Abstract
This study was aimed to evaluate the efficacy of a 3D-printed patient specific implant (PSI) made of polycaprolactone (PCL) in repairing orbital wall fractures. We retrospectively reviewed patients who underwent surgical repair for unilateral orbital wall fractures using a 3D-printed PCL PSI. Computed tomography scans were used to compare the orbital tissue volumes and the morphological similarity (root-mean-square [RMS] conformance distance) between the fractured wall and the mirrored counterpart before and after surgery. All orbital fractures (inferior wall, 19; medial wall, 9; and combined inferior and medial walls, 12) were successfully repaired without postoperative complications. The mean time for implant insertion during surgery was 19.8 s (range, 3–60). The mean orbital tissue volume ratio between the fractured orbit and the contralateral normal orbit significantly decreased after surgery (109.0% preoperatively vs. 100.6% at postoperative 6 months, P < 0.001, paired t-test). The median RMS conformance distance significantly decreased after surgery (3.426 mm preoperatively vs. 1.073 mm at postoperative 6 months, P < 0.001, Wilcoxon signed-rank test). Our findings suggest that using a 3D-printed PCL PSI could effectively restore the original volume and shape of the orbit, thus being a valuable addition to the surgeon’s armamentarium for managing orbital wall fractures.
Similar content being viewed by others
Introduction
Orbital wall fractures are a common result of facial trauma, with a prevalence ranging from 3–32%1. Surgical indications for orbital wall fracture repair typically include restrictive diplopia and significant enophthalmos2. An orbital wall implant is usually inserted during surgery to cover the defect and prevent re-herniation of orbital tissues3. The main goal of the surgery is to restore normal anatomical relationships of the internal orbit while avoiding complications associated with the procedure and implant.
However, precise reconstruction of orbital wall defects is challenging for surgeons owing to the individual variability in orbital anatomy. The orbit is not a simple cone-shaped structure; its 3D structure has complex concavities and convexities in contour. Therefore, intraoperative implant manipulation to fit these sophisticated contours is difficult and time-consuming, especially for inexperienced surgeons.
Advances in 3D printing technology offer a promising solution to this challenge. Our previous study demonstrated that applying a 3D-printed mold to the bending of titanium-embedded polyethylene 2D implants can achieve good orbital volume restoration4.Furthermore, recent developments in direct printing technology with biocompatible materials have enabled the reconstruction of various bony defects5,6,7. We have applied a 3D-printed patient specific implant (PSI) made of polycaprolactone (PCL) for orbital wall fracture repair. In this study, we evaluated the efficacy of PSI using computer program-aided volumetric and morphometric analyses.
Methods
We retrospectively reviewed the medical records of patients who underwent orbital wall fracture repair using a 3D-printed PSI made of PCL at Asan Medical Center between July 2019 and August 2021. Surgical indications were as follows: persistent diplopia, significant enophthalmos (≥ 2 mm) at presentation, and large fractures involving > 50% of the wall. Patients were informed about the PSI preoperatively, and those who agreed to underwent a surgery using PSI were included in the study. Patients with bilateral orbital wall fractures, fractures involving lateral or superior orbital wall, a previous history of orbital surgery, and a short postoperative follow-up period < 6 months were excluded from the study. The study was conducted according to the tenets of the Declaration of Helsinki. The protocol was approved by our institutional review board (approval no. 2020 − 0944). Written informed consents were obtained from all participants.
Manufacturing 3D-printed PSI
Preoperative facial computed tomography (CT) images with 1.5 mm thickness in axial and coronal planes were fully anonymised and exported in the DICOM format. The orbital wall fracture site was analysed using 3D modeling software (Mimics® version 20.0 and 3-Matic® version 14.0, Materialise, Leuven, Belgium). In the Mimics software, the boundary of the fracture was marked with more than 10 points, and then the area of the fracture was calculated. Using the surface construction tool of 3-Matic software, an orbital PSI model was designed to simulate the natural contour of the individual orbit and confirmed by a senior author (H-SS)4. An orbital PSI (TnR mesh, T&R Biofab Co., Ltd., Seoul, Korea) was fabricated with the fused deposition modeling (FDM) method using a 3D printer (T&R Biofab Co., Ltd.)8. The steel nozzle was 500 μm in size and maintained at 120 ˚C, 600 kPa. The PSI has a thickness of 0.8–1.2 mm and was made of biocompatible PCL (Corbion, Purac Biochem, Netherlands) and beta-tricalcium phosphate (Forster Corporation, USA) polymer in a ratio of 8:2. After smoothing of edges and Gamma irradiation sterilisation, the PSI was ready to be used for orbital wall fracture repair. Supplementary Video S1 demonstrates the manufacturing process of 3D-printed PSI.
