Abstract
Since the discovery of the turbulence drag reduction phenomenon over 70 years ago, it has been recognized that the addition of small quantities of drag-reducing agents to fluids can significantly decrease wall shear stress, thereby enhancing fluid pumpability. In many applications, the fluids often contain salts, such as those used in fracturing processes within the petroleum sector. The aim of this study is to experimentally investigate the effects of salinity, flow rate, and polymer concentration on the drag reduction performance of sodium alginate in circular pipes. The experimental process was designed using Response Surface Methodology (RSM), and a predictive regression model for the drag reduction rate (DR) was developed. By comparing the predicted results with the experimental outcomes, we found that the accuracy of the predictive model is high, with the error controlled within ± 20%. To provide a more intuitive understanding of the effect of salinity on the drag reduction performance of sodium alginate, this paper introduces the innovative concept of Drag Reduction Inhibition Rate (ε). The results indicate that the drag reduction rate in saline solutions is generally lower than that in salt-free solutions. However, during the initial stage of turbulence, a small amount of salt facilitates the unfolding of sodium alginate molecules, thereby enhancing the drag reduction effect (ε > 100%). Following this initial turbulent phase, the inhibitory effect of salinity on the polymer becomes pronounced, causing the drag reduction inhibition rate to decrease from 115 to 20%. We propose that high concentrations of salt may restrict the extent of polymer unfolding, resulting in concentrated solutions degrading into dilute solutions.
Similar content being viewed by others
Introduction
The drag reduction effect, also known as the Toms effect, refers to the phenomenon whereby the addition of small quantities of polymers (such as polyacrylamide) in high-speed turbulent pipe flows significantly diminishes pipeline resistance. Research into the drag reduction phenomenon has evolved continuously over many years. Initially observed by Toms in 1947 during studies on polymer mechanical degradation in pipe flows, he formally presented his findings at the 1st International Congress on Rheology in 19481. Since that time, drag reduction has found extensive applications in pipeline transportation and ship design, as reducing fluid resistance enhances transport efficiency and decreases energy consumption2,3,4. A deeper understanding of the mechanisms behind drag reduction can yield more efficient and energy-saving solutions in industrial production, energy utilization, and environmental protection5,6.
In operations such as hydraulic fracturing, well cleaning, and drilling within the oil industry, significant frictional pressure losses occur when fluids are pumped through straight pipes, thereby limiting pump capacity. Common methods to reduce drag include optimizing pipeline design, applying coatings, controlling fluid velocity, and incorporating drag-reducing agents7,8. The use of drag-reducing agents does not necessitate large-scale modifications to the pipeline system, rendering it a straightforward and cost-effective approach. These agents are applicable to various fluids and pipeline systems, including oil, natural gas, and drilling mud. By selecting suitable drag-reducing agents, the specific needs of different fluids and systems can be effectively met. Consequently, drag-reducing agents have remained a focal point of research, encompassing the in-depth exploration of drag reduction mechanisms, the development of new agents, the expansion into novel applications, and technical optimization9,10,11.
In drag reduction mechanism research, the drag reduction rate is the most fundamental and critical metric. Most studies have concentrated on salt-free fluids across varying Reynolds numbers and pressure conditions12,13,14,15,16,17,18. However, there is limited research on drag reduction in saline (high salinity) fluids. In practical engineering contexts, most solutions contain salt, such as groundwater or river water used in winter heating systems, high-salinity geothermal water in geothermal well development, and fracturing fluids that incorporate salts like sodium salicylate and potassium nitrate to enhance viscosity and stability10,19.
Researchers have primarily focused on the effects of salt on synthetic drag-reducing agents commonly employed in engineering applications. Kamel et al.20 examined the impact of salinity on the drag reduction efficiency of anionic polyacrylamide and found that increased salinity diminished drag reduction efficiency, particularly under low Reynolds number conditions. This indicates that salinity influences different drag-reducing agents in varied ways, warranting further investigation. Other researchers have corroborated this observation. Kostrzewa et al.21 utilized a rheometer to measure the micellar solution of cetyltrimethylammonium bromide and sodium salicylate (CTAB/NaCl) and discovered that excess salt altered micelle motion characteristics, resulting in shear thinning of the solution. Shetty et al.13 employed dynamic light scattering to quantify the effect of salinity on the drag reduction performance of polyethylene oxide (PEO). They concluded that magnesium sulfate could manipulate PEO’s aggregation state, altering the critical conditions for turbulence drag reduction by 2.5 times.
