Abstract
Actinobacteria are well-known producers of diverse secondary metabolites by the presence of biosynthetic gene clusters (BGCs). Biological control of banana pathogens using antagonistic actinomycetes is recently considered a promising strategy. Therefore, this study aimed to assess the plant growth-promoting activities and the antagonistic potential of the newly identified Streptomyces sp. VNUA74 strain that isolated from banana rhizosphere in Hung Yen province, Vietnam. The morphological, biochemical and physiological characteristics together with the whole genome and 16S rRNA based taxonomic analyses confirmed that VNUA74 strain belongs to Streptomyces parvulus. In silico genome mining revealed that S. parvulus VNUA74 contains rich source of potential BGCs for secondary metabolites involved in antagonistic activities. Notably, eleven BGCs showed 100% similarity in gene contents with the known clusters possessing antibacterial and antifungal activities such as actimomycin D, germicidin, istamycins, albaflavenone, and cyclic Lanthipeptide SapB. The functional genome analysis also revealed genes participated in plant growth-promoting. Furthermore, in vitro biochemical assays indicated that S. parvulus VNUA74 exhibited strong antagonistic activities against a range of important phytopathogens on banana, including Fusarium oxysporum f. sp. cubense Tropical race 4, F. solani, F. oxysporum, Colletotrichum gloeosporioides, Corynespora cassiicola, Xanthomonas axonopodis, Ralstonia solanacearum and Clavibacter michiganensis. Finally, the VNUA74 strain showed notable enhancements of all examined growth traits of banana plantlets in the pot experiment. In summary, the results showed that the S. parvulus VNUA74 strain possesses multiple characteristics of being the effective biocontrol and biofertilizer agents for the sustainable production of banana and other agricultural crops. In further, the genomic approaches will provide an opportunity to discover novel bioactive compounds as well as manipulating novel gene clusters from S. parvulus VNUA74 strain.
Similar content being viewed by others
Introduction
Banana (Musa spp.) is one of the world’s most important fruit crops, including Vietnam in terms of production and trade volume. In 2022, Vietnamese bananas exports reached 310 million USD1. Currently, the total banana growing area in Vietnam is more than 200,000 hectares, of which the banana growing area on a farm scale accounts for about 150,000 hectares2. For example, in Dong Nai Province, the banana cultivation area was only 7,300 hectares in 2016; however, by the end of 2023, it had doubled to reach 14,000 hectares3 (Fig. 1).
Bacterial, fungal and viral infectious diseases have been recognized as the primary causes of quality and yield losses of up to 47% in global banana production industry4. The major fungal and bacterial pathogens that have received significant attention are Fusarium oxysporum f. sp. cubense (Foc) causing Fusarium wilt (Panama disease), Ralstonia solanacearum causing Moko/Bugtok disease, Xanthomonas spp. causing Xanthomonas, and Colletotrichum spp. causing anthracnose of banana fruit5,6. Among these, Foc is one of the most serious pathogens for banana worldwide, having at least 24 distinct vegetative compatibility groups7. It is estimated that Foc Tropical race 4 (Foc TR4, the main fungal pathogen in Southeast Asia, Western Asia, Africa and South America) can affect 1.7 million hectares of banana by 2040 if it is not mitigated8,9.
The increase of both fungal and bacterial diseases in banana crop is steadily rising in many regions of the world, including Vietnam. These diseases not only decrease the yield but also result in higher expense for crop management. Numerous agricultural and biotechnological solutions have been developed to combat banana diseases; however, these solutions remain costly, environmentally unfavorable and time consuming to implement9,10. In recent years, antagonistic microorganisms have been acknowledged as highly effective agents due to their lower cost and their contribution to sustainable agriculture for the biological control of various phytopathogens11. In microorganisms, the species of Trichoderma, Bacillus and Pseudomonas have been proved and utilized as successful biological control agents for managing banana diseases12,13,14,15. Noteworthy, actinomycetes are ubiquitous microbes having various applications in sustainable agriculture16. In particular, actinomycetes are used as biological control agents because they are not harmful to human and animal health. Furthermore, they can help to improve plant yield as well as decrease the use of synthetic fungicides17,18. Additionally, actinomycetes have the ability to atmospheric nitrogen fixation, plant growth-promoting activity through the synthesis of phytohormones, production of various enzymes, breakdown of organic matters, bioremediation of different pollutants10,16.
Actinomycetes are well known to produce secondary metabolites with antifungal properties, which is one of the main antagonistic mechanisms of actinomycetes to control phytopathogenic fungi10. Among the different genera, Streptomyces which is the predominant genus of actinomycetes and consists of ca. 700 scientifically documented species has been recognized as one of the most important sources of antibiotic production19. Accumulating evidence indicates that polyketides, nonribosomal peptides and their hybrid compounds are the major secondary metabolites of Streptomyces, possessing a broad spectrum of antibiotic activities. Of particular interest are nonribosomal peptides (NRPs) such as actinomycin D and actinomycin X2, which have garnered significant attention for their potent and broad-spectrum antibacterial activities20,21. The gene clusters responsible for the biosynthesis of polypeptide antibiotics in Streptomyces such as polyketide synthases (PKSs) and nonribosomal peptide synthetases (NRPSs) have been well characterized22,23,24. Furthermore, a few studies have reported the antagonistic effects of Streptomyces in controlling banana diseases. However, most of the studies mainly focus on the antifungal activities specifically targeting Foc TR425,26,27,28,29. There is little information about the antibacterial and plant growth-promoting activities of Streptomyces on banana production.
Recently, whole genome sequencing and genome mining have emerged as an effective approach to discover novel and potentially secondary metabolites in Streptomyces30. An analysis of 1,110 publicly available Streptomyces genomes revealed high diversity of BGCs including nonribosomal peptide synthetases (NRPSs), type I polyketide synthases (PKS), terpenes and lantipeptides, even among very closely related strains. This observation suggests that different strains of the same species may vary tremendously in the BGCs they carry31. In our previous study, we successfully sequenced the entire circular chromosome of Streptomyces sp. strain VNUA74, however, the presence of BGCs that engaged in the antagonistic and plant growth promoting activities remains uncharacterized32.
The objectives of the present study were first to screen the antifungal activity of the isolated Streptomyces strains found in the rhizosphere of banana plants. The physiological and biochemical profiles of the selected VNUA74 strain were then characterized. Through in silico genome mining, the presence of BGCs responsible for promoting plant growth and exhibiting antagonistic activities in the VNUA74 strain were revealed. Subsequently, in vitro experiments were conducted to assess the abilities of the VNUA74 strain in promoting the growth of banana plantlets and its ability to counteract the prevalent phytopathogens. The study design was shown in Figure 1. Based on this extensive study, we propose utilizing the selected Streptomyces sp. VNUA74 as a promising biocontrol and plant growth-promoting agent for the global production of banana and other agricultural crops.
Results
Strain isolation and morphological observation
In this study, 222 Streptomyces isolates were isolated from 19 rhizosphere soil samples of banana root in six different locations in Vietnam (Table S1). The agar diffusion assay indicated that 17 isolates exhibited antifungal activity against C. gloeosporioides. Out of all the strains, the VNUA74 strain collected from Hung Yen province was selected for further analyses due to its strongest antifungal activity. The morphological characteristics of the VNUA74 strain grown in Gause’s No.1 medium were shown in Fig. S1a.
Morphologically, the VNUA74 colonies appeared as filamentous form with greyish white aerial mycelium on all ISP (International Streptomyces Project) media. In most cases, the colonies produced diffusible yellow or yellowish-brown pigments. No melanin was detected in the ISP-6 medium (Fig. S2f). The SEM observation revealed septation and disarticulation of the aerial mycelia. The spore chains are very characteristic simple spira with containing 10–50 arthrospores. Each arthrospores was approximately 0.7–1.2 μm size (width x length) (Fig. S1b-d). Notably, arthrospores characteristic of Streptomyces33, the physiological and biochemical profiles of the VNUA74 strain were shown in Table 1 and Figure S3, and S4. Therefore, based on the morphological characteristics, it was concluded that strain VNUA74 is Streptomyces sp.
