Abstract
To evaluate the diagnostic performance of ultrasound-derived fat fraction (UDFF) and clinical prediction models in assessing hepatic steatosis grades in MASLD patients, with liver biopsy as reference standard.A total of 85 obese patients who were found to have fatty liver by B-mode ultrasound and underwent UDFF measurement, with liver biopsy available, were enrolled. The diagnostic performance of UDFF, clinical prediction models including fatty liver index (FLI), hepatic steatosis index (HSI), ZheJiang University index (ZJU index) and lipid accumulation product (LAP) for hepatic steatosis was assessed. The areas under receiver operating characteristics curves (AUROCs) were utilized to determine the diagnostic efficacy. DeLong test was used to compare the diagnostic performance of different noninvasive methods for hepatic steatosis. The UDFF values of S1, S2 and S3 groups were 17.53 ± 7.49%, 25.00 ± 5.41% and 27.58 ± 6.55%, respectively (P < 0.001). UDFF values were significantly positively correlated with histologic steatosis grades (r = 0.531, P < 0.001). In the ≥ S2 group, the AUROC of UDFF was higher than that of FLI and LAP (P < 0.05), but not significantly different from that of HSI and ZJU index (P > 0.05). In the ≥ S3 group, the AUROC of UDFF was higher than that of FLI, ZJU index and LAP (P < 0.05), but not significantly different from that of HSI (P > 0.05). The UDFF proves effective in assessing hepatic steatosis in patients with MASLD, and its diagnostic efficacy exceeded that of FLI and LAP, but there was no significant difference with HSI, ZJU index in the ≥ S2 group, and with HSI in the ≥ S3 group.
Similar content being viewed by others
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease1, has emerged as the most common chronic liver disease worldwide and has become a significant global public health concern2. In recent years, the prevalence of MASLD has increased rapidly, with nearly 30% of patients with MASLD worldwide suffer from metabolic-associated steatohepatitis (MASH)3. A meta-analysis involving approximately 8 million individuals revealed that obese patients account for a significant proportion of MASLD and MASH cases, with 51% of MASLD patients being obese4.
Liver biopsy is currently the reference standard for diagnosing hepatic steatosis5. However, it has certain limitations, such as invasiveness, sampling error, infection risk and other issues6,7. It has been reported that Magnetic resonance imaging-proton density fat fraction (MRI-PDFF) is closely correlated to the hepatic histologic steatosis grades (HSG)8. Nevertheless, its high price, long scanning time and poor tolerability limit its clinical application.
Therefore, there is an urgent need for a noninvasive and highly accurate method of assessing hepatic steatosis. The UDFF is capable of reflecting the liver fat content by measuring the attenuation degree in liver tissue, providing a novel diagnostic and assessment tool for MASLD. Previous studies have demonstrated the good repeatability and reliability of UDFF9,10,11, but few studies have analyzed the correlation between UDFF values and clinical indicators, as well as conducting comparative analysis with liver biopsy results.
In addition, clinical prediction models, such as the fatty liver index (FLI)12, hepatic steatosis index (HSI)13, Zhejiang University (ZJU) index14, and lipid accumulation index (LAP)15, also play an important role in the assessment of MASLD. These models incorporate several clinical and biochemical indicators that, through specific calculation formulas, can be used to assess hepatic steatosis.
The aim of this study was to analyze the diagnostic performance in different degrees of hepatic steatosis using liver biopsy as the gold standard, as well as the correlation between these noninvasive assessment methods and various clinical indicators.
Materials and methods
Study cohort
The study protocol was in compliance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and was approved by the ethics committee of The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital (No. YXLL-KY-2024(014)). Obese individuals, who were found to have fatty liver by B-mode ultrasound and underwent UDFF measurement, along with liver biopsy results, were retrospectively selected from The First Affiliated Hospital of Shandong First Medical University between April 2023 and March 2024. B-mode ultrasound was used to assess hepatic steatosis by liver parenchymal brightness, hepatic and renal echo contrast, depth of attenuation, and vascular blurring based on the Hamaguchi score and to diagnose hepatic steatosis in patients with a Hamaguchi score of ≥ 216. The inclusion criteria were as follows: (1) BMI>30 kg/m²; (2) diffuse fatty liver or inhomogeneous fatty liver diagnosed by conventional B-mode ultrasound; (3) serologic test results were available; (4) Liver biopsy showed hepatic steatosis. The exclusion criteria include: (1) patients who have undergone liver resection or have liver space-occupying lesions; (2) pregnant women; (3) patients with incomplete medical history data.