In addition to synthetic agents, recent studies have focused on natural drag-reducing agents suitable for applications in food and agricultural irrigation. Xu et al.22 investigated the effect of NaCl on the drag reduction characteristics of xanthan gum (XG) solutions, finding that drag reduction efficiency significantly decreased with NaCl at high Reynolds numbers, while at low Reynolds numbers, drag reduction was enhanced. Bewersdorff et al.23 added NaCl to xanthan gum, a rigid drag-reducing agent, and found that salt reduced molecular self-association, enhancing the flexibility of the solution and modifying the drag reduction rate. Carmona et al.24 explored the effect of NaCl on the shear resistance of xanthan gum solutions under oscillatory shear and established a relationship between xanthan gum’s nonlinear viscoelasticity and NaCl content.
In summary, while previous research has examined the drag reduction characteristics of various agents in saline solutions, most have focused on synthetic agents like CTAB and xanthan gum. Few studies have investigated the drag reduction performance of sodium alginate. This study aims to explore the drag reduction properties of sodium alginate in saline solutions. Micron-sized straight circular tubes were selected for the drag reduction experiments, and Response Surface Methodology (RSM) was employed to establish a correlation equation between the drag reduction rate, flow rate, sodium alginate concentration, and salt mass fraction. To evaluate the effect of salinity on the drag reduction rate more intuitively, the novel concept of drag reduction inhibition rate (ε) was introduced. By analyzing the factors affecting the inhibition rate in sodium alginate saline solutions, this study offers guidance for engineering applications.
Experimental setup and fluid preparation
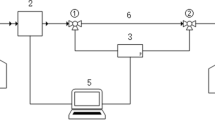
The experimental apparatus was modified based on the design of the research group’s previous equipment, as depicted in Fig. 125. The testing setup comprises the following components.
The setup includes a 5-liter transparent liquid storage tank, a constant-flow pump for circulating the liquid, and a flow system. The flow system features a test section that is 0.48 m long with an inner diameter of 1.85 millimeters. Additionally, the inlet and outlet sections of the flow system are 0.50 m and 0.40 m long, respectively, ensuring that the inlet section length exceeds 138 times the pipe diameter before reaching the test Sects26,27. A differential pressure sensor (EJA110E, YOKOGAWA) for measuring frictional resistance in straight pipe flow, with a maximum pressure measurement capacity of 50 kPa and an accuracy of ± 0.055%.
A thermostatic water bath to control the solution temperature, maintaining fluctuations within ± 0.5 °C and adjustable from room temperature to 80 °C. To prevent corrosion of the water bath and heating elements by the salts in the solution, a glass storage container was placed inside the water bath. Tests indicated that the solution reached the desired test temperature after being maintained in the water bath for 30 min. Data acquisition was conducted using a custom system to monitor temperature and pressure differential data. Experimental data were collected at one-second intervals, synchronized for storage in the controller, and displayed in real-time on the controller’s screen.
Sodium alginate is a natural high molecular weight polysaccharide composed of β-D-mannuronic acid (M units) and α-L-guluronic acid (G units) linked by (1,4)-glycosidic bonds, forming a linear, unbranched binary copolymer28,29. The research group has previously tested the drag reduction performance of sodium alginate aqueous solutions (salt-free), demonstrating excellent drag reduction capabilities30. However, no studies have been conducted on the biodegradability of sodium alginate. Therefore, the preparation of the solution should be completed as quickly as possible, and storage time should be minimized.