Strain identification and taxonomic analyses
We specifically amplified and sequenced the 16 S rRNA fragment of the VNUA74 strain. The BLAST result showed that the 16 S rRNA sequence of the VNUA74 strain exhibited 100% similarity with that of S. parvulus NBRC 13,193 (NR_041119). The 16 S rRNA sequence of S. parvulus VNUA74 was then deposited in GenBank under the accession number PP033922. The phylogenetic tree based on 16 S rRNA sequences revealed that all examined S. parvulus strains formed a single clade at species level with strong bootstrap support of 92% (Fig. 2).
The phylogenetic dendrogram obtained by neighbour-joining analysis based on 16 S rRNA sequences of the VNUA74 strain and other Streptomyces type strains. Numbers at the branches represent bootstrap percentages. The newly isolated strain in this study is shown in bold. Streptomyces ambofaciens ATCC 23,877 is used as an out group.
To verify the result of species identification and taxonomic classification, we utilized the complete genome sequence for the species demarcation of the strain. The genome of the VNUA74 strain was previously resolved at the chromosomal level by PacBio and DNBSEQ sequencing which produced a complete circular chromosome of 7,250,076 bp32. By Mash/MinHash based searching provided by BV-BRC server, we identified that the VNUA74 genome was highly similar to various S. parvulus strains with available whole genome data including S. parvulus 2297, JCM 4068, LP03, SX6, VCCM 22,513, JCM 4068 and BPPL-273 (Table S2). The genomes of those strains and the VNUA74 strain were submitted to TYGS typing server. The digital DNA-DNA hybridization (dDDH) values were used to measure the genome distance and reconstruct the phylogenetic position of the examined genomes. The phylogenetic data resolved by TYGS clearly showed that the VNUA74 genome clustered with its similar S. parvulus genomes to form a single clade at both species and subspecies levels (Fig. 3a). Out of the examined S. parvulus strains, the VNUA74 strain showed closest relationship to the S. parvulus strain LP03 and strain BPPL-273 (dDDH of 93.9% and 94.9%, orthoANI of 99.31% and 99.42%, respectively) (Fig. 3b, Table S2). Meanwhile, the other two strains including SX6 and VCCM 22,513 from Vietnam clustered with the 2297 strain, forming a sister subclade (Fig. 3a). The in silico G + C difference and ANI values also strongly supported the TYGS results. Among the similar genomes, the VNUA74 genome displayed the highest similarity to the BPPL-273 genome with the ANI, dDDH, and G + C difference values of 99.42%, 94.9%, and 0.05, respectively. Both indices were within the threshold for species identification (95–96 and 70% for ANI and dDDH, respectively)35,36.
Species demarcation of the VNUA74 strain using whole genome data. (a) The dendogram illustrating the taxonomic relationship between the VNUA74 strain (on top) and other Streptomyces type strains, obtained by the TYGS pipeline analysis. The tree was inferred by FastME2 from GDBP distances (d5) calculated from genome sequence34. The branches were numbered based on GBDP pseudo-bootstrap with support value > 60% from 100 replications. Mean branch support was 83.7% based on bootstrap data for all branches. The tree was rooted at the midpoint. (b) The phylogenetic position of the VNUA74 strain and its similar S. parvulus strains based on orthoANI algorithm. Streptomyces ambofaciens ATCC 23,877 was used as an outgroup. (c) The circular map of Streptomyces sp. VNUA74 with annotation done by PATRIC pipeline and viewed on the BV-BRC website.
The UPMA tree, built on the orthoANI value, also yielded consistent taxonomic position for the VNUA74 strain and its similar genomes (Fig. 3b). The assembled genome was annotated using various annotation tools including Prokka, Bakta, and RASTtk for exploring gene content and genome function (data not shown). The annotated results delivered consistent results with 6675 coding sequences, 66 tRNAs, and 18 rRNA genes. The genome also contains numerous putative genes (56 from PATRIC, seven from CARD, and 4 from NDARO) responsible for antimicrobial resistance capability (Fig. 3c).
By combining whole genome and 16 S rRNA sequence analyses with morphological and biochemical observations, we introduced the isolated actinomycete strain as Streptomyces parvulus VNUA74.
Genome mining of biosynthetic gene clusters in the VNUA74 strain
The AntiSMASH v7.1 tool (bacterial version) was used to detect BGCs for secondary metabolites, including non-ribosomal peptide synthetases (NRPSs), polyketide synthases (PKSs), ribosomally synthesized and post-translationally modified peptides (RiPPs), and other antimicrobial synthases. The antiSMASH analysis showed that the VNUA74 strain exhibited 21 potential BGCs, comprising of 5 terpenes, 2 NRPS types, 1 Type II PKS (T2PKS), 1 Type III PKS (T3PKS), 2 RiPP types, 3 siderophores, lanthipeptide-Class-III, and other well-known BGCs such as melanin, indole, ectonine, and others (Table 2). Out of these BGCs, eleven (52%) could be identified to contain 100% of the genes from the known clusters (Table 2, Fig. S5). The majority of these clusters are commonly observed in other S. parvulus strains (LP03 and BPPL-273) and other Streptomyces strains. The antiSMASH result indicated that the similar S. parvulus genome data with multiple contigs contained more BGCs than the genomes with single contigs (Table S3). However, the multiple contig genomes had fewer BGCs with high similarity levels to known clusters compared to the circular chromosomal genomes. For example, the VNUA74 strain contained 11 clusters showed 100% similarity to the known clusters which were significant higher than 6 clusters out of 29 and 10 clusters out of 35 in fragmented genomes of the SX6 and VCCM 22,513 strains, respectively (Table S3). This emphasized the importance of fully sequenced bacterial genomes in order to effectively and confidentially identify and analyze BGCs through mining genomic data.
The predicted BGC for actinomycin D in the VNUA74 strain, identified by antiSMASH and ARST showed 82% identical to the known actinomycin D gene cluster of Streptomyces anulatus (BGC000296) (Fig. S5). We analyzed the genomes of 6 different S. parvulus strains that are available on GenBank including 2297, SX6, VCCM 22,513, JCM 4068, LP03 and BPPL-273. All the genomes of S. parvulus strains harbored BGC of actinomycin D; however, the similarity level of the BGC of actinomycin D to its counterpart in MiBIG database varied among the examined S. parvulus strains. In addition to the cluster for actinomycin D, the VNUA74 genome also included clusters for the biosynthesis of other antibiotic compounds such as curamicin, germicidin, istamycins, albaflavenone, and cyclic lanthipeptide SapB which showed 100% similarity to the known clusters (Table 2).
Moreover, the KEGG assignment revealed several other genes coding for antimicrobial synthases including dTDP-glucose 4,6-dehydratase, 4-hydroxymandelate synthase, 4-hydroxymandelate oxidase, (S)-3,5-dihydroxyphenylglycine transaminase in vancomycin group; enediyne antibiotics: 2-hydroxy-5-methyl-1-naphthoate 7-hydroxylase, NDP-hexose 4-ketoreductase in enediyne antibiotic; and penicillin G amidase, cephalosporin-C deacetylase in beta-lactamase class A (Table 2). Together, genome data suggested that VNUA74 strain possessed a potential capacity to express antagonistic effects against vairous pathogenic agents.