Surgical procedures and postoperative follow-ups
All surgeries were performed by a single experienced oculoplastic surgeon (H-SS). According to a study showed that effective fracture repair can be performed up to 29 days after trauma9, we attempted to perform surgery within 4 weeks after the trauma. The overall surgical procedures were similar to those described in our previous study4. Combined inferior and medial wall fractures were accessed through a combined transconjunctival and transcaruncular incision. However, unlike the previously described procedures, intraoperative implant manipulation, including contouring and trimming using customised templates, was not performed as we used a 3D-printed PSI in this study. An implant insertion time (IIT), from exposing entire edges of the fracture defect to placing a PSI to cover the defect, was measured in seconds during each surgery.
All patients were followed up at 1 week, 3 months, and 6 months postoperatively and evaluated for surgical outcomes and complications. Extraocular movement limitation graded on a numerical scale of 0 (no limitation) to -4 (no movement)10, as confirmed by the Hess screen test. The number of patients with binocular diplopia in the central 10 and 30 degrees of the visual field evaluated using the Goldmann diplopia field test was counted10,11. Postoperative enophthalmos was evaluated using a Hertel exophthalmometer (Oculus Optikgeräte, Wetzlar, Germany), and an enophthalmos ≥ 2 mm compared with the contralateral eye was considered as a significant enophthalmos12. All patients underwent facial CT scans with 1.5 mm thickness at each visit.
Quantitative analysis of the repaired orbit
A 3D orbital tissue model was semi-automatically created in the fractured and contralateral normal orbits using the Mimics software by a single independent examiner (MKY). The mask threshold range was − 200 to + 200 Hounsfield units13. The following landmarks were used to delineate the orbital tissue model: frontozygomatic suture, inferior orbital rim, lateral orbital rim, anterior lacrimal crest, entrance of the optic canal, and pterygopalatine fossa14. The outline of herniated orbital fat at the fracture site was adopted as a margin of the orbital tissue model. Every pixel outside these landmarks and outline was erased, including pixels in the supraorbital notch and infraorbital groove.
Volumetric analysis
The Mimics software automatically provides model volume calculation in the mask properties section. The orbital volume ratio (OVR) was obtained by dividing the volume of the fractured orbit by that of the contralateral normal orbit to evaluate bilateral symmetry of the orbital volume15. The OVR was compared between preoperative and postoperative CT scans using the paired t-test and one-way ANOVA test using the MedCalc version 9.6.4.0 (MedCalc Software, Mariakerke, Belgium). As a subgroup analysis, the mean OVR in patients with combined wall fractures was compared to that in patients with a single-wall fracture using the independent t-test. Also, the mean OVR was compared between patients underwent surgery within 4 weeks and within 4 to 8 weeks. A P-value < 0.05 was considered statistically significant.
Morphometric analysis
Morphological symmetry between the two orbits’ postoperative contours was evaluated to assess surgical accuracy of the orbital fracture repair using a 3D-printed PSI. Surfaces of the fractured and contralateral normal orbital tissue models were generated in stereolithography files and then imported to the 3-Matic software. The midsagittal plane defined by nasion, anterior nasal spine, and basion was set to mirror the surface of the contralateral normal orbital tissue model to the surface of the fractured orbital tissue model16. Global registration was applied to align the two surfaces with maximum conformance.
In the coronal plane where the orbital rim is fully formed, three points were marked at the frontoethmoid suture, anterior orbital strut, and turning point between the lateral and inferior walls17,18,19. Two points were selected according to the location of the orbital wall fracture. Then, a cutting plane was made with these two points and the optic nerve entrance. For example, a vertical plane made with the frontoethmoid suture, anterior orbital strut, and optic nerve entrance was used for segmenting the medial surface.
