Abstract
Klotho has been importantly linked to atherosclerosis, but little is known about its specific role. This study investigates the mechanism by which Klotho enhances the stability of atherosclerotic plaques in chronic kidney disease. apoE-/- knockout mice and C57BL/6 mice underwent 5/6 nephrectomy and then klotho-NC and klotho-mimic groups were set up to be fed a high-fat chow diet and a dummy group was created to be fed a normal chow diet. qPCR detected relative mRNA expression of klotho. Oil Red O and HE staining assessed lipid proportion in the aorta. Masson staining evaluated renal failure pathology in mice. Immunohistochemistry measured MAC-2 and α-SMA expression in the aorta. ELISA quantified urea, cholesterol, calcium ions, and triglycerides in mouse plasma. Western blotting detected associated protein expression, followed by cell-based experiments for validation. Compared with the Klotho-NC group, the plaque area and aortic lipid and renal fibrosis area were reduced in the Klotho-mimic group. Klotho-mimic reduced macrophage area, plasma urea, cholesterol, calcium ions, and triglyceride levels, and decreased the expression of p-PERK, NOX2, NOX4, Caspase-3, Caspase-9, Bax, p-GRK2, p-PLCβ, p-Src, and p-IP3R. Without ox-LDL stimulation, Klotho expression increased in the Klotho-mimic group, with no significant differences in NOX2, p-SHP1, p-Src, p-PERK, p-GRK2, and p-PLCβ. With ox-LDL in high-calcium medium, Klotho and p-SHP1 increased, while NOX2, p-Src, p-PERK, p-GRK2, and p-PLCβ decreased in the Klotho-mimic group. After ox-LDL and TPI-1 treatment, Klotho increased, NOX2 decreased, and other proteins showed no significant changes. Adding shRNA-GRK2 reduced NOX2, p-Src, and p-PERK, increased p-SHP1, with no changes in p-GRK2 and p-PLCβ. Differences in NOX2, p-GRK2, p-PLCβ, and p-PERK between groups were reduced in high-calcium medium, while p-SHP1 differences increased. Klotho enhances chronic kidney disease atherosclerotic plaque stability by inhibiting GRK2/PLC-β-mediated endoplasmic reticulum stress in macrophages via the ROS/SHP1 pathway.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) is the most prevalent complication and foremost cause of mortality among patients with chronic renal failure (CRF), particularly those with end-stage renal disease (ESRD). The mortality rate from CVD in ESRD patients is 16.6–17.7 times higher than in the general population. Dialysis patients exhibit a mortality rate from CVD as high as 50%, which is 10–20 times greater than that of the general population. The incidence of atherosclerotic CVD, including myocardial infarction and stroke, is 5–10 times higher in CRF patients compared to the general population. The progression of atherosclerosis in CRF patients differs from that seen in the general population, with a higher incidence, earlier onset, and more extensive involvement. The underlying mechanisms of atherosclerosis in CRF patients remain poorly understood. While traditional risk factors such as hypertension, diabetes, and lipid metabolism disorders are prevalent among CRF patients, they do not fully account for the high incidence and mortality rate of CVD in these individuals, implying that other factors in CRF patients may accelerate the occurrence and progression of atherosclerosis1,2,3,4.
The pathogenesis of atherosclerosis (AS) is intricate. Numerous studies have corroborated that atherosclerosis is essentially a chronic inflammatory proliferative response, in which various inflammatory factors act on the vascular wall over an extended period, leading to the formation and progression of atherosclerotic lesions through the secretion of inflammatory mediators and activation of inflammatory cells. In the process of atherosclerosis, low-density lipoprotein (LDL) is oxidized within the vascular wall and taken up by phagocytic cells to form foam cells. These foam cells accumulate within the vascular wall, forming atherosclerosis5,6. Macrophages are one of the principal components, phagocytosing oxidized LDL and accumulating within the plaques. Immunotherapy primarily ameliorates the pathological process of atherosclerosis by modulating the immune system. It can enhance the activity of the immune system, suppress inflammation, and reduce plaque formation and progression. Specific immune therapies encompass the use of immunomodulators, cell therapy, and gene therapy7,8,9.