The sodium alginate utilized in this study was supplied by Fuchen Chemical Reagent Co., Ltd. (Tianjin). The preparation of the sodium alginate solution involved mixing sodium alginate with deionized water at room temperature using a low-speed stirrer until completely dissolved. For salinity tests, anhydrous Na₂SO₄ was added to the fully dissolved sodium alginate solution and stirred until the salt was completely dissolved. After stirring, the solution was allowed to stand for two hours to ensure complete dissolution of the polymer in water, thereby enhancing the accuracy of the experiments. The prepared sodium alginate saline solution was subsequently placed in a beaker within a thermostatic water bath, where the solution was maintained at 24 °C.
During the test, deionized water was initially passed through the test section, and the friction coefficient f, calculated from the flow pressure differential, was utilized as the system’s calibration baseline. The stabilized sodium alginate saline solution was subsequently pumped into the test system using a constant-flow pump, with the flow rate set to the required value. Once the pressure differential stabilized, data recording commenced. The test solution was collected in a waste tank and not recirculated, as the drag-reducing fluid is subject to degradation after shear exposure.
Results and discussions
Drag reduction flow analysis
The measured pressure differential data were converted to the Darcy friction factor fs using the following equation:
Where: l is the length of the test section, ΔP is the pressure differential in the test section (Pa), Ub is the average flow velocity in the pipe (m/s), d is the pipe diameter (m), and ρ is the fluid density (kg/m3). The density ρ is not treated as a variable in this experiment31.
The drag reduction rate (DR) is calculated as follows:
fs represents the friction factor of deionized water at a specific flow rate, and fp represents the friction factor of the sodium alginate solution at the same flow rate. The difference between fs and fp facilitates the calculation of the drag reduction rate, which quantitatively describes the drag-reducing effect under varying salt concentrations.
The flow velocity of the solution is characterized by the average Reynolds number (Re) :
\(\mu\) represents the viscosity of the solution. The solvent density and viscosity are considered under the same conditions as those of the experimental fluid. At 24 °C, the viscosity (\(\mu\)) of deionized wat er is 0.9142 mPa·s32.
Q represents the volumetric flow rate of the solution (ml/min).
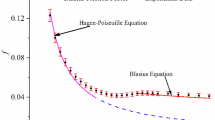
According to previous research33, for millimeter-scale circular pipes, the friction factor adheres to the Blasius equation34 when the Reynolds number exceeds 3000, marking the onset of the turbulent regime. Figure 2 illustrates the correlation between the friction factor of clean water and the Reynolds number. The friction factor of water aligns well with the Blasius equation, exhibiting errors within ± 5%. Furthermore, the friction factor of water also corresponds to the prediction of the Drew correlation35 for Newtonian fluids in turbulent flow within a smooth pipe. This confirms the strong consistency between the experimental data and theoretical predictions, thereby validating the reliability of the experimental results.
To investigate the effect of salinity on the drag reduction performance of sodium alginate, a series of solutions with varying salt concentrations were prepared, with salt content gradually increasing from 0 to 9%. Figure 3 presents the measured drag reduction rates under different experimental conditions.
In Fig. 3a, at low flow rates (Q < 300 mL/min), the drag reduction rate is negative, indicating no drag reduction effect. This phenomenon may arise because, near the transition region, the shearing forces between fluid micro-clusters are insufficient to fully extend the polymer chains of sodium alginate. Additionally, interactions among sodium alginate molecules may increase flow resistance. As the flow rate increases, turbulence intensity rises, allowing the coiled sodium alginate molecules to extend and unfold. The extended polymer chains subsequently interact with the turbulent eddies, weakening the disordered micro-clusters and reducing flow resistance, which results in an increased drag reduction rate. Figure 3a also illustrates that under low Reynolds number conditions, the drag reduction rate of lower-concentration sodium alginate solutions is higher. As the Reynolds number increases, the drag reduction rate of higher-concentration solutions gradually exceeds that of lower concentrations. This suggests that in higher concentration environments, greater shear forces are required to induce polymer chain extension, while turbulent eddies may further facilitate the diffusion and rearrangement of the drag-reducing molecules, thereby enhancing the drag reduction effect.