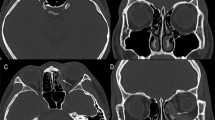
Both models were superimposed and then segmented at once along the coronal plane and a suitable cutting plane. Geometric comparison analysis in 3-Matic software provided the 25% and 75% quartile, median, mean, standard deviation, and root-mean-square (RMS) conformance distances between segmented surfaces of the two orbits. Detailed procedures of the morphometric analysis are presented in Fig. 1. The median RMS conformance distance was compared between preoperative and postoperative CT scans using the Wilcoxon signed-rank test and Kruskal–Wallis test. The median RMS conformance distance was also compared among subgroups using the Mann–Whitney U test. The association between the area of fracture and postoperative 6 months RMS conformance distance was investigated using the univariate linear regression analysis.
Morphometric analysis of the two orbits in a patient with a left inferior orbital wall fracture. (a, b). The surface of the normal orbital tissue model (blue colour) was mirrored based on the mid-sagittal plane (blue grid) and then aligned to the surface of the fractured orbital tissue model (red colour). (c) In the coronal plane, where the orbital rim is fully formed, the frontoethmoid suture, anterior orbital strut, and turning point between the lateral and inferior walls were marked with red dots. (d) A horizontal cutting plane (green grid) was made with the optic nerve entrance, anterior orbital strut, and turning point between the lateral and inferior walls. Conformance distances were calculated between two inferior surfaces simultaneously segmented along the coronal plane in (c) (red grid) and horizontal plane.
Results
Forty patients were included in the present study, with a mean age of 38.4 ± 14.8 years (range: 18–79). Twenty-seven (67.5%) patients were male. Among the patients, 19 had an inferior wall fracture, 9 had a medial wall fracture, and 12 had combined inferior and medial wall fractures. Seven of twelve patients with combined fractures had fractures involving the anterior bony strut. Twenty-five patients underwent surgery for orbital wall fractures within 4 weeks, and other 15 patients (37.5%) underwent surgery within 8 weeks due to their late presentation to our hospital. Revisional case was not included.
The mean IIT was 19.8 ± 24.7 s (range: 3–60). For patients with combined inferior and medial wall fractures, the mean IIT was 20.0 ± 17.0 s (N = 12, range: 10–60). All implants were well supported on the intact ledge at the border of the fracture defect without using the intraoperative navigation system.
The mean follow-up period was 12.0 ± 7.8 months. Clinical outcomes evaluated at 1 week, 3 months, and 6 months postoperatively are presented in Table 1. No complications associated with surgery or implants, including hematoma, infection, implant-related persistent inflammation, implant displacement, or visual disturbance, were observed. Representative preoperative and 6 months postoperative computed tomography coronal images are presented in Supplementary Fig. S1.
The orbital tissue volume of the fractured orbit significantly decreased after surgery (26.57 cm3 vs. 24.51 cm3; P < 0.001, paired t-test; Fig. 2A). There was no significant difference in orbital tissue volume between the fractured and contralateral orbits (24.51 cm3 vs. 24.36 cm3 at 6 months postoperatively; P > 0.99). The mean OVR also significantly decreased after surgery (109.0% vs. 100.6% at 6 months postoperatively; P < 0.001) and remained unchanged throughout follow-ups (P > 0.99, one-way ANOVA test, Fig. 2B).
In the subgroup with combined inferior and medial wall fractures, the mean preoperative OVR was significantly higher than that of the subgroup with a single-wall fracture (113.4% vs. 107.1%; P = 0.005, independent t-test). However, the mean postoperative OVR was not significantly different in both subgroups (102.3% vs. 99.6% at 6 months postoperatively; P = 0.105). Also, the mean postoperative 6 months OVR was similar in patients underwent surgery within 4 weeks and within 4 to 8 weeks using the independent t-test (99.9% vs. 101.7%, P = 0.136) (Table 2).
Morphometric analysis demonstrated a high similarity between the repaired orbital wall and the mirrored counterpart (Table 3). The median RMS conformance distance significantly decreased after surgery (3.426 mm vs. 1.073 mm at 6 months postoperatively; P < 0.001, Wilcoxon signed-rank test). The median postoperative RMS conformance distance remained stable throughout 6-month follow-ups (range: 0.896–1.073 mm; P = 0.958, Kruskal–Wallis test).