Kuro-o et al. discovered that disruption of the Klotho gene in transgenic mice resulted in signs of aging, including growth retardation, hypogonadotropic hypogonadism, rapid thymus degeneration, skin atrophy, muscle wasting, vascular calcification, osteoporosis, emphysema, and hearing impairment. Conversely, overexpression of the Klotho gene can delay tissue aging and extend the lifespan of mice, suggesting that the Klotho gene may regulate the aging process. Klotho has a wide range of functions, with most Klotho expressed in the kidneys and choroid plexus of the brain, where it exerts protective effects. However, there are also tissues or organs, such as the vascular endothelium, that do not express Klotho. Studies have demonstrated that elevated levels of soluble Klotho can protect endothelial cells and cardiovascular function, mitigate vascular calcification, intimal proliferation, endothelial dysfunction, arterial stiffness, hypertension, and impaired angiogenesis, and have hormone-like effects10,11,12,13,14.
Oxidative stress is a critical mechanism in the formation and progression of atherosclerosis. Reactive oxygen species (ROS) produced by oxidative stress can lead to inactivation of SHP-115. SHP-1 (Src homology 2 domain-containing protein tyrosine phosphatase-1) plays a significant role in atherosclerosis. SHP-1 is a tyrosine phosphatase that affects the function of vascular endothelial cells and immune cells by regulating multiple signaling pathways. Studies have shown that SHP-1 may have a protective effect in the development of atherosclerosis. Some studies have found that defects in SHP-1 may exacerbate atherosclerosis. Specifically, SHP-1 defects may affect the function of vascular endothelial cells, leading to increased inflammation, elevated expression of vascular cell adhesion molecules, and accelerated formation of atherosclerotic plaques. Additionally, SHP-1 is involved in the regulation of immune cell activation and inflammatory response16,17,18,19. Defects in SHP-1 may lead to abnormal activation of immune cells and an enhanced inflammatory response, thereby affecting the progression of atherosclerosis.
Therefore, in this study, we investigated the mechanism by which Klotho enhances the stability of atherosclerotic plaques in patients with chronic kidney disease.
Methods
Experimental animals
24 male apoE-/- mice and eight normal C57BL/6 mice were purchased from Hunan Sckbios Technology Co., Ltd. All mice were acclimated for one week in a temperature-controlled environment (22 ± 1℃), with a humidity of 45–55%, a 12-hour light/dark cycle, and free access to water and food. Mice are randomly assigned by weight and cage order. This study has been approved by the Ethics Committee of Hebei North University and conducted in accordance with guidelines for the protection of animal subjects. The study was performed in accordance with ARRIVE guidelines.
Model construction and treatment
The kidneys of all mice were surgically removed by 5/6 nephrectomy. The mice were divided into sham group, Klotho-NC group, and Klotho-mimic group. 8 pieces per group. Lentiviral vectors carrying full-length mouse Klotho cDNA (LV-KL) labeled with green fluorescent protein (GFP) were obtained from OriGene Technologies and accompanied by a negative control. The lentiviral particles were then packaged by GeneChem. After 24 h, the supernatant was replaced with complete medium. RAW264.7 cells infected with the lentiviral particles were subsequently injected into the Klotho-NC group and Klotho-mimic group mice via the tail vein. The Klotho-NC group and Klotho-mimic group mice were fed with a diet containing 21% fat and 0.15% cholesterol for 12 weeks to establish the AS model, while the sham group mice were fed a normal diet for 12 weeks.The mice were then euthanized with 20% isoflurane to minimize anxiety during the euthanasia process. The remaining mice of the experiment were also euthanized.
Oil red O staining
The aorta was removed from the fixative solution, rinsed twice with PBS, meticulously dissected longitudinally along the vessel wall under a dissecting microscope, and immersed in the oil red O staining solution (Sigma Product No.: O0625-25 g) at 37 °C for 60 min. After staining, the aorta was differentiated in 75% alcohol until the bright red plaque tissue in the lumen remained, while the other parts were nearly colorless. It was then washed twice with distilled water, affixed on a back plate, and photographed.
HE staining
Paraffin-embedded sections were deparaffinized and stained with hematoxylin for 3–5 min. After rinsing with tap water, differentiation was performed, followed by bluing, rinsing, and counterstaining with eosin for 5 min. The staining results were observed under a microscope.
Immunohistochemistry staining
Sections were retrieved with pH 6.0 sodium citrate solution for 3 min, followed by incubation at 30 °C for 30 min. After blocking endogenous peroxidase with hydrogen peroxide, the sections were incubated with primary antibodies MAC-2 and α-SMA overnight at 4 °C. The following day, the sections were washed and incubated with horseradish peroxidase-conjugated secondary antibodies for 30 min. After washing with PBS, the sections were stained with DAB, counterstained with hematoxylin, dehydrated, and mounted.