When comparing salt-free solutions (Fig. 3a) with saline solutions (Fig. 3b–d), it is evident that as the salt concentration increases, the drag reduction rate tends to decrease. This may be attributed to the added salts altering the ionic strength of the solution and the charge screening effect, which limits the ability of sodium alginate molecules to extend, resulting in a reduced drag reduction rate. Notably, when the salt concentration reaches 9%, the drag reduction rate becomes negative at high flow rates, indicating an increase in flow resistance.
Response surface methodology (RSM) results and analysis
Response Surface Methodology (RSM) is a statistical analysis technique employed to investigate the influence of multiple factors on response variables36,37. RSM constructs mathematical models to predict and optimize response variable values and is extensively utilized in engineering, science, and experimental design38. RSM establishes the relationship between response and independent variables using a design matrix and polynomial regression models.
To reduce the number of experimental runs while avoiding unrealistic or unsafe conditions during fluid preparation—such as those caused by extreme flow rates or salt concentrations—we adopted the Box-Behnken design using the free version of Design Expert software. This experimental design focused on three key factors that significantly influence the drag reduction rate (DR): flow rate (Q), sodium alginate concentration (Cp), and salt mass fraction (Cs). A three-factor, three-level response surface analysis was performed, with the drag reduction rate (DR) set as the response variable, as detailed in Table 1.
Table 2 summarizes the design scheme generated by the Design Expert software. In accordance with statistical requirements, 15 distinct experimental runs were performed. Analysis of Fig. 3 indicates that the relationship between DR and the three variables is complex and nonlinear. Consequently, a second-order polynomial model was selected to fit the relationship between the response variable and independent variables to obtain an accurate mathematical model for the drag reduction rate (DR) as a function of Q, Cs, and Cp. Equation (7) represents the obtained multivariate second-order response surface regression model.
The credibility and accuracy of the regression equation were assessed through an analysis of variance (ANOVA), with results presented in Table 3. The regression model’s correlation coefficient (R2 = 0.9963) indicates that 99.63% of the variation in the response variable (drag reduction rate) can be explained by the selected independent variables. A smaller p-value indicates a more significant influence of the factor on the response variable. The p-value for the total regression term is less than 0.0001, indicating that the regression model is highly significant and that the experiment exhibits high reliability and accuracy. The individual factors influencing the drag reduction rate, in order of importance, are as follows: salt mass fraction, flow rate, and drag-reducing agent concentration. Under the interaction of multiple factors, all cross-term p-values were less than 0.05, indicating that the interaction between drag-reducing agent concentration and flow rate has the greatest influence on the drag reduction rate.
Plots illustrating the three assumptions of ANOVA. (a) The normal probability plot assesses whether the residuals (the differences between experimental data and predicted values) follow a normal distribution. (b) The constant variance plot demonstrates the relationship between predicted values and residuals, allowing for the assessment of residuals within a specified range. (c) The plot of residuals versus run order examines potential time-dependent trends; if a noticeable trend is present, it suggests that a time-related variable may influence the response, indicating a need for experimental revision. Otherwise, the data should appear randomly distributed.
The three key assumptions for ANOVA in Response Surface Methodology (RSM)—normality, homoscedasticity (constant variance), and randomness—were validated in this study. The normal probability plot of the residuals (Fig. 4a) shows that the residual data points are distributed along both sides of a straight line without forming an S-shaped curve, indicating that the residuals meet the assumption of normality. In Fig. 4b, the residuals remain within the range of ± 6.25, and the variance of the experimental values does not show any divergence as the predicted values increase, fulfilling the assumption of homoscedasticity. To evaluate the assumption of randomness, the correlation between the run order and residuals was examined. As shown in Fig. 4c, no significant trends related to time or sequence were observed, confirming that the experimental data exhibit randomness and satisfy the assumption of independence. Therefore, based on this analysis, the drag reduction rate data comply with all three ANOVA assumptions, affirming the validity of the experimental method and the reliability of the collected data.