Genes contributing to plant growth-promoting traits
The RASTtk with SEED database analysis of the VNUA74 genome identified target genes across 23 subsystem categories (Fig. 4). The annotation revealed multiple genes associated with various well-known pathways that promote plant growth, including nitrogen metabolism, phosphate solubilization, citrate utilization, urea utilization, and various types of hydrolytic enzymes. In addition, S. parvulus VNUA74 genome contains genes involved in homeostasis of metal ions, including multiple siderophore BGCs such as desferrioxamine B/desferrioxamine E, paulomycin, kinamycin and coelichelin. These siderophores can chelate metal ions, forming soluble complexes that can be easily absorbed by plant roots, thereby promoting plant growth (Fig. 4, Table S4).
Cluster of Orthologous Groups of protein (KOG) function classification in the VNUA74 strain. Subsystem category annotated by RAST server and displayed in SEED server. In subsystem coverage, green bar represented percentage of annotated gene (20%) in the Subsystem. Pie chart in the middle represented the distribution of subsystem category which was given as gene counting in the right-side listing. The numbers in parentheses showed the counts of genes with specific functions.
Furthermore, the VNUA74 genome contains various types of enzymes responsible for the IAA production. Among them the presence of the two tryptophan synthase chains in the VNUA74 strain suggests that the IAA biosynthesis in this strain is likely tryptophan-dependent pathway. However, the identity of other essential elements for this IAA biosynthesis pathway remains to be characterized in our strain as the genome mining results did not reveal other components for IAA metabolism such as indole-3-pyruvate decarboxylase, tryptophan aminotransferase, acetaldehyde oxidase/dehydrogenase (IpyA pathway), IAM pathway (tryptophan 2 monooxygenase, IaaM, IAM hydrolase), and IAN pathway (Nitrilase, tryptophan decarboxylase) (Table S4).
Genes associated with fungal cell wall and other carbohydrate degrading enzymes
Streptomyces bacteria are renowned for their extensive genetic repertoire and diverse array of glycoside hydrolase enzymes. Consequently, Streptomyces plays a crucial role not only in producing a variety of secondary metabolites but also in degrading plant-derived and fungal carbohydrates. Possessing enzymes capable of breaking down fungal cell wall components like chitin, Streptomyces serves as a valuable tool in combating fungal pathogens that affect crops37,38.
Genomic analysis with dbCAN database revealed that the VNUA74 strain exhibited a wide range of carbohydrate metabolism pathways, with a total of 272 genes encoding carbohydrade active enzymes (CAZymes). This included 12 auxiliary activities (AA), 43 carbohydrate-binding modules (CBM), 26 carbohydrate esterases (CE), 130 glycoside hydrolases (GH), 54 glycosyltransferases (GT), and 7 polysaccharide lyases (PL) (Fig. 4, Table S4). Notably, the strain genome contains 14 genes encoding enzymes involved in chitin degradation, including chitinase (09 genes), secreted chitinase (02 genes), chitodextrinase (01 gene), and glucanase glgE (02 genes). Furthermore, the VNUA74 strain confers potentials to degrade various biomass components, as evidenced by the presence of genes encoding enzymes like amylases (07 genes), pectinases (04 genes), and xylanases (03 genes) (Table S4). These CAZymes coding genes were likely attributed to VNUA74’s catalytic activities targeting various carbohydrate substances such as xylan, chitin, cellulose and amylopectin (Table 1).
Effect of S. parvulus VNUA74 on the growth of banana plantlets
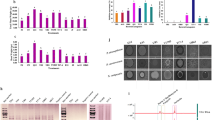
A pot experiment was conducted to test whether the VNUA74 strain can enhance the growth of banana plantlets. As shown in Fig. 5; Table 3, at 90 days post-inoculation with the VNUA74 strain, there were significant enhancements in all growth traits of banana plantlets in the treatment pots compared to the control pots. Among them, root fresh weight showed the highest level of enhancement of 47.6%.
Antibacterial and antifungal activities
The result of agar diffusion assay revealed that S. parvulus VNUA74 could produce secondary metabolites which were able to inhibit the growth of all examined pathogenic bacteria (Fig. S6). Particularly, the zone of inhibition (ZOI) of the VNUA74 strain against X. axonopodis, R. solanacearum and C. michiganensis were 9.85 ± 3.22 (mm), 10.25 ± 1.27 (mm) and 14.63 ± 4.48 (mm), respectively.
In this study, we assess whether S. parvulus VNUA74 has a broad-spectrum antifungal activity by selecting five common phytopathogenic fungi for testing. As shown in Fig. 6, S. parvulus VNUA74 exhibited significant inhibitory activity against the mycelial growth of all tested fungal pathogens in the dual culture experiments, with inhibition percentages ranging from 51.82 to 82.07%. The highest inhibition was observed against C. cassiicola (82.07%), while the lowest was recorded against Foc TR4 (51.82%). In addition, S. parvulus VNUA74 significantly inhibited the mycelial growth of other phytopathogenic fungi, including F. solani (66.67%), F. oxysporum (55.56%) and C. gloeosporioides (66.25%).
Antifungal activities of S. parvulus VNUA74 against phytopathogenic fungi. Five pathogenic fungi were used in the dual culture test including Fusarium oxysporum f. sp. cubense Tropical race 4, Fusarium solani, Fusarium oxysporum, Colletotrichum gloeosporioides and Corynespora cassiicola. Control plate consists solely of pathogenic fungus. Numbers are mean values and standard deviation based on n = 3, independent observations.
Moreover, the secondary metabolites in the culture filtrate of S. parvulus VNUA74 effectively inhibited the mycelial growth and the spore germination of C. gloeosporioides as shown in Fig. 7. The results revealed that the mycelia of C. gloeosporioides appeared distorted after incubating with culture filtrate of the VNUA74 strain. Specifically, in culture filtrates the hyphal morphology swelling and frequent septa shrank (Fig. 7d). On the contrary, structure of the hyphae where observed in the control treatment was normal (Fig. 7a). In addition, the conidia in samples treated with the culture filtrate of the VNUA74 strain was observed a large percentage of the conidia either did not germinate or developed swollen germ tubes with a slow growth rate (Fig. 7f). Only 13.51% (± 1.24) spores were recorded as germinated in the treatment group (Fig. 7e), compared to 98.48% (± 0.58) germination observed in the control group (Fig. 7b). Therefore, the percentage of spore germination inhibition (PSGI) in this experiment was 86.28%.
Effects of the culture filtrate of S. parvulus VNUA74 on morphological characteristics of C. gloeosporioides after 9 h of treatment. The morphology of mycelia and spore germination in the control (a-c) and in the treatment (d-f); (g) Percentage of spore germination in the control and treatment with culture filtrate of the VNUA74 strain. The mean values and standard deviation were calculated from n = 3, independent experiments. Arrows indicate the distortion of mycelium.
Discussion
Biological management of soil ecosystems by actinomycetes through their diverse biological characteristics greatly contributes to the development of sustainable agriculture39. For effective biological control, it is important to screen broad-spectrum and novel antagonistic actinomycete strains as biocontrol agents, and incorporate their other impressive features40. In the current study, we aimed to isolate Streptomyces strains with strong antagonistic and plant growth-promoting activities for banana plantations in Vietnam. Our finding showed that the isolated strain VNUA74 showed the most antifungal activity against C. gloeosporioides and other important phytopathogenic fungi (Fig. 6). GenBank database of 16 S rRNA sequences is available for the type strains of prokaryotic species, allowing rapid identification of a strain at the genus or higher taxonomic level. In our present study, the BLAST result showed that of the strain VNUA74 exhibited the highest similarity to S. parvulus NBRC 13,193 (NR_041119) (100%). Simultaneously, the 16 S rRNA phylogenetic analysis revealed all examined S. parvulus strains formed a single clade at species level with strong bootstrap support of 92%. Consequently, the phylogeny of the 16 S rRNA gene was able to differentiate strain VNUA 74 from its closely related species and the VNUA74 strain showed closest relationship to the S. parvulus. However, identification to species based on phylogenetic trees of the 16 S rRNA gene is unreliable for species in the genus Streptomyces because of the high level of sequence conservation28,41,42. With the advances in next generation sequence, the whole bacterial genome can be pratically achieved and provides a more precise taxonomic analysis of bacterial strains43. In the overall genomic relatedness indices (OGRIs), including average nucleotide identity (ANI) and digital DNA–DNA hybridization (dDDH) are two commonly used indices that are usually applied to define genomic species. Both indices have proposed thresholds that can be used for species definition are around 95–96% and 70%, respectively35,36. Both values were within the respective threshold values. Therefore, we introduced the newly isolated actinomycete strain as Streptomyces parvulus VNUA74.