Clinical data and the acquisition of liver biopsy results
Clinical data collection: General clinical data including gender, age, body mass index (BMI), waist circumference (WC) and hip circumference (HC) were obtained from medical records. Serologic test results include: fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT).
Liver biopsy results were obtained by reviewing the patient’s medical records, and the corresponding HSG was recorded. The histologic hepatic fat fraction was graded as follows: S1, 5–33%; S2, 33–66%; S3, > 66%. Liver biopsy tissues were from patients who underwent bariatric surgery at the First Affiliated Hospital of Shandong First Medical University. Surgeons performed wedge liver biopsies from the margins of the left lobe of the liver under direct visualisation after bariatric surgery and subsequently sent the liver tissue to the pathology department for analysis by an experienced pathologist. The indications for liver biopsy were: patients with a definitive diagnosis of MASLD and preoperative examinations suggesting fatty liver.
UDFF evaluation
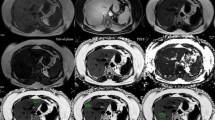
In the present study, all UDFF examinations were performed by two physicians, with over five years of experience in ultrasound imaging. The Siemens ACUSON Sequoia diagnostic ultrasound system, equipped with a standard abdominal probe and a specialized DAX probe designed for deep penetration, was utilized. Prior to the UDFF measurement, participants were instructed to fast for 4 h and then examined in the supine position. Following the conventional B-mode ultrasound examination, patients were coached through a series of breath-holding exercises. During the examination, the DAX probe was positioned in the right intercostal space, and the UDFF mode was selected to place a fixed-size region of interest (ROI) vertically in the right lobe of the liver, 1.5 cm away from the liver capsule, avoiding the intrahepatic duct structure. Measurements were acquired after the patient maintained a calm breathing pattern, and the system automatically determined the shear wave velocity (Vs), elastic modulus (E), and UDFF values (Fig. 1). A total of five separate measurements were performed in the right lobe of the liver, and the median UDFF value was recorded for subsequent analysis.
Vs, velocity; E, elasticity; UDFF, ultrasound-derived fat fraction.
Clinical prediction models for MASLD
FLI was calculated using BMI, WC, TG and GGT as the main parameters, with a total score of 0–100. The FLI is valuable for the initial screening of MASLD in the general population. When the FLI is < 30, MASLD can be ruled out, and when the FLI is ≥ 60, MASLD can be diagnosed (from an Italian population)12.
FLI=(e0.953*loge (TG)+0.139*BMI+0.718*loge (GGT)+0.053*(WC)−15.745)/(1 + e0.953*loge (TG)+0.139*BMI+0.718*loge(GGT)+0.053*(WC)−15.745)*100.
HSI is related to AST/ALT ratio, BMI, gender and diabetes mellitus, and can be used to evaluate the possibility of MASLD. An HSI < 30 usually rules out MASLD, whereas an HSI > 36 may indicate the presence of MASLD (from Korean population)13. Diabetes diagnosis: fasting blood glucose ≥ 7.0 mmol/L or 2 h postprandial blood glucose ≥ 11.1 mmol/L or random blood glucose ≥ 11.1 mmol/L, and accompanied with the typical symptoms of diabetes mellitus (e.g., polydipsia, polyuria, weight loss, etc.).
HSI = 8 * ALT/AST ratio + BMI (+ 2, if female;+ 2, if diabetes mellitus).
ZJU index consists of 4 parameters: BMI, FPG, TG and ALT/AST ratio.
ZJU index = BMI + FPG + TG + 3 * ALT/AST ratio (+ 2, if female).
The ZJU index is a useful model for detecting MASLD by community physicians in China. When the value is < 32, the ZJU index can exclude MASLD with a sensitivity of 92.2%. Conversely, when the value is > 38, the ZJU index can detect MASLD with a specificity of 93.4%14.
LAP is a simplified algorithm that considers gender, WC and TG in order to identify patients with hepatic steatosis. The risk of MASLD is found to increase with higher LAP levels15.