Masson staining
Paraffin sections were subjected to deparaffinisation and then operated according to the instructions of Masson trichrome staining kit, stained with Lichun’s magenta staining solution for 5 min, washed with weak acidic working solution for 1 min, washed with phosphomolybdic acid for 1 min, stained in aniline blue staining solution, dewatered with ethanol and then transparent with xylene, and sealed with neutral gum for image acquisition.
qPCR
Total RNA was extracted from mouse aortic tissue using Trizol reagent. Reverse transcription was performed with a reverse transcription kit under the following conditions: 42 ℃, 50 min; 85 ℃, 5 min, using qPCR kit with real-time quantitative PCR instrument for detection, 10 µL reaction system was selected for amplification: 95 ℃ pre-denaturation for 3 min; Two-step cyclic reaction: 95 ℃, 10 s; 60 ℃, 30 s; 40 cycles, all reactions repeated 3 times. After the amplification is completed, the CT values of the target gene and the internal reference gene of each group of samples can be obtained, the dissolution curve is plotted, and the final data is analyzed by 2−△△Ct. The primers for qPCR are shown below. Klotho: Forward primer (5’−3’):GGCTTTCCTCCTTTACCTGAAAA; Reverse primer (3’−5’):CACATCCCACAGATAGACATTCG. GAPDH: Forward primer (5’−3’):CAAGCTCATTTCCTGGTATGACA; Reverse primer (3’−5’):TCTCTTGCTCAGTGTCCTTGCT.
ELISA
Blood samples were collected in anticoagulant tubes and centrifuged. The concentrations of plasma urea, cholesterol, calcium ions, and triglycerides were measured using ELISA kits according to the manufacturer’s instructions. To determine specificity, the antigen was coated on ELISA plates (100 µL per well) and incubated at 37 °C for 4 h, followed by discarding the liquid. Samples were then blocked with 5% bovine serum at 37 °C for 40 min, washed three times, and reacted with antibodies in each well (100 µL per well) at 37 °C for 40–60 min. After washing three times, chromogenic substrate (TMB) was added. Finally, the OD values were measured at 450 nm wavelength using an ELISA reader, and the concentrations of urea, cholesterol, calcium ions, and triglycerides were determined based on the OD values.
Cell culture and treatment
RAW264.7 cells were purchased from Wuhan Pricella Biotechnology Co., Ltd. They were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin/streptomycin, 1× non-essential amino acids (NEAA), and 100 U/ml streptomycin. The cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. RAW264.7 cells were infected with Klotho overexpression lentivirus and corresponding negative control, and then stimulated with ox-LDL to establish an atherosclerosis cell model. High-calcium medium was used to simulate a cellular model of renal failure, and cells were treated with the SHP1 inhibitor THP1 and infected with shRNA-GRK2 as well as its negative control (Vectors without shRNA sequences were used as controls to ensure that any observed effects were caused by specific inhibition of the shRNA and not by the vector itself), respectively.
Western blotting
Tissues and cells were digested with trypsin, washed with PBS, and centrifuged. After discarding the supernatant, the cells were thoroughly lysed with an appropriate amount of RIPA lysis buffer to extract total proteins. BCA assay was used to determine protein concentrations, and the buffer was boiled in a metal bath for 10 min. Protein samples (20 µg/well) were separated by SDS-PAGE, transferred onto PVDF membrane, blocked with 5% skim milk for 2 h, and incubated with primary antibodies Klotho, NOX2, p-PERK, NOX4, Caspase-3, Caspase-9, Bax, p-GRK2, p-PLCβ, p-Src, p-SHP1, and p-IP3R overnight at 4 °C. The following day, the membrane was washed with TBST and incubated with HRP-conjugated secondary antibodies at room temperature for 2 h. After washing with TBST, the membrane was incubated with ECL solution and exposed in a gel imaging system. Finally, the grayscale values were analyzed.
Statistical analysis
Data and image analysis were performed using Prism 9.0 software. Experimental data were expressed as mean ± standard deviation and analyzed using t-test. The data satisfied the assumptions of the statistical method, and if the data did not meet the requirements, it was directly excluded. A p-value less than 0.05 was considered statistically significant.