The biochemical analysis revealed that the VNUA74 strain can produce various types of extracellular enzymes with xylanase, chitinase, cellulase and pectinase exhibiting particularly strong activities. These results aligned with previous findings that xylanase, chitinase, and cellulase are among the major extracellular enzymes produced by Streptomyces spp44. Additionally, the genome analysis of the VNUA74 strain further supports our in vitro results, revealing a diverse repertoire of carbohydrate-active enzymes. These enzymes have numerous applications in industrial and agricultural practices45. Chitin is the major structural components of fungal cell walls. Chitinases produced by biocontrol microbes and plants can inhibit fungal growth46,47. The study conducted by Qi et al.28 showed that the chitinase and β-1,3-glucanase produced by Streptomyces luomodiensis sp. nov (SCA4-21T) can inhibit the growth of Foc TR4 banana wilt disease. Interestingly, in strain SCA4-21T genome contained 14 chitinase genes, which various chitinases can lyse fungal cell wall, therefore potentially contributing to the antifungal capabilities of Streptomyces spp44,48,49.
The antibacterial assays showed that both tested Gram-positive (C. michiganensis) and Gram-negative (X. axonopodis, R. solancearum) phytopathogenic bacteria were particularly inhibited by the VNUA74 strain which infers that this newly isolated strain can produce broad-spectrum antibiotics. Among the tested bacteria, R. solancearum and X. axonopodis are the world’s most important phytopathogens due to their lethality, broad geographic distribution and wide host range, including banana5. In several countries, R. solacearum is considered a major threat to banana, leading to yield losses up to 100% (4). Regarding antifungal activity, S. parvulus VNUA74 exhibited strongly inhibitory effects against all common phytopathogenic fungi, including some of the most dangerous banana pathogens, such as Foc TR4, F. oxysporum and C. gloeosporioides. In addition, the secondary metabolile BCGs identified in the VNUA74 genome could be reponsible for the significant inhibition of spore germination of C. gloeosporioides. The fungus C. gloeosporioides causing anthracnose has been reported as one of the most important pathogens worldwide, which can infect more than 1000 plant species. C. gloeosporioides mainly causes anthracnose on fruits such as bananas, mangoes, avocados, peppers, and strawberries causing serious impacts on production, marketing and export50. Therefore, S. parvulus VNUA74 hold promise as a potential biocontrol agent not only against serious pathogens of banana but also against important pathogens of many agricultural crops worldwide.
Many studies have revealed that S. parvulus is the important source of various antimicrobial secondary metabolites such as actinomycin, streopovaricin, polyoxypeptin and others20,51,52,53. Among these, actinomycin D, actinomycin X2 and actinomycin X0β are the main secondary metabolites exhibiting the broad-spectrum antibiotic activities against both bacterial and fungal pathogens54,55. The actinomycins possess a wide range of biological activities by attaching to DNA and inhibiting transcription, thereby suppressing the growth and activities of all the disease-causing microbials56. The production and resistance mechanisms of actinomycin D are well understood57,58. In silico mining of the VNUA74 genome also showed the presence of NRPS cluster responsible for the biosynthesis of actinomycin D. However, only 82% of genes in this cluster showed identical to the known actinomycin D gene cluster of S. anulatus. In addition, it is possible that the specific conditions employed in this study did not activate some of the known BGCs within the VNUA74 genome. Furthermore, the strain might contain antimicrobial compounds whose BGCs cannot be identified by antiSMASH.
Recent studies on genome mining of Streptomyces have focused mostly on the discovering antibiotic BGCs30,31,59. This study, however, not only reveals the presence of common antibiotic BGCs but also highlights the existence of numerous genes involved in various well-known pathways that promote plant growth, including nitrogen metabolism, phosphate solubilization, siderophore production, IAA production, and various extracellular enzymes. The presence of genes encoding plant growth-promoting traits coupled with the biochemical profile of S. parvulus VNUA74 strongly supports the enhancement effects of this actinomycete strain on banana plantlets in this study. Among the enhanced traits, root fresh weight showed the most significant improvement. Generally, as root weight increases, all other growth traits also improve, highlighting the importance of well-developed root system for healthy plant growth and development. The IAA produced by bacteria can promote the development of the plant shoot and root system60. Thus, the high level of IAA production ability of S. parvulus VNUA74 may explain for the marked improvement in the root and shoot of inoculated banana plantlets. While several strains of S. parvulus have been reported to produce IAA, they typically do so at lower levels48,52. Interestingly, siderophores secreted by Streptomyces spp. can chelate metal ions, forming soluble complexes that can be easily absorbed by plant roots, and at the same time inhibit the phytopathogens that rely on iron for their growth61.
In summary, we provide novel insight into the antagonistic activity and the plant growth-promoting properties of the newly isolated Streptomyces parvulus strain VNUA74 based on both in silico and in vitro methods. Our study underscores the potential of this actinomycete strain as a biocontrol agent and a biofertilizer for the sustainable production of banana and other agricultural crops worldwide. However, the efficacy of this actinomycete strain can vary due to biotic and abiotic factors, such as environmental conditions, the farming techniques and the growth stage of the pathogen. Furthermore, it is only applicable for long-term cultivation.
Methods
Isolation of Actinobacteria
Nineteen rhizosphere soil samples of Musa acuminata (Cavendish cultivar) fields in six different provices in Vietnam were collected and used for the isolation of actinomycete strains according to the method described by Rahman et al.62 with some small modifications. In brief, 10 g (g) of dried rhizosphere soil were suspended in 90 mL sterile ddH2O and then serially diluted up to 10− 6. An aliquot of 100 µL of each dilution was spread over the surface of Gause’s No. 1 agar plate (20 g L− 1 soluble starch; 0.5 g L− 1 K2HPO4; 0.5 g L− 1 MgSO4.7H2O; 0.5 g L− 1 NaCl; 0.5 g L− 1 KNO3; 0.01 g L− 1 FeSO4; 20 g L− 1 agar; Adjust pH to 7.4) supplemented with nystatin (50 µg mL− 1) to inhibit the growth of other fungi. Plates were incubated at 30 °C for 7 days. The selected isolates were recultivated several times for purity and preserved at −20oC in the addition of glycerol (30% v/v).
Screening of Streptomyces isolates for antifungal activity
Two hundred and twenty-two actinobacteria isolates were isolated and evaluated for their antifungal activities against Colletotrichum gloeosporioides. This C. gloeosporioides strain was identified for virulent characteristics before use. Antifungal activities of Streptomyces isolates were screened by agar diffusion assay on PDA medium according to method described by Sakthivel & Gnanamanickam63 with some modifications. First, Streptomyces isolates were grown separately on Gause’s No. 1 at 30 °C for 7 days. Second, 100 µL of spore suspension of C. gloeosporioides, with a concentration of 1 × 106 spores mL− 1, were spread onto PDA plates (90 mm in diameter). Third, four plugs of agar (5 mm in diameter) which contained tested Streptomyces isolates, grown previously on Gause’s No. 1, were placed as four symmetrical points on PDA plates. All plates were incubated at 30 °C for 3 days. The antifungal activities of Streptomyces isolates were determined by measuring the inhibition zone surrounding the agar plug by using the following formula: Inhibition zone (mm) = D − d, Where, D is the diameter (mm) of the inhibition zone; d is the diameter (mm) of the agar plug. All experiments were performed in triplicates. The VNUA74 strain was selected for further analyses based on its strongest antifungal activity against C. gloeosporioides.