LAP = (WC-65)×TG, if male; LAP = (WC-58)×TG, if female.
Statistical analysis
Spearman’s correlation coefficient was employed to evaluate correlations between UDFF and HSG, MASLD clinical prediction models and HSG, while the correlation with each of the other clinical indicators was analyzed using Pearson’s correlation coefficient. An r-value ≥ 0.7 indicates a high positive correlation, 0.3 ≤ r-value < 0.7 indicates a moderate positive correlation, and an r-value < 0.3 indicates a low positive correlation. Comparisons between groups were made by one-way ANOVA, and two-way comparisons between groups were made using the Mann-Whitney U test. Independent predictors of UDFF were identified by multivariable linear regression models. Multicollinearity was defined by a variance inflation factor higher than 5. When steatosis was diagnosed according to HSG rather than UDFF, it was defined as an inconsistency between HSG and UDFF. In the inconsistency assessment, the UDFF cutoff identified in this study was examined. Discordant risk factors were identified by multivariate logistic regression models. To test the diagnostic value of UDFF and clinical prediction models, receiver operating characteristic curves (ROC) were constructed by liver biopsy as the reference method. DeLong test was used to compare the diagnostic performance of different non-invasive methods for the same grade of fatty liver degeneration. Statistical analysis was performed with SPSS version 25.0 and GraphPad Prism version 5.0. Data were presented as mean ± SD. A P-value < 0.05 indicates significant difference.
Results
Clinical features of the study cohort
A total of 85 participants were enrolled in this study, aged 18–61 years, with a mean age of 30.70 ± 8.37 years; BMI 32.20–60.94 kg/m2, with a mean BMI of 40.99 ± 6.25 kg/m2. Of the participants, 22 (26%) were male and 63 (74%) were female. According to the histopathologic analysis, there were 28 cases of S1 grade (S1 group), 26 cases of S2 grade (S2 group), and 31 cases of S3 grade (S3 group). The UDFF values gradually increased in the S1, S2 and S3 groups and the difference was statistically significant (all P < 0.01) (Fig. 2). A two-way comparison between groups showed that the differences in UDFF measurements between groups S1, S2 and S3 were statistically significant (P < 0.05). The differences in age, HC, FPG, TG, TC, HDL, LDL, ALT, AST, ALP, GGT and LAP were not statistically significant between groups (all P > 0.05) (Table 1).
Horizontal brackets over plots indicate statistical significance as determined by the Mann–Whitney test. *P < 0.05; **P < 0.01; ***P < 0.001; and NS, not significant.
UDFF, ultrasound-derived fat fraction.
BMI, body mass index; WC, Waist circumference; HC, Hip circumference; FPG, fasting plasma glucose; TG, Triglyceride; TC, Total Cholesterol; HDL, high-density lipoprotein; LDL, low‐density lipoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; FLI, fatty liver index; HSI, hepatic steatosis index; ZJU: ZheJiang University; LAP, lipid accumulation product; UDFF, ultrasound‐derived fat fraction. Compared with the S1 group, aP<0.05;Compared with the S2 group, bP<0.05;Compared with the S3 group, cP<0.05; Values in bold are statistically significant figures.
Correlation of UDFF with clinical variables
Spearman correlation test showed that there was a significant positive correlation between UDFF and HSG (r = 0.531, P < 0.001), whereas FLI, HSI and ZJU index were weakly correlated with HSG (r = 0.265, 0.312, 0.259, P < 0.05). And there was no correlation between LAP and HSG (r = 0.100, P = 0.362).
Pearson correlation analysis showed that UDFF was weakly correlated with BMI, HSI and ZJU index (r = 0.224, 0.236, 0.264, P = 0.039, 0.030, 0.015), but there was no correlation with other clinical indicators. The correlation analysis between UDFF value and variables is shown in Table 2.
In the multivariate regression model including age, gender, HSG, BMI, waist circumference, hip circumference and AST, the independent predictors of UDFF were HSG (beta coefficient 0.535, P < 0.001) and BMI (beta coefficient 0.224, P = 0.039) (Table 3).