Results
Klotho can inhibit the progression of atherosclerosis in chronic renal failure
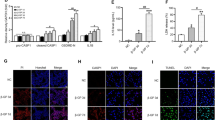
First, to study the effect of klotho on the progression of atherosclerosis in chronic renal failure. The results of qPCR experiments showed that the relative mRNA expression of klotho in the klotho-NC group was significantly lower than that in the sham group. However, the relative mRNA expression of klotho in the klotho-mimic group was significantly increased. The results of oil red O staining showed that the aortic wall of the mice in the sham operation group was smooth, and there was no obvious red coloring area. The aortic arch to the abdominal aorta region of mice in the klotho-NC group were distributed with red plaques of different sizes, and the coloring area was wide. Compared with the klotho-NC group, the klotho-mimic group of mice with reduced areas of red plaques in the aorta, lighter coloration, and reduced lipid deposition (Fig. 1).
Klotho can reduce the content of macrophages in atherosclerotic plaques in chronic kidney disease
Further observation of the morphological features of aortic plaques was conducted through HE staining and immunohistochemistry. The results of HE staining showed that the sham group had the morphology of normal vascular intima with intima, intima, media, and neat arrangement, and no obvious plaque formation. The aortic root plaque formation in the klotho-NC group was disordered, inflammatory cell infiltration was visible, there was a local fibrous plaque cap, and the core necrotic area in the lumen was formed. Compared with the klotho-NC group, the aortic root plaque was reduced, the cell arrangement disorder was improved, the inflammatory cell infiltration was reduced, the middle membrane array disorder and the plaque fiber cap were improved in the klotho-mimic group. Immunohistochemistry results revealed a significant increase in the expression of MAC-2 and α-SMA in the Klotho-NC group and Klotho-mimic group compared to the sham group. However, the expression of MAC-2 was significantly reduced in the Klotho-mimic group compared to the Klotho-NC group, while the expression of α-SMA showed no significant difference. This indicates that Klotho regulates atherosclerosis in chronic kidney disease through macrophages rather than smooth muscle cells (Fig. 2).
Klotho reduces the content of macrophages in atherosclerotic plaques in chronic renal failure. A HE staining results and statistical analysis of atherosclerotic plaque area; B Immunohistochemical staining results for MAC-2 and statistical analysis of expression data; C Immunohistochemical staining results for α-SMA and statistical analysis of expression data. N = 8;**P < 0.01; nsP > 0.05.
Klotho can decrease the concentrations of urea, cholesterol, calcium ions, and triglycerides in atherosclerosis in chronic kidney disease
ELISA was employed to quantify the concentrations of urea, cholesterol, calcium ions, and triglycerides in mouse plasma. The findings indicated a substantial increase in the concentrations of urea, cholesterol, calcium ions, and triglycerides in both the Klotho-NC group and the Klotho-mimic group compared to the sham group. However, these component concentrations were significantly reduced in the Klotho-mimic group relative to the Klotho-NC group. Masson staining showed significant renal interstitial fibrosis in the Klotho-NC group relative to the sham group, with larger areas staining blue and the presence of large collagen deposits. Fibrosis was significantly reduced in the Klotho-mimic group relative to the Klotho-NC group (Fig. 3).
Klotho reduces the concentrations of urea, cholesterol, calcium ions, and triglycerides in chronic renal failure with atherosclerosis. A ELISA detection of urea concentrations in mouse plasma; B ELISA detection of cholesterol concentrations in mouse plasma; C ELISA detection of calcium ion concentrations in mouse plasma; D ELISA detection of triglyceride concentrations in mouse plasma. E: Masson staining results were plotted and the area of positive MASSON staining (blue area) was counted. N = 8;**P < 0.01; *P < 0.05.
Klotho enhances atherosclerotic plaque stability in patients with chronic kidney disease
Furthermore, Western blot analysis revealed a significant elevation in the relative protein expression of p-PERK, NOX2, NOX4, Caspase-3, Caspase-9, Bax, p-GRK2, p-PLCβ, p-Src, and p-IP3R, along with a significant reduction in the relative protein expression of SHP1 in the Klotho-NC group and Klotho-mimic group compared to the sham group. Compared to the Klotho-NC group, the Klotho-mimic group exhibited a marked decrease in the relative protein expression of p-PERK, NOX2, NOX4, Caspase-3, Caspase-9, Bax, p-GRK2, p-PLCβ, p-Src, and p-IP3R, and a significant increase in the relative protein expression of SHP1 (Fig. 4).
Klotho enhances the stability of atherosclerotic plaques in chronic renal failure. A Protein bands and relative protein expression levels of NOX2, NOX4, Caspase-3, Caspase-9, Bax and p-PERK as well as statistical analysis; B Protein bands and relative protein expression levels of p-GRK2, p-PLCβ and p-IP3R, as well as statistical analysis. C Protein bands and relative protein expression levels of p-SHP1 and p-Src, as well as statistical analysis.GAPDH as the control protein. N = 8; **P < 0.01.