Morphological and biochemical analyses
The morphology of the VNUA74 strain was analyzed by using a scanning electron microscopy (S-4800, Hitachi) after culture on Gause’s No. 1 medium at 30 °C for 7 days. The growth characteristics of the VNUA74 strain were characterized on six standard ISP (International Streptomyces Project) media including: Tryptone-yeast extract broth-ISP1; yeast extract-malt extract agar-ISP2; oatmeal agar-ISP3; inorganic salts-starch agar-ISP4; glycerol-asparagine agar-ISP5 and peptone yeast-iron agar-ISP664. The formation of melanin pigment was observed on ISP6 and the colors of the substrate, aerial mycelia, and diffusible pigments were determined after 14 days by using the ISCC-NBS color system. The biochemical properties of the VNUA74 strain were investigated as methods described by Abbasi et al.65 and Williams et al.66.
Phosphate and zinc solubilization
In order to identify the ability to solubilize phosphorus of the VNUA74 strain, we placed a mycelial block (5 mm in diameter) containing the VNUA74 colonies on Pikovskayas (PKV) agar plate according to the method described by Nautiyal67. The ability of the VNUA74 strain to solubilize zinc was evaluated on Bunt and Rovira agar plate as described by Suriyachadkun et al.68. The solubilization halo zone (mm) of the VNUA74 strain was measured after 14 days of the incubation of the plates at 30 °C. All experiments were performed in triplicates. The solubilization index (SI) was calculated by the following formula: SI = (colony diameter + halo zone diameter)/ (colony diameter).
Siderophores production
The ability to produce siderophores of the VNUA74 strain was evaluated according to the method described by Louden et al.69. In brief, a mycelial block (5 mm in diameter) containing colonies of the VNUA74 strain was placed on CAS agar plate. The plate was incubated at 30 °C for 14 days and the siderophore production index (SI) was determined by the following formula: SI = (colony diameter + halo zone diameter)/ (colony diameter).
IAA production
The capacity of the VNUA74 strain to produce the auxin phytohormone IAA (indole-3-acetic acid) was assessed according to the method of Glickmann & Dessaux70. Firstly, colonies of the VNUA74 strain were inoculated in 100 mL of Gause’s No.1 medium supplemented with 0.1% L-tryptophan (v/v) at 30 °C, 200 rpm for 7 days. A 2 mL of cell culture supernatant was collected by centrifugation and mixed with Salkowski reagent (2:1). The mixture was kept in dark at room temperature for 30 min. The IAA concentration in the mixture was measured by comparing the absorbance at 530 nm of the sample to the standard curve of IAA (0–100 µg mL− 1). The mean value and standard deviation were calculated from three independent cultivations.
Whole genome based taxonomic analyses
For the taxonomic analysis of the VNUA74 strain using whole genome data, we first identified bacterial genomes that were similar to VNUA74 by using Mash/MinHash distance71 implemented in BV-BRC genome finder. The cut-off value for the Mash distance was set at 0.05. The VNUA74 and its similar genomes were then submitted to analyze phylogenetic position in DSMZ Type Strain Genome Sever (TYGS, https://tygs.dsmz.de) and Genome to Genome Distance Calculator (GGDC 3.0, https://ggdc.dsmz.de)72,73. The phylogenetic trees generated by TYGS were visualized using the interactive tree of life (iTOL) V6.943 and further refined in Inkscape 1.2. The dDDH values between VNUA74 and its closely related genomes were calculated by using the recommended default settings of the GGDC 4.0. Average nucleotide identity (ANI) was pairwise computed among the genomes by using conventional FastANI tool74 and OrthoANI algorithm75.
Strain identification and 16 S rRNA sequence-based phylogenetic analysis
In order to identify the bacterial strain, we performed the genomic DNA extraction and purification from the selected VNUA74 strain by using the GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific) following the manufacturer’s protocol. The purified genomic DNA was then used as template to amplify the 16 S rRNA by the universal primer pairs: 27 F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′)76. The amplification reaction was performed in 20 µL reaction volume containing 1 µL of genomic DNA solution (20 ng µL− 1), 1 µL of each forward and reverse primers (100 µM), 10 µL 2×DreamTaq PCR Master Mix (Thermo Fisher Scientific Baltics UAB, Lithuania) and 7 µL of ddH2O. The PCR condition was as follow: an initial denaturation at 95 °C for 5 min, followed by 28 cycles of denaturation at 95 °C for 30 s, annealing at 53 °C for 30 s, and extension at 72 °C for 1 min. The reaction was completed with a final extension step at 72 °C for 10 min. The PCR product of approximately 1500 bp was visualized by electrophoresis on a 1.5% agarose gel and subsequently purified by using GeneJET Gel Extraction Kit (Thermo Fisher Scientific). The purified PCR product was sent for sequencing at 1st BASE company (Singapore). The nucleotide sequence obtained from sequencing was first edited by MEGA 11 software version 11.0.1377 and then aligned with reference sequences of 16 S rRNA retrieved from the GenBank database by using BLAST (NCBI, USA).
The 16 S rRNA sequences of the VNUA74 strain and related species were retrieved from NCBI GenBank (Table S5) and aligned using the ClustalW algorithm78. The phylogenetic tree was then constructed using the Neighbour-joining method and the reliability of the tree was estimated by the bootstrap method with 1000 replications. The phylogenetic analysis was conducted in MEGA 11 (ver. 11.0.13)77.
Secondary metabolite gene cluster analysis and RAST annotation for subsystem category
Analyses of genomes for potential secondary metabolite BGCs was performed by antiSMASH 7.1 bacterial version with a “relaxed” strict prediction setting79. The identified clusters and their genes contents were queried against the MiBIG database to identify the level of similarity in gene contents to the known clusters80,81. The putative BGCs were also verified and comparatively analyzed among S. parvulus strains by ARTS 2.0 which employs self-resistance based genome mining approach (assessed in May 2024)82.
The functional subsystem of the VNUA74 genome was analyzed by Rapid Annotations using Subsystems Technology tool kit (RASTtk) pipeline in RAST server83. The result was then visualized as a subsystem category in SEED viewer81. The annotation file of the VNUA74 genome from RASTtk was manually mined for genes associated with plant growth promotion and antagonistic attributes after analyzed by BlastKOALA84. Genes encoding carbohydrate active enzymes in the VNUA74 genome were mined from the KofamKOALA ver. 2024-05-01 (KEGG release 110.0) search85 and predicted using dbCAN 3.086.
Plant growth promotion in the pot experiment
The plant growth stimulating ability of strain VNUA74 was evaluated according to the method described by Zhu et al.87 with the following modifications. In this experiment, we used the in vitro banana plantlets (Musa acuminata AAA Group) of the same size (average height of about 7 cm with 3–4 leaves) at sixty-day-old to assess the plant growth-promoting effect of the VNUA74 strain. Each banana plantlet was first transplanted in an individual pot filled with 1 kg of mixed soil (rice field clay : smoked rice husks, 4:1, w/w), then the planted pots were devided into the control and the treatment groups, each group containing 10 pots. The pot experiment was proceeded in a green house of the Faculty of Biotechnology, Vietnam National University of Agriculture, Hanoi, Vietnam at a temperature of 28–30 °C with photoperiod condition was 12 h light/12 hours dark, watering was done once every 3 days. After 7 days of plantation, a 100 mL of S. parvulus VNUA74 spore suspension, with a concentration of 1 × 106 spores mL− 1, were added to the treatment pots. The same volume of tap water was applied to the control pots. At 90 days post-transplanting, plant fresh weights, root fresh weights, plant height, number of leaves, and stem diameter were measured. The experiment was performed in six replicates.