Effects of salt content, sodium alginate concentration, and volumetric flow rate on the drag reduction rate (DR). (a) Response surface plot of DR at a salt mass fraction Cs = 6% under varying concentrations of the drag-reducing agent and flow rates. (b) Response surface plot of DR at a flow rate Q = 400 ml/min under varying salt mass fractions and drag-reducing agent concentrations. (c) Response surface plot of DR at a drag-reducing agent concentration Cp = 1000 ppm under varying salt mass fractions and flow rates.
Under experimental conditions with sodium alginate concentrations ranging from 500 to 1500 ppm, flow rates between 350 and 450 ml/min, and salt mass fractions from 3 to 9%, Eq. (7) effectively predicts the drag reduction rate (DR) of saline sodium alginate solutions. As indicated by Eq. (7), all three experimental conditions include interaction and quadratic terms. The presence of quadratic terms signifies the existence of critical values for DR, which lead to extremum points in the drag reduction rate. The coefficient of the quadratic term for flow rate (Q) is positive, whereas those for salt mass fraction (Cs) and drag-reducing agent concentration (Cp) are negative. This implies that the curve for flow rate (Q) is concave up, while the influence curves of (Cs) and (Cp) are concave down, resulting in a complex three-dimensional response surface (Fig. 5). Figure 5a illustrates the response surface for the drag reduction rate (DR) with a salt mass fraction of 7.74%, depicting the relationship between flow rate (Q) and drag-reducing agent concentration (Cp). It is evident that at a constant flow rate, the drag reduction rate (DR) initially increases with rising sodium alginate concentration before decreasing, indicating the presence of a maximum point for drag reduction. Additionally, the overall variation presents a saddle shape; as the flow rate (Q) increases from low to high, the drag reduction initially decreases and then rises, contrasting with the trend shown in Fig. 3a and highlighting the complexity of salt’s effect on drag reducers. Figure 5b presents the response surface for salt mass fraction (Cs) and drag-reducing agent concentration (Cp). It is observed that, once the salt mass fraction is established, a critical point exists for the drag reduction rate (DR). Notably, lower salt concentrations correspond to higher concentrations of sodium alginate and increased drag reduction rates (DR). Figure 5c depicts the three-dimensional response surface for salt mass fraction (Cs) and flow rate (Q), showing that the drag reduction rate (DR) exhibits a similar linear variation trend concerning both salt content (Cs) and flow rate (Q).
Figure 6 illustrates the complete dataset predicted by Design Expert software using Eq. (7) (see Table 2). Most data points are closely clustered around the line y = x, indicating that the drag reduction Eq. (6) accurately captures the dynamics of the drag reduction phenomenon and provides highly consistent predictions for the experimental data. Figure 7 demonstrates the predictive capability of the RSM correlation Eq. (7) for additional experimental datasets. The predictive values align closely with the experimental values, exhibiting a fitting accuracy of ± 20%, suggesting a strong correlation between the experimental and predicted data.
Analysis of drag reduction suppression rate
To assess the impact of salt concentration on the drag reduction rate of sodium alginate solutions, this study introduces the concept of the drag reduction suppression rate (as defined by Eq. (8)). In this equation, DR represents the drag reduction rate when deionized water is used as a solvent, while DRs denotes the drag reduction rate of saline solutions under identical sodium alginate concentrations and flow conditions. When \(\epsilon\) exceeds 1, it indicates that the presence of salt enhances drag reduction, with higher values reflecting a stronger promoting effect. Conversely, when ε is less than 100%, it suggests that salt exerts a suppressive effect on the drag reduction capability of sodium alginate, with lower values indicating a stronger suppression.
Figure 8 illustrates the variations in the drag reduction suppression rate of sodium alginate saline solutions with flow rate (Q) under three different salt mass fractions. Figure 8a and b clearly show that, at different flow rates, the drag reduction suppression rate decreases with increasing salt mass fraction, indicating that higher salt concentrations diminish the drag reduction effectiveness of sodium alginate solutions. Figure 8a reveals that the suppression rate initially decreases with increasing flow rate before rising again, with the curve for the 3% salt concentration being especially pronounced. In contrast, Fig. 8b shows that at a higher sodium alginate concentration (Cp=1250 ppm), the decline in the drag reduction suppression rate becomes more gradual as the flow rate increases. This suggests that elevated sodium alginate concentrations mitigate the suppressive effect of salt on the polymer chains, necessitating higher turbulence intensity to fully extend the sodium alginate molecules and activate their drag reduction properties. The increase in drag reduction suppression rate may result from the weakening of polymer chain integrity due to salt, which, under turbulent conditions, can lead to chain breakage and a loss of drag reduction effectiveness.