HSG, hepatic histologic steatosis grades; BMI, body mass index; WC, Waist circumference; HC, Hip circumference; FPG, fasting plasma glucose; TG, Triglyceride; TC, Total Cholesterol; HDL, high-density lipoprotein; LDL, low‐density lipoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; FLI, fatty liver index; HSI, hepatic steatosis index; ZJU: Zhe Jiang University; LAP, lipid accumulation product ; UDFF, ultrasound‐derived fat fraction.
Values in bold are statistically significant figures.
HSG, hepatic histologic steatosis grades; BMI, body mass index; WC, Waist circumference; HC, Hip circumference; AST, aspartate aminotransferase; UDFF, ultrasound-derived fat fraction.
Values in bold are statistically significant figures.
Performance of UDFF and clinical prediction models for different grades of hepatic steatosis
The diagnostic performance of various noninvasive methods for different degrees of hepatic steatosis was performed by ROC curves using liver biopsy as the reference standard. In the ≥ S2 group, the AUROC of UDFF was significantly higher than that of FLI and LAP(P < 0.05), but not significantly different from that of HSI and ZJU index(P > 0.05). In the ≥ S3 group, the AUROC of UDFF was higher than that of FLI, ZJU index and LAP(P < 0.05), but there was no significant difference between UDFF and HSI(P > 0.05) (Table 3; Fig. 3). The optimal cut-off values of UDFF based on the Youden index in the diagnosis of hepatic steatosis in ≥ S2 group and ≥ S3 group were 20.5% and 23.5%, the sensitivity were 86.0% and 81.0%, and the specificity were 68% and 65%, respectively. The diagnostic performance of UDFF and clinical prediction models was shown in Table 4.
AUROC, areas under receiver operating characteristics curve; CI, confidence interval; UDFF, ultrasound-derived fat fraction; FLI, fatty liver index; HSI, hepatic steatosis index; ZJU: Zhe Jiang University; LAP, lipid accumulation product.
P value is calculated using the DeLong test. Values in bold are statistically significant figures.
a. b.
ROC, receiver operating characteristic curve; UDFF, ultrasound-derived fat fraction; FLI, fatty liver index; HSI, hepatic steatosis index; ZJU: Zhe Jiang University; LAP, lipid accumulation product.
Discordance factors between hepatic steatosis diagnosed by UDFF and HSG
Using the threshold values derived from this study (≥ S2, UDFF = 20.5%; ≥S3, UDFF = 23.5%), discordance between HSG and UDFF was observed in 30 (35%) individuals. The results showed that UDFF underestimated steatosis in 9 patients and overestimated steatosis in 21 patients. The results of the multivariate regression analyses indicated that lower age was an independent risk factor for underestimation of steatosis, while no risk factors were associated with overestimation of steatosis (Table 5).
BMI, body mass index; WC, Waist circumference; HC, Hip circumference; AST, aspartate aminotransferase; UDFF, ultrasound-derived fat fraction.
Values in bold are statistically significant figures.
Discussion
A number of studies have reported that the prevalence of MASLD is on the rise, with a strong correlation to the emergence of various ailments, including metabolic syndrome and cardiovascular disease17. This is one of the main causes for the progression of chronic liver disease into cirrhosis and hepatocellular carcinoma18. Consequently, it is of paramount importance to implement early identification, diagnosis and treatment of fatty liver, which can effectively prevent the progression of MASLD and reduce adverse disease outcomes.