Antibacterial assay
In this experiment, the antibacterial activities of the VNUA74 strain were identified by agar-diffusion method as described in detail by Balouiri et al.88. The bacterial pathogens used in the assays were Xanthomonas axonopodis, Ralstonia solanacearum and Clavibacter michiganensis. First, the VNUA74 strain was inoculated in 50 mL Gause’s No. 1 medium at 30 °C, 200 rpm for 7 days. Single colony of test bacteria was cultivated separately in 50 mL Luria Broth (LB) medium at 30 °C, 200 rpm for 24 h. Second, the LB agar plates were inoculated with a standardized inoculum of the test bacteria (McFarland standard 0.5, approximately cell density of 1 × 108 CFU mL− 1). Third, two holes (5 mm in diameter) were bored into the agar with a sterile stainless-steel tube. Then, 100 µL of the VNUA74 growing supernatant was added into one of the holes, while the other hole was filled with the equal volume of sterile Gause’s No. 1 medium as a control and kept the plates at 4 °C for 4 h. The plates were thereafter incubated at 30 °C for 24 h. The zone of inhibition (ZOI) was calculated by the equation: ZOI (mm) = D−d. Where, D is the diameter (mm) of the inhibition zone; d is the diameter (mm) of the hole. The experiment was performed in triplicates.
Antifungal assay
The dual culture plate assay using PDA medium described by Dhanasekaran et al.89 was applied to identify the antifungal activities of the VNUA74 strain against common fungal pathogens of banana such as Foc TR4, F. solani, F. oxysporum, C. cassiicola and C. gloeosporioides. In brief, a mycelial block (5 mm in diameter) of each pathogenic fungus was placed at about 20 mm away from the edge of the Petri plate (90 mm in diameter). The same mycelial block (5 mm in diameter) from the VNUA74 strain was placed opposite the pathogenic fungus block and at about 20 mm away from the edge of the plate. In the control plate, a mycelial block (5 mm in diameter), consisting solely of the pathogenic fungus, was placed at the center of the Petri plate. The growth inhibition of the VNUA74 strain against pathogenic fungi was recorded after incubation at 30 °C for 7 days. The antifungal activities were determined by measuring the growth radius of tested pathogens on the control and treated plates using ImageJ ver. 1.54 g software and the percentages of growth inhibition were calculated by the equation: PI (%) = [(d0−d1)/d0]×100. Where, d0 is the radius of the untreated colony (mm); d1 is the radius of the treated colony (mm) at the interface with the VNUA74 strain.
Data availability
The 16 S rRNA sequence of Streptomyces parvulus VNUA74 is available on GenBank databases with the GenBank accession number: PP033922 (https://www.ncbi.nlm.nih.gov/nuccore/PP033922.1/). Other data and analyses generated during the current study are included in this published article and in the supplementary materials.
References
Available online at (2024). https://vietnamagriculture.nongnghiep.vn/japanese-consumers-are-increasing-their-purchase-of-vietnamese-bananas-d362809.html. Accessed April 26th.
Pham, H. H. et al. Molecular identification of Fusarium oxysporum f. sp. cubense (Foc) causing Fusarium wilt disease of Tieu hong banana cultivar in the Red River Delta of Vietnam. Arch. Phytopathol. Plant. Protect 57, 470–490 (2024).
Available online at (2024). https://vietnamagriculture.nongnghiep.vn/dong-nai-promotes-banana-exports-to-many-markets-d349299.html. Accessed May 1st.
Almadhoun, H. R. & Abu-Naser, S. S. Banana knowledge based system diagnosis and treatment. Int. J. Acad. Pedagog Res. 2, 1–11 (2018).
Blomme, G. et al. Bacterial diseases of bananas and enset: Current state of knowledge and integrated approaches toward sustainable management. Front. Plant. Sci. 8, 1290. https://doi.org/10.3389/fpls.2017.01290 (2017).
Zakaria, L., Sahak, S., Zakaria, M. & Salleh, B. Characterisation of Colletotrichum species associated with anthracnose of banana. Trop. Life Sci. Res. 20, 119–125 (2009).
Dita, M., Barquero, M., Heck, D., Mizubuti, E. S. G. & Staver, C. P. Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Front. Plant. Sci. 9, 1468. https://doi.org/10.3389/fpls.2018.01468 (2018).
Sheerer, L., Pemsl, D., Dita, M., Perez Vicente, L. & Staver, C. A quantified approach to projecting losses caused by Fusarium wilt Tropical race 4. Acta Hort. 1196, 211–218 (2018).
de Figueiredo Silva, F. et al. Estimating worldwide benefits from improved bananas resistant to Fusarium wilt tropical race 4. J. Agric. Appl. Econ. Assoc. 2, 20–34 (2023).
Torres-Rodriguez, J. A., Reyes-Pérez, J. J., Quiñones-Aguila, E. E. & Hernandez-Montiel, L. G. Actinomycete potential as biocontrol agent of phytopathogenic fungi: Mechanisms, source, and applications. Plants 11, 3201. https://doi.org/10.3390/plants11233201 (2022).
Mon, Y. Y., Bidabadi, S. S., Oo, K. S. & Zheng, S. J. The antagonistic mechanism of rhizosphere microbes and endophytes on the interaction between banana and Fusarium oxysporum f. sp. cubense. Physiol. Mol. Plant. Pathol. 116, 101733. https://doi.org/10.1016/j.pmpp.2021.101733 (2021).
Damodaran, T. et al. Biological management of banana Fusarium wilt caused by Fusarium oxysporum f. sp. cubense Trobical race 4 using antagonistic fungal isolate CSR-T-3 (Trichoderma reesei). Front. Microbiol. 11, 595845. https://doi.org/10.3389/fmicb.2020.595845 (2020).
Lin, C. P. & Ho, Y. C. Beneficial microbes and fertilization in antagonism of banana Fusarium wilt. Agronomy. 11, ; (2043). https://doi.org/10.3390/agronomy11102043 (2021).
Samuelian, S. Potential of Trichoderma harzianum for control of banana leaf fungal pathogens when applied with a food source and an organic adjuvant. 3 biotech. 6, 8. https://doi.org/10.1007/s13205-015-0327-0 (2016).
Lv, N. et al. Root-associated antagonistic Pseudomonas spp. contribute to soil suppressiveness against banana Fusarium wilt disease of banana. Microbiol. Spectr. 11, e03525–e03522. https://doi.org/10.1128/spectrum.03525-22 (2023).
Gousia, J., Ishfaq, S., Uqab, B. & Mudasir, S. Actinomycetes as biofertilisers for sustainable agriculture. In Microbiomes for the Management of Agricultural Sustainability (eds. Dar, G. H., Bhat, R. A., Mehmood, M. A.) 183–192 (Springer, Switzerland, 2023).
Li, X. et al. Biocontrol efficacy and possible mechanism of Streptomyces sp. H4 against postharvest anthracnose caused by Colletotrichum fragariae on strawberry fruit. Postharvest Biol. Technol. 175, 111401. https://doi.org/10.1016/j.postharvbio.2020.111401 (2021).
Zhou, D. et al. Biocontrol potential of a newly isolated Streptomyces sp. HSL-9B from mangrove forest on postharvest anthracnose of mango fruit caused by Colletotrichum gloeosporioides. Food Control 135, 108836. https://doi.org/10.1016/j.foodcont.2022.108836 (2022).
Komaki, H. Recent progress of reclassification of the genus Streptomyces. Microorganisms 11, 831. https://doi.org/10.3390/microorganisms11040831 (2023).