Klotho enhances the stability of atherosclerotic plaques in chronic kidney disease by inhibiting the ROS/SHP1 pathway and downregulating the ER stress mediated by GRK2/PLC-β in macrophages
Western blot results demonstrated that, without ox-LDL stimulation, the relative protein expression of Klotho was significantly elevated in the Klotho-mimic group compared to the Klotho-NC group. There were no significant differences in the relative protein expression of NOX2, p-SHP1, p-Src, p-PERK, p-GRK2, and p-PLCβ. Following ox-LDL stimulation in a high-calcium medium, the Klotho-mimic group exhibited a significant increase in the relative protein expression of Klotho and p-SHP1, while the relative protein expression of NOX2, p-Src, p-PERK, p-GRK2, and p-PLCβ was significantly diminished compared to the Klotho-NC group. After administering the SHP1 inhibitor TPI-1, the Klotho-mimic group showed a significant rise in the relative protein expression of Klotho, a substantial decrease in the relative protein expression of NOX2, and no significant differences in the relative protein expression of p-SHP1, p-Src, p-PERK, p-GRK2, and p-PLCβ compared to the Klotho-NC group. Post-infection with shRNA-GRK2 and negative controls, the Klotho-mimic group exhibited a significant increase in the relative protein expression of Klotho and p-SHP1, a notable decrease in the relative protein expression of NOX2 and p-Src, and no significant differences in the relative protein expression of p-PERK, p-GRK2, and p-PLCβ compared to the Klotho-NC group.
Additionally, a comparison was made between high-calcium medium and calcium-free medium, both with ox-LDL stimulation. Western blot results indicated that, compared to the Klotho-NC group, the Klotho-mimic group exhibited a significant decrease in the relative protein expression of NOX2, p-GRK2, p-PLCβ, and p-PERK, and a substantial increase in the relative protein expression of p-SHP1 in both high-calcium medium and calcium-free medium. Compared to high-calcium medium, calcium-free medium showed a significant decrease in the relative protein expression of NOX2, p-GRK2, p-PLCβ, and p-PERK, and a marked increase in the relative protein expression of p-SHP1 (Fig. 5).
Klotho enhances the stability of atherosclerotic plaques through ROS/SHP1 pathway inhibition, downregulating GRK2/PLC-β-mediated ER stress. A Protein bands and relative protein expression levels of Klotho, NOX2, p-SHP1, p-Src, p-PERK, p-GRK2, and p-PLCβ, as well as statistical analysis; B Protein bands and relative protein expression levels of NOX2, p-SHP1, p-GRK2, p-PLCβ, and p-PERK, as well as statistical analysis. GAPDH as the control protein. N = 3; **P < 0.01; nsP > 0.05.
In summary, Klotho enhances the stability of atherosclerotic plaques in chronic renal failure combined with atherosclerosis by inhibiting the ROS/SHP1 pathway and downregulating GRK2/PLC-β-mediated ER stress in macrophages. This offers new therapeutic approaches and targets for the study of chronic renal failure combined with atherosclerosis (Fig. 6).
Discussion
Cardiovascular complications are prevalent in patients with chronic renal failure, exhibiting a high incidence. Arterial stiffness is a precise and sensitive marker of early vascular disease and a high-risk factor for cardiovascular disease, significantly decreasing the survival rate of patients with chronic renal failure. Patients with end-stage renal disease (ESRD) face a 15–20 times higher risk of cardiovascular events and atherosclerotic cardiovascular disease than the general population, with approximately 80% experiencing complications like hypertension and primarily succumbing to cardiovascular events. Diminished arterial elasticity has also been confirmed as an independent predictor of ESRD cardiovascular mortality1,2,20.
Atherosclerosis is a chronic inflammatory disease characterized by lipid accumulation and fibrous tissue proliferation in the vascular wall. It results in the narrowing, hardening, and loss of elasticity of arterial blood vessels, impeding normal blood flow. Patients with chronic renal failure typically present multiple risk factors such as hypertension, hypercholesterolemia, and diabetes, all of which contribute to atherosclerosis. When atherosclerosis affects the renal artery, it can lead to diminished renal blood flow, hence impairing the kidney’s normal function. Vascular stenosis or occlusion may result in renal ischemia or necrosis. Moreover, atherosclerosis-induced blood circulation disorders can further reduce the glomerular filtration rate, accelerating the progression of renal failure21,22,23,24. Consequently, chronic renal failure and atherosclerosis have a reciprocal and promotive relationship, with atherosclerosis potentially hastening the progression of chronic renal failure.