Figure 9a and b present the variation curves of drag reduction suppression rates for sodium alginate saline solutions at varying mass fractions. The data clearly indicate that under low flow conditions (Q = 350 ml/min) and low salt content (Cs = 3%), the suppression rate exceeds 100%, demonstrating that salt acts to enhance drag reduction under these conditions. This effect may arise during the initial turbulence phase, where a lower salt mass fraction reduces interfacial tension, thereby weakening the interaction between the fluid and the solid interface, and decreasing friction between the fluid and the solid wall during flow.
As the salt content increases, the drag reduction suppression rate experiences a sharp decline under low flow conditions. This phenomenon can be attributed to the increased interfacial tension between the fluid and solid interface caused by the elevated salt concentration during the initial turbulence phase. Furthermore, we posit that a small amount of salt aids in the extension of polymer chains, while excessive salt restricts their movement, reducing their extension length and resulting in a more compact polymer structure. Consequently, the concentrated solution behaves more like a dilute solution, diminishing the polymers’ regulatory effect on turbulence and leading to a rapid decrease in the drag reduction suppression rate.
Effect of salt content on the drag reduction inhibition rate of sodium alginate solutions. (a) Variation of the drag reduction inhibition rate at a salt mass fraction of 3% for different sodium alginate concentrations. (b) Variation of the drag reduction inhibition rate at a salt mass fraction of 6% for different sodium alginate concentrations.
Conclusion
In this study, we investigated the effects of salinity, flow rate, and concentration on the drag reduction characteristics of sodium alginate flowing through a circular pipe. We employed Response Surface Methodology (RSM) to establish an experimental design that correlates drag reduction with micro-pipe flow conditions, along with a correlation equation for predicting drag reduction. A comparison of experimental and predicted data indicated that the predictive model is highly accurate, with an error margin within ± 20%.
Additionally, we introduced the concept of drag reduction suppression rate (ε) to evaluate the impact of salinity on drag reduction. The analysis revealed opposing effects of salt during the initial turbulence phase and the vigorous turbulence phase. We suggest that adding a small amount of salt (less than 3%) during the initial turbulence phase facilitates the extension of polymer chains, thereby enhancing drag reduction. However, in regions of more vigorous turbulence, the presence of salt significantly suppresses the extension of polymer chains, resulting in a transition from concentrated to dilute solutions. Furthermore, salt may reduce the strength of polymer chains, leading to chain breakage under turbulent conditions and a subsequent loss of drag reduction effectiveness. This phenomenon is reflected in the drag reduction suppression rate, which initially decreases and then increases with rising flow rates.
Data availability
The datasets supporting the findings of this study are available from the corresponding author upon reasonable request. For further inquiries, please contact Zhensong Cheng at 2019045@sdipct.edu.cn.
Abbreviations
- HVAC:
-
Heating ventilation and air conditioning
- C s :
-
Quality fraction of salt (%)
- C p :
-
Sodium alginate concentration(ppm)
- Q :
-
Volume flow (ml/min)
- \(\Delta p\) :
-
Pressure drop (Pa)
- f :
-
Darcy friction coefficient (−)
- \(\rho\) :
-
Density of the solution (kg m− 3)
- \(U_{b}\) :
-
Mean velocity (m/s)
- D :
-
Inner diameter (m)
- l :
-
Tube length (m)
- DR :
-
Drag reduction rate of deionized water solution (−)
- DR s :
-
Drag reduction rate of saline solution (−)
- \(\mu\) :
-
Viscosity (mPa·s)
- Re :
-
Reynolds number (−)
- ANOVA:
-
Analysis of variance
- ε :
-
Drag reduction suppression rate (−)
References
Toms, B. A. Some observations on the flow of linear polymersolutions through straight tubes at large Reynolds numbers. In Proceedings of the 1st International Congress on Rheology Vol. 2, 135–141. (1949).