Shetty, P. R., Buddana, S. K., Tatipamula, V. B., Naga, Y. V. V. & Ahmad, J. Production of polypeptide antibiotic from Streptomyces parvulus and its antibacterial activity. Braz J. Microbiol. 45, 303–312 (2014).
Wang, D. et al. Identification, bioactivity, and productivity of actinomycins from the marine-derived Streptomyces heliomycini. Front. Micobiol 8, 1147. https://doi.org/10.3389/fmicb.2017.01147 (2017).
Ayuso-Sacido, A. & Genilloud, O. New PCR primers for the screening of NRPS and PKS-I systems in actinomycetes: Detection and distribution of these biosynthetic gene sequences in major taxonomic groups. Microb. Ecol. 49, 10–24 (2005).
Wawrik, B., Kerkhof, L., Zylstra, G. J. & Kukor, J. J. Identification of unique type II polyketide synthase genes in soil. Appl. Environ. Microbiol. 71, 2232–2238 (2005).
Wei, Y., Zhang, L., Zhou, Z. & Yan, X. Diversity of gene clusters for polyketide and nonribosomal peptide biosynthesis revealed by metagenomic analysis of yellow see sediment. Front. Microbiol. 9, 295. https://doi.org/10.3389/fmicb.2018.00295 (2018).
Jing, T. et al. Newly isolated Streptomyces sp. JBS5-6 as a potential biocontrol agent to control banana Fusarium wilt: Genome sequencing and secondary metabolite cluster profiles. Front. Microbiol. 11, 602591. https://doi.org/10.3389/fmicb.2020.602591 (2020).
Li, X. et al. Biological control of banana wilt disease caused by Fusarium oxysporum f. sp. cubense using Streptomyces sp. H4. Biol. Control 155, 104524. https://doi.org/10.1016/j.biocontrol.2020.104524 (2021).
Kawicha, P. et al. Evaluation of soil Streptomyces spp. for the biological control of Fusarium wilt disease and growth promotion in tomato and banana. Plant. Pathol. J. 39, 108. https://doi.org/10.5423/PPJ.OA.08.2022.0124 (2023).
Qi, D. et al. Taxonomic identification and antagonistic activity of Streptomyces luomodiensis sp. nov. against phytopathogenic fungi. Front. Microbiol. 15, 1402653. https://doi.org/10.3389/fmicb.2024.1402653 (2024).
Perez, J. V. et al. Antarctic streptomyces: Promising biocontrol agents for combating Fusarium oxysporum f. sp. cubense. Biotechnol. Rep. 43, e00852. https://doi.org/10.1016/j.btre.2024.e00852 (2024).
Ward, A. C. & Allenby, N. E. Genome mining for the search and discovery of bioactive compounds: The Streptomyces paradigm. FEMS Microbiol. Lett. 365. https://doi.org/10.1093/femsle/fny240 (2018).
Belknap, K. C., Park, C. J., Barth, B. M. & Andam, C. P. Genome mining of biosynthetic and chemotherapeutic gene clusters in Streptomyces bacteria. Sci. Rep. 10, 2003. https://doi.org/10.1038/s41598-020-58904-9 (2020).
Nguyen, T. T., Dinh, S. T. & Nguyen, C. X. Genome characterization of Streptomyces sp. strain VNUA74, a potential biocontrol against pathogenic fungus Colletotrichum spp. Microbiol. Resour. Announc 12, e0087323. https://doi.org/10.1128/MRA.00873-23 (2023).
Hazarika, S. N. & Thakur, D. Actinobacteria. In Beneficial Microbes in Agro-ecology (eds. Amaresan, N., Kumar, M. S., Annapurna, K., Kumar, K., Sankaranarayanan, A.) 443–476 (Academic Press, 2020).
Lefort, V., Desper, R. & Gascuel, O. FastME 2.0: A comprehensive, accurate, and fast distance-based phylogeny inference program. Mol. Biol. Evol. 32, 2798–2800 (2015).
Riesco, R. & Trujillo, M. E. Update on the proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 74, 006300. https://doi.org/10.1099/ijsem.0.006300 (2024).
Meier-Kolthoff, J. P., Auch, A. F., Klenk, H. P. & Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 14, 1–14 (2013).
Sabaratnam, S. & Traquair, J. A. Mechanism of antagonism by Streptomyces griseocarneus (strain Di944) against fungal pathogens of greenhouse-grown tomato transplants. Can. J. Plant. Pathol. 37, 197–211 (2015).
Pacios-Michelena, S. et al. Application of Streptomyces antimicrobial compounds for the control of phytopathogens. Front. Sustain. Food Syst. 5, 696518. https://doi.org/10.3389/fsufs.2021.696518 (2021).
Shanthi, V. & Actinomycetes Implications and prospects in sustainable agriculture in Biofertilizers: Study and Impact (eds Inamuddin, Ahamed, M. I., Boddula, R. & Rezakazemi, M.) 335–370 (Scrivener Publishing, (2021).
Ribeiro, H. G. & van der Sand, S. T. Exploring the trends in actinobacteria as biological control agents of phytopathogenic fungi: A (mini)-review. Indian J. Microbiol. 64, 70–81 (2024).
Mispelaere, M. et al. Whole genome–based comparative analysis of the genus Streptomyces reveals many misclassifications. Appl. Microbiol. Biotechnol. 108, 1–12 (2024).
Komaki, H. Reclassification of 15 Streptomyces species as synonyms of Streptomyces albogriseolus, Streptomyces althioticus, Streptomyces anthocyanicus, Streptomyces calvus, Streptomyces griseoincarnatus, Streptomyces mutabilis, Streptomyces pilosus or Streptomyces rochei. Int. J. Syst. Evol. Microbiol. 71, 004718. https://doi.org/10.1099/ijsem.0.004718 (2021).
Meier-Kolthoff, J. P. & Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 10, 2182. https://doi.org/10.1038/s41467-019-10210-3 (2019).
Talamantes, D., Biabini, N., Dang, H., Abdoun, K. & Berlemont, R. Natural diversity of cellulases, xylanases, and chitinases in bacteria. Biotechnol. Biofuels 9, 133. https://doi.org/10.1186/s13068-016-0538-6 (2016).
Kumar, M., Kumar, P., Das, P., Solanki, R. & Kapur, M. K. Potential applications of extracellular enzymes from Streptomyces spp. in various industries. Arch. Microbiol. 202, 1597–1615 (2020).
Li, W. et al. Antifungal activity and biocontrol mechanism of Fusicolla violacea J-1 against soft rot in kiwifruit caused by Alternaria alternata. J. Fungi 7, 937. https://doi.org/10.3390/jof7110937 (2021).
Ruangwong, O. U., Kunasakdakul, K., Chankaew, S., Pitija, K. & Sunpapao, A. A rhizobacterium, Streptomyces albulus Z1-04-02, displays antifungal activity against Sclerotium rot in mungbean. Plants 11, 2607. https://doi.org/10.3390/plants11192607 (2022).
Mingma, R. & Duangmal, K. Characterization, antifungal activity and plant growth promoting potential of endophytic actinomycetes isolated from rice (Oryza sativa L). Chiang Mai J. Sci. 45, 2652–2665 (2018).
Sanjivkumar, M., Vijayalakshmi, K., Silambarasan, T., Sholkamy, E. N. & Immanuel, G. Biosynthesis, statistical optimization and molecular modeling of chitinase from crab shell wastes by a mangrove associated actinobacterium Streptomyces olivaceus (MSU3) using Box-Behnken design and its antifungal effects. Bioresour Technol. Rep. 11, 100493. https://doi.org/10.1016/j.biteb.2020.100493 (2020).
Phoulivong, S. et al. Colletotrichum gloeosporioides is not a common pathogen on tropical fruits. Fungal Divers. 44, 33–43 (2010).