Klotho is a protein predominantly expressed in the kidneys, brain, and other tissues, existing in two key forms: membrane-bound Klotho (mKlotho) and soluble Klotho (sKlotho). mKlotho primarily functions on cell membranes, whereas sKlotho is released into the bloodstream. Klotho protein fulfills several critical physiological roles. It is involved in regulating phosphate metabolism in the kidneys, maintaining phosphate balance in the body by inhibiting phosphate absorption and promoting urinary excretion. Additionally, Klotho modulates sodium transport in renal tubules, influencing water and salt balance. It also participates in calcium metabolism and skeletal growth regulation25,26,27,28,29,30.
Moreover, Klotho exhibits systemic anti-aging and anti-inflammatory properties. It can inhibit inflammatory responses, reduce oxidative stress, and decrease cell apoptosis, thereby protecting cells and tissues from damage. Research has increasingly linked Klotho to the pathogenesis and progression of various diseases, including cardiovascular disease, diabetes, chronic kidney disease, and Alzheimer’s disease. In this study, Oil Red O and HE staining demonstrated that Klotho reduced lipid accumulation in the aorta. Immunohistochemical staining revealed that Klotho attenuated the size of atherosclerotic plaques in chronic renal failure via macrophages, but did not affect smooth muscle cells.
Urea, a metabolic byproduct of amino acids produced by the liver, is primarily excreted by the kidneys to maintain nitrogen balance in the body. Elevated urea levels often indicate impaired kidney function or other renal disorders; thus, urea levels are frequently utilized to assess kidney health31. Cholesterol, a lipid essential for building cell membranes and synthesizing hormones and vitamin D, can lead to atherosclerosis when excessively deposited in the arterial walls7. Calcium ions are crucial signaling molecules involved in nerve transmission, muscle contraction, and cell apoptosis. However, vascular intima damage can cause calcium ions to deposit in the vessel wall, contributing to atherosclerotic plaque formation32. Triglycerides, synthesized from fatty acids and glucose absorbed by the intestines, are stored in adipocytes for energy. Elevated triglyceride levels, associated with dietary habits, obesity, and metabolic disorders, may increase the risk of atherosclerosis and cardiovascular disease30,33. In this study, ELISA results showed that Klotho decreased plasma concentrations of urea, cholesterol, calcium ions, and triglycerides in mice with chronic renal failure and atherosclerosis.
To elucidate Klotho’s mechanism of action, we employed Western blotting in in vivo experiments to detect the relative protein expression levels of p-PERK, NOX2, NOX4, p-SHP1, Caspase-3, Caspase-9, Bax, p-GRK2, p-PLCβ, p-Src, and p-IP3R. Results indicated that Klotho significantly inhibited the relative protein expression levels of p-PERK, NOX2, NOX4, Caspase-3, Caspase-9, Bax, p-GRK2, p-PLCβ, p-Src, and p-IP3R in AS. It also increased p-SHP1 expression. Studies have shown that Klotho can mitigate oxidative stress by enhancing superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) activities. Oxidative stress can modify SHP1 through cysteine disulfide oxidation, reducing its tyrosine phosphatase activity and, thus, its substrate phosphorylation regulation. SHP1 can directly interact with Src and phosphorylate its tyrosine sites, inhibiting Src activity16. GRK2, a protein kinase in the G protein-coupled receptor kinase family, can be phosphorylated by Src, promoting phosphorylation of PLCβ and IP3R, which are related to cell signaling and calcium influx34,35,36. This relieves ER stress, thereby inhibiting oxidative stress and cell apoptosis. Furthermore, we validated the conclusions drawn from in vivo experiments through in vitro experiments. However, this study also needs to conduct clinical experiments to verify the results to enhance the accuracy of the experimental results.
In summary, Klotho enhances the stability of atherosclerotic plaques in chronic renal failure combined with atherosclerosis by inhibiting the ROS/SHP1 pathway and downregulating GRK2/PLC-β-mediated ER stress in macrophages. This provides new therapeutic methods and targets for the study of chronic renal failure combined with atherosclerosis.
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
References
Kłosowicz, M. et al. Biomarkers that seem to have the greatest impact on promoting the formation of atherosclerotic plaque in current scientific research [J]. Curr. Issues Mol. Biol. 46(9), 9503–9522. (2024).