As the current first-line approach for the detection of MASLD in adults5, B-mode ultrasound examination is widely used in clinical practice. Nevertheless, conventional B-mode ultrasound diagnosis of fatty liver is significantly influenced by operator subjectivity and lacks consistency in grading the diagnosis of fatty liver19. In recent years, several methods for hepatic fat quantification have been developed, including attenuation imaging (ATI)20, controlled attenuation parameter (CAP)21, and ultrasound-guided attenuation parameter (UGAP)22. Among these techniques, ATI, a method developed by Canon in Japan, is characterized by simplicity and reproducibility among operators for the quantitative detection of hepatic fat content23. However, ATI needs to manually adjust the region of interest for measurement, and ATI displays the average attenuation coefficient (AC) in dB/cm/MHz, which cannot directly output the percentage of hepatic fat fraction. FibroScan can be used to measure CAP, a noninvasive and rapid technique to assess the degree of hepatic fibrosis and steatosis. Nevertheless, CAP has limitations, such as the varying criteria for fatty liver grading reported in different studies24. Compared to CAP, UGAP has a higher sensitivity and specificity for diagnosing grades of hepatic steatosis. However, it requires a reference phantom method for later calculation and does not provide attenuation imaging25. In contrast, the UDFF carried by the Siemens Sequoia ultrasound system can automatically calculate the attenuation coefficient and backscatter coefficient, integrate the model data into the ultrasound system, and directly output the percentage of hepatic fat fraction without external verification after scanning. It is an ultrasound technology that uses the same measurement unit (%) as MRI-PDFF and has good consistency with MRI-PDFF9. In addition, patients with fatty liver have a larger liver volume and less interference by intrahepatic pipelines. UDFF can perform breath-holding training on the premise of ultrasound visualization, minimize out-of-plane motion artifacts, and greatly improve the stability of operation.
Previous studies have shown that the FLI, HSI, ZJU index, LAP and other clinical diagnostic models each have their own advantages. The FLI can rapidly and easily evaluate the degree of liver steatosis, while the HSI can accurately reflect the state of liver fibrosis. The ZJU index and LAP have demonstrated unique value in the early screening of MASLD12,13,14,15.
The result of our study showed that UDFF values were significantly correlated with hepatic steatosis grade (r = 0.531, P < 0.001). As the degree of steatosis increases, the UDFF values correspondingly increase, and there are statistically significant differences in UDFF values between different groups (P< 0.05), which indicated that UDFF values were reliable indicators of different degrees of hepatic steatosis. This is consistent with the results of previous studies9,10,11. The results of this study show that FLI, HSI, and ZJU had a weak correlation with HSG (r = 0.265, 0.312, 0.259, P < 0.05), whereas LAP had no statistically significant correlation with HSG (r = 0.100, P= 0.362). This may be because LAP has mainly been studied in the United States and the formula for calculating LAP is simple, taking into account only WC, TG and gender, and is not accurate enough for people with MASLD who have metabolic abnormalities in a variety of serological markers. Previous studies have described LAP as a simple screening indicator for the presence of MASLD, and therefore it may not be suitable for stratified diagnosis of MASLD. In addition, the use of ultrasound rather than liver biopsy results to determine the presence or absence of MASLD in almost all previous studies may be an important reason for the discrepancy in results15,26. Further analysis of the correlation revealed that UDFF had a weak correlation with BMI (r = 0.224, P = 0.039) and no correlation with other indicators (P < 0.05), as reported by Dillman et al.22. This indicates that UDFF can better quantify the degree of hepatic steatosis than the aforementioned indicators. Similarly, we demonstrate that hepatic histologic steatosis grades and BMI are independent predictors of UDFF after correction for several confounders.
In addition to these findings, when constructing the ROC curves for UDFF and clinical prediction models respectively, we found that the AUROC of UDFF in diagnosing different grades of fatty liver was ≥ 0.75, which was consistent with the findings of Yassin Labyed10et al. It indicated that UDFF may provide a better way to diagnose hepatic steatosis grade. In addition, the diagnostic performance of UDFF was higher than that of FLI and LAP in the ≥ S2 group, and that of UDFF was higher than that of FLI, ZJU index and LAP in the S3 group; however, there was no statistically significant difference between the AUC of HSI and that of UDFF in the ≥ S2 and ≥ S3 groups, and there was no statistically significant difference between the AUCs of UDFF and the ZJU index for the diagnosis of hepatic steatosis in the ≥ S2 group. A study by Tavaglione et al. showed that UDFF performed better than FLI and HSI in diagnosing high-risk populations with MASLD27, but there was no statistically significant difference between the AUC of HSI and the AUC of UDFF in the present study (P> 0.05). This may be due to the fact that Tavaglione’s study population was a European population at high risk of MASLD, whereas the present study was an Asian population with MASLD, and Lee JH has shown that HSI may perform better in Asian populations13. However, UDFF, as a non-invasive and convenient ultrasound technique, facilitates the screening and monitoring of fatty liver compared to HSI, as UDFF measurements do not require the patient’s general clinical characteristics and serological indices to be obtained, and the fat content of the liver can be quickly obtained without calculations while the liver is being visualised. Furthermore, the difference between the AUC of UDFF in diagnosing hepatic steatosis in the ≥ S2 group and the AUC of ZJU index in diagnosing hepatic steatosis was not statistically significant (P< 0.05). In a study of 148 patients with liver biopsy results, the ZJU index was considered suitable for screening for MASLD28, but did not grade hepatic steatosis. The results of this study showed that the ZJU index did not perform significantly differently from UDFF in the ≥ S2 group, but performed poorly in the ≥ S3 group. However, the study results of Yassin Labyed et al. demonstrated that the cut-off values of ≥ S2 group and ≥ S3 group were 15.9% and 16.1%, respectively, which were significantly lower than those in our study. The reason for this can be analysed as the inclusion of subjects with BMI > 30 kg/m2 and all diagnosed with MASLD in our study, which is related to the difference in the inclusion of subjects and the degree of hepatic steatosis.