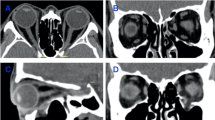
In the subgroup with combined inferior and medial wall fractures, the median preoperative RMS conformance distance was larger than that of the subgroup with a single-wall fracture (4.153 mm vs. 3.191 mm, P = 0.012, Mann–Whitney U test). At 6 months postoperatively, the median RMS conformance distance of the subgroup with combined fractures remained larger than that of the subgroup with a single-wall fracture (1.238 mm vs. 0.895 mm; P = 0.021); however, it was not significantly different from that of the subgroup with inferior wall fracture (1.238 mm vs. 0.998 mm; P = 0.139). CT images and colour-coded maps depicting RMS conformance distances of a representative case with combined inferior and medial wall fractures are presented in Fig. 3.
A representative case with combined inferior and medial wall fractures in the left orbit. (Top row) Preoperative computed tomography images in coronal and sagittal planes. (Middle row) Six months postoperative computed tomography images. (Bottom row) Preoperative and 1 week, 3 months, and 6 months postoperative 3D colour-coded maps of the conformance distance between the fractured orbital tissue model and the mirrored counterpart.
The median area of fracture was 258 (interquartile range [IQR] 169–302) mm2 in inferior wall fracture, 177 (IQR 147–200) mm2 in medial wall fracture, and 504 (IQR 451–549) mm2 in combined inferior and medial wall fracture, respectively. Each area was not associated with postoperative 6 months RMS conformance distance (P = 0.076, 0.992, and 0.279, respectively).
Discussion
This study retrospectively reviewed outcomes of orbital wall fracture repair using a 3D-printed PSI. Clinical outcomes were satisfactory without postoperative complications. In addition, computer program-aided volumetric and morphometric analyses demonstrated that the repaired orbit is symmetrical to the contralateral normal orbit in size and contour throughout 6-month follow-ups.
Applications of the 3D printing are advantageous in patient-customised surgery, especially for areas with structural complexity5,20,21,22,23. Orbital PSI using a 3D printing technology can be generated using molding or direct printing techniques. In general, the direct printing technique enables the reproduction of more complex structures than the molding technique24. There are various methods for direct 3D printing, such as stereolithography, selective laser sintering, and FDM. FDM has been increasingly used in the biomedical field because it is a simple, inexpensive technology that can be applied to various materials, including bioabsorbable polymers20,25. A previous study reported the efficacy of an FDM-generated orbital implant8; however, it was a population-based implant that cannot be individually customised to a patient’s orbital characteristics.
Due to the structural complexity and curvature of the orbital walls, reconstruction with the classical ‘manual cutting and bending’ method using 2D implants is challenging in most cases of orbital wall fractures. The reconstruction requires several sessions of tailoring implants by trial and error, and surgical outcomes vary depending on surgeons’ experience. Although the present study is not a comparative study, orbital reconstruction using a 3D-printed PSI may have several theoretical advantages compared to those of reconstructions using classical methods26. A meta analysis showed that the 3D-printed models are superior than free-hand-shaped implants for an accurate orbital wall reconstruction with fewer complications27. Furthermore, fully reproducing the original curvature and stability using this classical method might be impossible in cases of combined inferior and medial wall fractures, especially in those involving the inferomedial bony strut28. Previous studies have reported that postoperative diplopia in patients with combined wall fractures was worse than in those with a single-wall fracture regarding incidence, deviation angle, and Hess area ratio29,30,31. Other studies reported higher postoperative OVR in combined wall fractures than in a single-wall fracture32,33. Our results showed that 3D-printed PSIs enable satisfactory clinical outcomes without diplopia and minimal volume differences for combined wall fractures, similar to those for single-wall fractures. Additionally, even if the surgery was performed within 4 to 8 weeks, there was no difference in clinical outcomes and volume restoration compared to surgery performed within 4 weeks.