Quach, N. T. et al. Genome-guided investigation provides new insights into secondary metabolites of Streptomyces parvulus SX6 from Aegiceras corniculatum. Pol. J. Microbiol. 71, 381–394 (2022).
Kadaikunnan, S., Alharbi, N. S., Khaled, J. M. & Alobaidi, A. S. Biocontrol property of Streptomyces parvulus VRR3 in green gram plant (Vigna radiata L.) against Fusarium solani in greenhouse. Physiol. Mol. Plant. Pathol. 128, 102128. https://doi.org/10.1016/j.pmpp.2023.102128 (2023).
Naine, S. J., Devi, C. S., Mohanasrinivasan, V. & Vaishnavi, B. Antimicrobial, antioxidant and cytotoxic activity of marine Streptomyces parvulus VITJS11 crude extract. Braz Arch. Biol. Technol. 58, 198–207 (2015).
Rahman, A., Islam, M. Z., Khondkar, P. & Islam, A. U. Characterization and antimicrobial activities of a polypeptide antibiotic isolated from a new strain of Streptomyces parvulus. Bangladesh Pharm. J. 13, 14–16 (2010).
Chandrakar, S. & Gupta, A. K. Actinomycin-producing endophytic Streptomyces parvulus associated with root of Aloe vera and optimization of conditions for antibiotic production. Probiotics Antimicrob. Proteins 11, 1055–1069 (2018).
Worsley, S. F. et al. Streptomyces endophytes promote host health and enhance growth across plant species. Appl. Environ. Microbiol. 86, e01053–e01020. https://doi.org/10.1128/AEM.01053-20 (2020).
Inbar, L. & Lapidot, A. Metabolic regulation in Streptomyces parvulus during actinomycin D synthesis, studied with 13 C- and 15 N-labeled precusors by 13 C and 15 N nuclear magnetic resonance spectroscopy and by gas chromatography-mass spectometry. J. Bacteriol. 170, 4055–4064 (1988).
Lin, Y. et al. Multi-omics analysis reveals anti-Staphylococcus aureus activity of actinomycin D originating from Streptomyces parvulus. Int. J. Mol. Sci. 22, 12231. https://doi.org/10.3390/ijms222212231 (2021).
Nishizawa, T. et al. Complete genome sequence of Streptomyces parvulus 2297, integrating site-specifically with actinophage R4. Genome Announc 4, e00875-16 (2016).
Srivastava, L. M. Auxins. In Plant Growth and Development: Hormones and Environment. 155–169 (eds Srivastava, L. M.) (Academic, 2002).
Expert, D., Franza, T. & Dellagi, A. Iron in plant-pathogen interactions. In Molecular Aspects of Iron Metabolism in Pathogenic and Symbiotic Plant-Microbe Associations (ed. Expert, D. & O’Brian, M.) 7–39 (Springer, 2012).
Rahman, A., Islam, M. Z. & Islam, A. U. Antibacterial activities of actinomycete isolates collected from soils of Rajshahi, Bangladesh. Biotechnol. Res. Int. 2011, 857925. https://doi.org/10.4061/2011/857925 (2011).
Sakthivel, N. & Gnanamanickam, S. S. Evaluation of Pseudomonas fluorescens for suppression of sheath rot disease and for enhancement of grain yields in rice (Oryza sativa L). Appl. Environ. Microbiol. 53, 2056–2059 (1987).
Shirling, E. B. & Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Evol. Microbiol. 16, 313–340 (1966).
Abbasi, S., Safaie, N., Sadeghi, A. & Shamsbakhsh, M. Streptomyces strains induce resistance to Fusarium oxysporum f. sp. lycopersici race 3 in tomato through different molecular mechanisms. Front. Microbiol. 10, 1505. https://doi.org/10.3389/fmicb.2019.01505 (2019).
Williams, S. T. et al. Numerical classification of Streptomyces and related genera. J. Gen. Microbiol. 129, 1743–1813 (1983).
Nautiyal, C. S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 170, 265–270 (1999).
Suriyachadkun, C., Chunhachart, O., Srithaworn, M., Tangchitcharoenkhul, R. & Tangjitjareonkun, J. Zinc-solubilizing Streptomyces spp. as bioinoculants for promoting the growth of soybean (Glycine max (L.) Merrill). J. Microbiol. Biotechnol. 32, 1435–1446 (2022).
Louden, B. C., Haarmann, D. & Lynne, A. M. Use of blue agar CAS assay for siderophore detection. J. Microbiol. Biol. Educ. 12, 51–53 (2011).
Glickmann, E. & Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 61, 793–796 (1995).
Ondov, B. D. et al. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 17, 132. https://doi.org/10.1186/s13059-016-0997-x (2016).
Meier-Kolthoff, J. P., Carbasse, J. S., Peinado-Olarte, R. L. & Göker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 50, D801–D807. https://doi.org/10.1093/nar/gkab902 (2022).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296. https://doi.org/10.1093/nar/gkab301 (2021).
Jain, C., Rodriguez-R, L. M., Phillippy, A. M., Konstantinidis, K. T. & Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 9, 5114. https://doi.org/10.1038/s41467-018-07641-9 (2018).
Lee, I., Kim, Y. O., Park, S. C. & Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 66, 1100–1103 (2016).
Weisburg, W. G., Barns, S. M., Pelletier, D. A. & Lane, D. J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173, 697–703 (1991).
Tamura, K., Stecher, G. & Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 38, 3022–3027 (2021).
Chenna, R. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31, 3497–3500 (2003).
Blin, K. et al. antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 51, W46–W50. https://doi.org/10.1093/nar/gkad344 (2023).
Medema, M. H. et al. Minimum information about a biosynthetic gene cluster. Nat. Chem. Biol. 11, 625–631 (2015).
Kautsar, S. A. et al. MIBiG 2.0: a repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 48, D454–D458. https://doi.org/10.1093/nar/gkz882 (2020).
Mungan, M. D. et al. ARTS 2.0: feature updates and expansion of the Antibiotic Resistant Target Seeker for comparative genome mining. Nucleic Acids Res. 48, W546–W552. https://doi.org/10.1093/nar/gkaa374 (2020).
Brettin, T. et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 5, 8365. https://doi.org/10.1038/srep08365 (2015).
Kanehisa, M., Sato, Y. & Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 428, 726–731 (2016).
Aramaki, T. et al. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 36, 2251–2252 (2020).
Zheng, J. et al. dbCAN3: automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Res. 51, W115–W121. https://doi.org/10.1093/nar/gkad328 (2023).
Zhu, Z., Tian, Z. & Li, J. A. Streptomyces morookaensis strain promotes plant growth and suppresses Fusarium wilt of banana. Trop. Plant. Pathol. 46, 175–185 (2021).
Balouiri, M., Sadiki, M. & Ibnsouda, S. K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 6, 71–79 (2016).
Dhanasekaran, D., Thajuddin, N. & Panneerselvam, A. Applications of actinobacterial fungicides in agriculture and medicine. In Fungicides for Plant and Animal Diseases (ed. Dhanasekaran, D) 29−54 (IntechOpen, 2012).
Acknowledgements
We thank Vietnam National University of Agriculture and Duy Tan University for providing access to the laboratory facilities to carry out this research.
Author information
Authors and Affiliations
Contributions
C.X.N. designed all experiments. T.T.N. carried out almost all experiments. Strain identification and taxonomic analysis were done by T.H.N. Genome sequence was analyzed by D.T.T. and S.T.D. Genome mining was conducted by T.L.N. and T.M.V. Data was analyzed and the manuscript was written and revised by T.T.N., T.M.V. and C.X.N. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nguyen, T.T., Nguyen, T.T., Nguyen, T.H. et al. In silico and in vitro analyses reveal the potential use of Streptomyces parvulus VNUA74 as bioagent for sustainable banana production. Sci Rep 15, 7049 (2025). https://doi.org/10.1038/s41598-024-83520-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83520-2