Finally, we examined independent risk factors associated with discrepancy (over or underestimation) between steatosis diagnosed by UDFF and HSG. We found that UDFF may overestimate or underestimate the degree of hepatic steatosis when HSG is used as a diagnostic criterion, with overestimation being more common. We found that lower age was an independent risk factor for underestimation of steatosis, while no risk factors were associated with overestimation of steatosis. In a study by Captan et al. of hospitalised patients with type 2 diabetes, liver fat was found to average 8.9% in the right lobe and 7.7% in the left lobe (P< 0.001)29.Similarly, another study showed that in non-diabetic subjects, the right lobe of the liver contains more fat than the left lobe and correlates better with components of the metabolic syndrome, and that quantification of fat in the right lobe may allow a more accurate diagnosis of MASLD30. While UDFF measurements were performed on the right lobe in the present study, liver biopsy tissue was obtained from the left lobe, which may explain the greater tendency of UDFF to overestimate the degree of hepatic steatosis compared to liver biopsy in inconsistent cases.
The advanced nature of our study is as follows: (1) According to the liver biopsy as a standard, the superiority of UDFF in diagnosing different degrees of hepatic steatosis has been validated; (2) We have compared the diagnostic performance of UDFF and clinical prediction models based on serological indicators for diagnosing hepatic steatosis.
This study also has some limitations. (1) The sample size of this study is small and the study population was relatively homogeneous. Future studies should further expand the sample size to cover a wider range of study populations and evaluate the application value of UDFF and clinical indicators more comprehensively. (2) Other ultrasound indicators, such as shear wave velocity, were not included in this study, and these indicators can be combined for multimodal analysis in subsequent studies.
In conclusion, UDFF, as a noninvasive ultrasound technique, significantly correlates with the degree of hepatic histologic steatosis grades, and has a higher diagnostic performance than FLI, and LAP for grading fatty liver in MASLD, which is expected to provide more information for non-invasive clinical quantification of the degree of hepatic steatosis.
Data availability
The data that support the findings of this study are available from our Ultrasound Clinic but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of our Ultrasound Clinic. If anyorganization or individual wants to request the data from this study, please contact Meng Sun at simplesimon0224@163.com.
References
Fowler, K. J. & Chernyak, V. What Is in a Name: Understanding the Nomenclature Updates for Fatty Liver Disease. Radiology 310 (1), e232771 (2024).
Younossi, Z. M. et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 77 (4), 1335–1347 (2023).
Williams, C. D. et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140 (1), 124–131 (2011).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64 (1), 73–84 (2016).
Ferraioli, G. et al. Quantification of Liver Fat Content with Ultrasound: A WFUMB Position Paper. Ultrasound Med. Biol..
Ratziu, V. et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 128 (7), 1898–1906 (2005).
Bravo, A. A., Sheth, S. G. & Chopra, S. Liver biopsy. N Engl. J. Med. 344 (7), 495–500 (2001).
Tang, A. et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology.
Labyed, Y. & Milkowski, A. Novel Method for Ultrasound-Derived Fat Fraction Using an Integrated Phantom. J. Ultrasound Med. 39 (12), 2427–2438 (2020).
Gao, J. et al. Reliability of performing ultrasound derived SWE and fat fraction in adult livers. Clin. Imaging. 80, 424–429 (2021).