ZWAENEPOEL, B. et al. Predictive value of protein-bound uremic toxins for heart failure in patients with chronic kidney disease [J] (ESC heart failure, 2023).
ZUBOVIC S V, KRISTIC, S. et al. PREVLJAK S, Chronic Kidney Disease and Lipid Disorders [J]. Medical archives (Bosnia Herzegovina), 70(3): 191–192. (2016).
XU, C. et al. Novel perspectives in chronic kidney disease-specific cardiovascular disease [J]. Int. J. Mol. Sci., 25(5), 2658 (2024).
MOHAMED O N et al. The Relationship of Fetuin-A with Coronary Calcification, Carotid Atherosclerosis, and Mortality Risk in Non-Dialysis Chronic Kidney Disease [J]. J. lipid atherosclerosis. 13 (2), 194–211 (2024).
HUANG P Y, HSU B G, LIN, Y. L. et al. Serum Lipoprotein(a) Levels as a Predictor of Aortic Stiffness in Patients on Long-Term Peritoneal Dialysis [J]. Med. Sci. monitor: Int. Med. J. experimental Clin. Res. 30, e944348 (2024).
ZYSSET, D. & WEBER, B. TREM-1 links dyslipidemia to inflammation and lipid deposition in atherosclerosis [J]. Nat. Commun. 7, 13151 (2016).
ZUO, P. et al. Protease-Activated Receptor-2 Deficiency Attenuates Atherosclerotic Lesion Progression and Instability in Apolipoprotein E-Deficient Mice [J]. Front. Pharmacol. 8, 647 (2017).
ZUNIGA, M. C. & RAGHURAMAN, G. PKC-epsilon and TLR4 synergistically regulate resistin-mediated inflammation in human macrophages [J]. Atherosclerosis 259, 51–59 (2017).
ZUBKIEWICZ-KUCHARSKA A, WIKIERA, B. Soluble Klotho Is Decreased in Children With Type 1 Diabetes and Correlated With Metabolic Control [J]. Front. Endocrinol. 12, 709564 (2021).
ZOU, D. et al. The role of klotho in chronic kidney disease [J]. BMC Nephrol. 19 (1), 285 (2018).
ZHU, Y. et al. DNA methylation-mediated Klotho silencing is an independent prognostic biomarker of head and neck squamous carcinoma [J]. Cancer Manage. Res. 11, 1383–1390 (2019).
ZHU, H. et al. Klotho Improves Cardiac Function by Suppressing Reactive Oxygen Species (ROS) Mediated Apoptosis by Modulating Mapks/Nrf2 Signaling in Doxorubicin-Induced Cardiotoxicity [J]. Med. Sci. monitor: Int. Med. J. experimental Clin. Res. 23, 5283–5293 (2017).
BI, J. et al. Relationships of serum FGF23 and α-klotho with atherosclerosis in patients with type 2 diabetes mellitus [J]. Cardiovasc. Diabetol. 23 (1), 128 (2024).
YAN, Y. et al. 1-Pyrroline-5-carboxylate released by prostate Cancer cell inhibit T cell proliferation and function by targeting SHP1/cytochrome c oxidoreductase/ROS Axis [J]. J. Immunother. Cancer. 6 (1), 148 (2018).
ZHUANG, X. et al. SHP-1 knockdown suppresses mitochondrial biogenesis and aggravates mitochondria-dependent apoptosis induced by all trans retinal through the STING/AMPK pathways [J]. Molecular medicine (Cambridge, Mass), 28(1): 125. (2022).
ZHUANG, X. et al. SHP-1 suppresses endotoxin-induced uveitis by inhibiting the TAK1/JNK pathway [J]. J. Cell. Mol. Med. 25 (1), 147–160 (2021).
ZHOU, X. et al. Leishmania infantum-chagasi activates SHP-1 and reduces NFAT5/TonEBP activity in the mouse kidney inner medulla [J]. Am. J. Physiol. Ren. Physiol. 307 (5), F516–F524 (2014).
ZHOU, R. & CHEN, Z. HAO D, et al. Enterohemorrhagic Escherichia coli Tir inhibits TAK1 activation and mediates immune evasion [J]8734–748 (Emerging microbes & infections, 2019). 1.
ZOCCALI, C. et al. Cardiovascular complications in chronic kidney disease: a review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association [J]. Cardiovascular. Res. 119 (11), 2017–2032 (2023).
ZHAO, B. et al. The relationship between sclerostin and carotid artery atherosclerosis in patients with stage 3–5 chronic kidney disease [J]. Int. Urol. Nephrol. 52 (7), 1329–1336 (2020).
