiol. 11, 774613. https://doi.org/10.3389/fcimb.2021.774613 (2021).
Zheng, N. & Shabek, N. Ubiquitin ligases: Structure, function, and regulation. Annu. Rev. Biochem. 86, 129–157. https://doi.org/10.1146/annurev-biochem-060815-014922 (2017).
Bacmeister, L. et al. Inflammation and fibrosis in murine models of heart failure. Basic Res. Cardiol. 114, 19. https://doi.org/10.1007/s00395-019-0722-5 (2019).
Rao, M. et al. Resolving the intertwining of inflammation and fibrosis in human heart failure at single-cell level. Basic Res. Cardiol. 116, 55. https://doi.org/10.1007/s00395-021-00897-1 (2021).
Rai, A. et al. Adaptive immune disorders in hypertension and heart failure: focusing on T-cell subset activation and clinical implications. J. Hypertens. 38, 1878–1889. https://doi.org/10.1097/hjh.0000000000002456 (2020).
Peng, S., Xu, J., Ruan, W., Li, S. & Xiao, F. PPAR-γ activation prevents septic cardiac dysfunction via inhibition of apoptosis and necroptosis. Oxid. Med. Cell. Longev. 2017, 8326749. https://doi.org/10.1155/2017/8326749 (2017).
Montaigne, D., Butruille, L. & Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 18, 809–823. https://doi.org/10.1038/s41569-021-00569-6 (2021).
Wang, S., Dougherty, E. J. & Danner, R. L. PPARγ signaling and emerging opportunities for improved therapeutics. Pharmacol. Res. 111, 76–85. https://doi.org/10.1016/j.phrs.2016.02.028 (2016).
Heymans, S., Lakdawala, N. K., Tschöpe, C. & Klingel, K. Dilated cardiomyopathy: causes, mechanisms, and current and future treatment approaches. Lancet 402, 998–1011. https://doi.org/10.1016/s0140-6736(23)01241-2 (2023).
Acknowledgements
We thank all of the patients in this study for their cooperation. This study was supported by the National Natural Science Foundation of China (82300395, 82170326).
Funding
The National Natural Science Foundation of China, 82300395, 82170326.
Author information
Authors and Affiliations
Contributions
Y.L. and H.Y. designed the study and performed the experiments; Z.W. and Y.X. collected and analyzed the data; Y.D. and H.Y. reviewed and edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, H., Wang, Z., Xu, Y. et al. Prognostic signature and therapeutic drug identification for dilated cardiomyopathy based on necroptosis via bioinformatics and experimental validation. Sci Rep 15, 319 (2025). https://doi.org/10.1038/s41598-024-83455-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83455-8