De Robertis, R. et al. Ultrasound-derived fat fraction for detection of hepatic steatosis and quantification of liver fat content. Radiol. Med. 128 (10), 1174–1180 (2023).
Bedogni, G. et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33 (2006). Published 2006 Nov 2.
Lee, J. H. et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 42 (7), 503–508 (2010).
Wang, J. et al. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci. Rep. 5, 16494 (2015). Published 2015 Nov 16.
Bedogni, G. et al. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 10, 98 (2010). Published 2010 Aug 25.
Hamaguchi, M. et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am. J. Gastroenterol. 102 (12), 2708–2715 (2007).
Tariq, R., Axley, P. & Singal, A. K. Extra-Hepatic Manifestations of Nonalcoholic Fatty Liver Disease: A Review. J. Clin. Exp. Hepatol. 10 (1), 81–87 (2020).
Eslam, M. et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 73 (1), 202–209 (2020).
European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 63 (1), 237–264 (2015).
Tada, T. et al. Usefulness of Attenuation Imaging with an Ultrasound Scanner for the Evaluation of Hepatic Steatosis. Ultrasound Med. Biol. 45 (10), 2679–2687 (2019).
Zeng, Q. et al. Controlled attenuation parameter by vibration-controlled transient elastography for steatosis assessment in members of the public undergoing regular health checkups with reference to magnetic resonance imaging-based proton density fat fraction. Hepatol. Res. 50 (5), 578–587 (2020).
Tada, T. et al. Utility of Attenuation Coefficient Measurement Using an Ultrasound-Guided Attenuation Parameter for Evaluation of Hepatic Steatosis: Comparison With MRI-Determined Proton Density Fat Fraction. AJR Am. J. Roentgenol. 212 (2), 332–341 (2019).
Yoo, J. et al. Reproducibility of ultrasound attenuation imaging for the noninvasive evaluation of hepatic steatosis. Ultrasonography 39 (2), 121–129 (2020).
Sirli, R. & Sporea, I. Controlled Attenuation Parameter for Quantification of Steatosis: Which Cut-Offs to Use? Can J Gastroenterol Hepatol. ; 2021:6662760. Published 2021 Mar 26. (2021).
Fujiwara, Y. et al. The B-Mode Image-Guided Ultrasound Attenuation Parameter Accurately Detects Hepatic Steatosis in Chronic Liver Disease. Ultrasound Med. Biol. 44 (11), 2223–2232 (2018).
Ebrahimi, M. et al. Lipid accumulation product (LAP) index for the diagnosis of nonalcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Lipids Health Dis. 22 (1), 41 (2023).
Tavaglione, F. et al. Non-invasive assessment of hepatic steatosis by ultrasound-derived fat fraction in individuals at high-risk for metabolic dysfunction-associated steatotic liver disease. Diabetes Metab. Res. Rev. 40 (3), e3787 (2024).
Wang, J. et al. ZJU index: a novel model for predicting nonalcoholic fatty liver disease in a Chinese population. Sci. Rep. 5, 16494 (2015).
Capitan, V. et al. Macroscopic heterogeneity of liver fat: an MR-based study in type-2 diabetic patients. Eur. Radiol. 22, 2161–2168 (2012).
Hua, B., Hakkarainen, A., Zhou, Y., Lundbom, N. & Yki-Järvinen, H. Fat accumulates preferentially in the right rather than the left liver lobe in non-diabetic subjects. Dig. Liver Dis. 50 (2), 168–174 (2018).
Author information
Authors and Affiliations
Contributions
All authors were involved in the conceptualisation and design of the study. The experiment design and optimization completed by M.Z. Patients contact and surgery were performed by Z.M.W. Case collection was completed by L.F.Q., L.M., and S.M. Data analysis was performed by L.F.Q. and S.M. The data collection completed by Z.X.R., S.M. Contacting patients was done by N.W. And the first draft of the manuscript was written by S.M., all authors commented on the previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, M., Zhong, M., Luo, F. et al. Noninvasive assessment of hepatic steatosis grades by ultrasound derived fat fraction in metabolic dysfunction associated steatotic liver disease. Sci Rep 14, 32044 (2024). https://doi.org/10.1038/s41598-024-83606-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-83606-x