MATHEW A V, Z. E. N. G. L. et al. Myeloperoxidase-derived oxidants damage artery wall proteins in an animal model of chronic kidney disease-accelerated atherosclerosis [J]. J. Biol. Chem. 293 (19), 7238–7249 (2018).
YU et al. Association Between Midlife Obesity and Kidney Function Trajectories: The Atherosclerosis Risk in Communities (ARIC) Study [J]. Am. J. kidney diseases: official J. Natl. Kidney Foundation. 77 (3), 376–385 (2021).
WATRAL, J. et al. Comprehensive proteomics of monocytes indicates oxidative imbalance functionally related to inflammatory response in chronic kidney disease-related atherosclerosis [J]. Front. Mol. Biosci. 11, 1229648 (2024).
ZHOU, X. et al. Klotho, an anti-aging gene, acts as a tumor suppressor and inhibitor of IGF-1R signaling in diffuse large B cell lymphoma [J]. J. Hematol. Oncol. 10 (1), 37 (2017).
ZHOU, S. et al. The Anti-aging hormone klotho promotes retinal pigment epithelium cell viability and metabolism by activating the AMPK/PGC-1α pathway [J]. Antioxid. (Basel Switzerland), 12(2), 385 (2023).
ZHOU W, CHEN M M, L. I. U. H. L. et al. Dihydroartemisinin suppresses renal fibrosis in mice by inhibiting DNA-methyltransferase 1 and increasing Klotho [J]. Acta Pharmacol. Sin. 43 (10), 2609–2623 (2022).
ZHOU, L. et al. Wnt/β-catenin links oxidative stress to podocyte injury and proteinuria [J]. Kidney Int. 95 (4), 830–845 (2019).
EDMONSTON, D. & GRABNER, A. FGF23 and klotho at the intersection of kidney and cardiovascular disease [J]. Nat. reviews Cardiol. 21 (1), 11–24 (2024).
LIU, J. et al. Klotho exerts protection in chronic kidney disease associated with regulating inflammatory response and lipid metabolism [J]. Cell. bioscience. 14 (1), 46 (2024).
ZUO, Z. et al. Acupuncture attenuates renal interstitial fibrosis via the TGF–β/Smad pathway [J]. Mol. Med. Rep. 20 (3), 2267–2275 (2019).
ZHOU, Y. & GREKA, A. Calcium-permeable ion channels in the kidney [J]. Am. J. Physiol. Ren. Physiol. 310 (11), F1157–F1167 (2016).
ZIMMER, M. et al. CAT-2003: A novel sterol regulatory element-binding protein inhibitor that reduces steatohepatitis, plasma lipids, and atherosclerosis in apolipoprotein E*3-Leiden mice [J]. Hepatol. Commun. 1 (4), 311–325 (2017).
YU, L. et al. Src couples estrogen receptor to the anticipatory unfolded protein response and regulates cancer cell fate under stress [J]. Biochim. et Biophys. acta Mol. cell. Res. 1867 (10), 118765 (2020).
SALYER L G et al. Troponin I Tyrosine Phosphorylation Beneficially Accelerates Diastolic Function [J]. Circul. Res. 134 (1), 33–45 (2024).
VROMAN, R. et al. FULLAM S,. Reduced Capsaicin-Induced Mechanical Allodynia and Neuronal Responses in the DRG in the Presence of Ptpn6 Overexpression [J]. Molecular pain, : 17448069241258106. (2024).
Acknowledgements
The authors thank all the participants of the present study for their contribution.
Funding
This work was funded by the Science and Technology Projects in Baoding (NO.2241ZF302).
Author information
Authors and Affiliations
Contributions
Z.L. and J.Li. were jointly responsible for the experimental design, data collection and analysis, and co-authored the research paper. Q.W. and Q.Z. assisted in the processing and analysis of the experimental data and provided important technical support. L.L. was responsible for supervising and guiding the experimental program, reviewing and revising the research methods and results. L.T. and C.L. were responsible for the design and execution of the overall research program, monitoring the experimental progress, and ultimately reviewing and revising the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Supplementary Information
The data for this research institute is in Supplementary File 1.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Z., Li, J., Li, L. et al. Klotho enhances stability of chronic kidney disease atherosclerotic plaques by inhibiting GRK2/PLC-β-mediated endoplasmic reticulum stress in macrophages via modulation of the ROS/SHP1 pathway. Sci Rep 14, 32091 (2024). https://doi.org/10.1038/s41598-024-83596-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83596-w