Fukagata, K., Iwamoto, K. & Hasegawa, Y. Turbulent drag reduction by streamwise traveling waves of wall-normal forcing. Annu. Rev. Fluid Mech. 56, 69–90 (2024).
Kameneva, M. V. Microrheological effects of drag-reducing polymers in vitro and in vivo. Int. J. Eng. Sci. 59, 168–183 (2012).
Yang, H. et al. Eco-friendly polysaccharide coatings for antifouling and drag-reduction and potential application for marine devices. Friction 12(4), 726–744 (2024).
Zhang, X., Duan, X. & Muzychka, Y. New mechanism and correlation for degradation of drag-reducing agents in turbulent flow with measured data from a double-gap rheometer. Colloid Polym. Sci. 296, 829–834 (2018).
Wyatt, N. B., Gunther, C. M. & Liberatore, M. W. Drag reduction effectiveness of dilute and entangled xanthan in turbulent pipe flow. J. Nonnewton. Fluid Mech. 166(1–2), 25–31 (2011).
Ahmad, M., Baig, M. F. & Anwer, S. F. Turbulent drag reduction in compressible flows using streamwise traveling waves. Phys. Fluids 36(3). (2024).
Gattere, F., Chiarini, A. & Quadrio, M. Dimples for skin-friction drag reduction: Status and perspectives. Fluids 7(7), 240 (2022).
Jia, Z. et al. A salt-resistant sodium carboxymethyl cellulose modified by the heterogeneous process of oleate amide quaternary ammonium salt. Polymers 14(22), 5012 (2022).
Blaauw, L. J., Lohse, D. & Huisman, S. G. Sodium chloride inhibits effective bubbly drag reduction in turbulent bubbly Taylor–Couette flows. Philos. Trans. R. Soc. A. 381(2243), 20220127 (2023).
Xing, L., Ke, Y., Hu, X. & Liang, P. Preparation and solution properties of polyacrylamide-based silica nanocomposites for drag reduction application. New J. Chem. 44(23), 9802–9812 (2020).
Al-Yaari, M., Soleimani, A., Abu-Sharkh, B., Al-Mubaiyedh, U. & Al-Sarkhi, A. Effect of drag reducing polymers on oil–water flow in a horizontal pipe. Int. J. Multiph. Flow. 35(6), 516–524 (2009).
Shetty, A. M. & Solomon, M. J. Aggregation in dilute solutions of high molar mass poly (ethylene) oxide and its effect on polymer turbulent drag reduction. Polymer 50(1), 261–270 (2009).
Yang, S. Q. Drag reduction in turbulent flow with polymer additives (2009).
Quan, Q. et al. Experimental study on the effect of high-molecular polymer as drag reducer on drag reduction rate of pipe flow. J. Petrol. Sci. Eng. 178, 852–856 (2019).
Virk, P. S. Drag reduction fundamentals. AIChE J. 21(4), 625–656 (1975).
Virk, P. S., Mickley, H. S. & Smith, K. A. The ultimate asymptote and mean flow structure in Toms’ phenomenon (1970).
Zhang, X., Duan, X., Muzychka, Y. & Wang, Z. Experimental correlation for pipe flow drag reduction using relaxation time of linear flexible polymers in a dilute solution. Can. J. Chem. Eng. 98(3), 792–803 (2020).
Habibpour, M., Koteeswaran, S. & Clark, P. E. Drag reduction behavior of hydrolyzed polyacrylamide/polysaccharide mixed polymer solutions—effect of solution salinity and polymer concentration. Rheol. Acta. 56, 683–694 (2017).
Kamel, A. & Shah, S. N. Effects of salinity and temperature on drag reduction characteristics of polymers in straight circular pipes. J. Petrol. Sci. Eng. 67(1–2), 23–33 (2009).
Kostrzewa, M., Delgado, A. & Wierschem, A. Particle settling in micellar solutions of varying concentration and salt content. Acta Mech. 227(3), 677–692 (2016).
Shengxuan, X. U., Wenbin, Z. H. A. O., Mingyi, L. I., Yuying, L. I. N. & Changfeng, L. I. Experimental study on pipe flow transition of XG solution and drag reduction characteristics with different mass fractions of NaCl. J. Exp. Fluid Mech. 35 (4), 34–40 (2021).
Bewersdorff, H. W. & Singh, R. P. Rheological and drag reduction characteristics of xanthan gum solutions. Rheol. Acta 27, 617–627 (1988).
Carmona, J. A., Ramírez, P., Calero, N. & Muñoz, J. Large amplitude oscillatory shear of xanthan gum solutions. Effect of sodium chloride (NaCl) concentration. J. Food Eng. 126, 165–172 (2014).
Cheng, Z. et al. Investigation of drag reduction by slurry-like drag-reducing agent in microtube flow using response surface methodology (RSM). Sci. Rep. 13(1), 22433 (2023).
White, F. M. & Majdalani, J. Viscous Fluid Flow Vol. 3, 433–434 (McGraw-Hill, 2006).
White, F. M. & Xue, H. Fluid Mechanics Vol. 3 (McGraw-hill, 2003).
Gasljevic, K., Hall, K., Chapman, D. & Matthys, E. F. Invariant type-B characteristics of drag-reducing microalgal biopolymer solutions. Exp. Fluids 58, 1–16 (2017).
Paswan, M., Chandel, A. K. S., Malek, N. I. & Dholakiya, B. Z. Preparation of sodium alginate/Cur-PLA hydrogel beads for curcumin encapsulation. Int. J. Biol. Macromol. 254, 128005 (2024).
Cheng, Z. et al. Drag reduction and degradation by sodium alginate in turbulent flow. Sci. Rep. 14(1), 16854 (2024).
Rudd, M. J. Measurements made on a drag reducing solution with a laser velocimeter. Nature 224(5219), 587–588 (1969).
Wagner, W. & Kretzschmar, H. J. IAPWS industrial formulation 1997 for the thermodynamic properties of water and steam. In International Steam Tables: Properties of Water and Steam Based on the Industrial Formulation IAPWS-IF97 7–150. (2008).
Ghajar, A. J., Tang, C. C. & Cook, W. L. Experimental investigation of friction factor in the transition region for water flow in minitubes and microtubes. Heat Transf. Eng. 31(8), 646–657 (2010).
Dean, R. B. Reynolds number dependence of skin friction and other bulk flow variables in two-dimensional rectangular duct flow (1978).
Drew, T. B., Koo, E. C. & McAdams, W. H. The friction factor for clean round pipes. AIChE 28, 56–72 (1932).
Box, G. E. & Wilson, K. B. On the experimental attainment of optimum conditions. In Breakthroughs in Statistics: Methodology and Distribution 270–310 (Springer, New York, 1992).
Zhang, X., Duan, X. & Gao, Z. Experimental correlation of gas–liquid–solid mass transfer coefficient in a stirred tank using response surface methodology. Heat Mass Transf. 53, 3109–3118 (2017).
Veza, I., Spraggon, M., Fattah, I. R. & Idris, M. Response surface methodology (RSM) for optimizing engine performance and emissions fueled with biofuel: Review of RSM for sustainability energy transition. Results Eng. 101213 (2023).
Acknowledgements
This research was supported by the Dongying Science Development Fund under Grant Numbers 2023SS019 and 2023SS017.
Author information
Authors and Affiliations
Contributions
Z.C. wrote the main manuscript text; X.Z. and X.D. prepared figures; L.L. and L.G. performed experiment; X.W. and X.S. revised the manuscript. G.Z. and Y.L. performed the validation. X.Y. , J.Z. and H.Z. performed the data analysis. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, Z., Zhang, X., Dai, X. et al. Experimental study on the drag reduction performance of sodium alginate in saline solutions. Sci Rep 14, 32123 (2024). https://doi.org/10.1038/s41598-024-83910-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-83910-6