Another strength of the present study is that we performed computer program-aided volumetric and morphometric analyses to evaluate the efficacy of 3D-printed orbital PSIs. Several previous studies reported results of volumetric analysis after orbital fracture repair4,8,34, but only few studies conducted morphometric analysis to determine whether the repaired wall was 3-dimensionally similar to the original wall. Davies et al. reported that the RMS conformance distance after inferior orbital wall fracture repair using autologous iliac or scapular bone graft (N = 10) ranged from 1.72 to 2.87 mm35. Our results showed better accuracy of reconstruction in isolated inferior wall fractures (N = 19, median RMS distance of 0.895 mm) and even in all types of fractures (N = 40, median RMS distance of 1.073 mm). Furthermore, in our morphometric analysis, we first conducted globe registration prior to segmentation as it can reflect the contour and position differences between the fractured orbital wall and the mirrored counterpart. Our morphometric analysis results revealed that orbital reconstruction using 3D-printed PSIs is highly accurate in restoring the original curvature of fractured orbits and is stable throughout 6 months. Additionally, the accuracy of the reconstruction was not associated with the fracture size, which is thought to be because the implant was designed to reflect the shape and size of the individual fracture and to ensure sufficient support on the undamaged orbital wall. True-to-original orbital reconstruction is advantageous in avoiding possible surgical complications, such as orbital tissue incarceration or implant displacement36. Further long-term studies investigating the association between clinical outcomes and morphometric symmetry will help clarify the benefits.
Another advantage of PSIs is that it requires much less effort to obtain better outcomes37. Previous comparative studies showed that 3D printing-assisted orbital reconstruction could reduce surgical duration and intraoperative bleeding than conventional surgeries using manual-bending implants, especially for large orbital wall fractures8,36. Accordingly, the mean IIT was as short as 20 s in our series because PSIs did not required intraoperative tailoring by trial and error. Although some large implants for combined fractures were divided and inserted separately, IIT for patients with combined fractures was comparable and less than 1 min in most cases.
There were some limitations of the present study, including the retrospective design. The present study lacked a comparison with the results of conventional methods using non-customised implants in terms of clinical outcome, orbital tissue volume, and conformance distance. In addition, the accuracy of volumetric and morphometric analyses could be limited due to the nature of automatic segmentation. For example, the anterior surface of segmentation was delineated between the lateral orbital rim and anterior lacrimal crest; however, it may be influenced by head position.
In conclusion, using a 3D-printed PSI for orbital wall fracture repair may enable easier procedures and achieve satisfactory clinical outcomes without significant complications. Moreover, it could restore the original volume and curvature of the orbit in the surgical repair of orbital fractures, including combined wall fractures.
Data availability
All data relevant to the study are available upon reasonable request (corresponding author: Ho-Seok Sa, e-mail: lineblue@hanmail.net, ORCID 0000-0003-3043-7222).
References
Oliver, J. D., Saba, E. S., Gupta, N., Hendricks, T. M. & Singh, D. J. Alloplastic reconstruction of orbital floor fractures: a systematic review and pooled outcomes analysis. Eur. J. Plast. Surg. 43, 109–116. https://doi.org/10.1007/s00238-019-01614-x (2020).
Ramesh, S., Hubschman, S. & Goldberg, R. Resorbable Implants for Orbital Fractures: a systematic review. Ann. Plast. Surg. 81, 372–379. https://doi.org/10.1097/SAP.0000000000001504 (2018).
Jordan, D. R., Onge, S., Anderson, P., Patrinely, R. L., Nerad, J. A. & J. R. & Complications associated with alloplastic implants used in orbital fracture repair. Ophthalmology 99, 1600–1608. https://doi.org/10.1016/s0161-6420(92)31760-9 (1992).
Kang, S. et al. Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction. Eye 32, 1864–1870. https://doi.org/10.1038/s41433-018-0193-1 (2018).
Kim, S. Y. Application of the three-dimensionally printed biodegradable polycaprolactone (PCL) mesh in repair of orbital wall fractures. J. Craniomaxillofac. Surg. 47, 1065–1071. https://doi.org/10.1016/j.jcms.2019.03.009 (2019).
Lee, S., Choi, D., Shim, J. H. & Nam, W. Efficacy of three-dimensionally printed polycaprolactone/beta tricalcium phosphate scaffold on mandibular reconstruction. Sci. Rep. 10, 4979. https://doi.org/10.1038/s41598-020-61944-w (2020).
Kim, D. H. et al. Long-term efficacy and safety of 3D printed implant in patients with nasal septal deformities. Eur. Arch. Otorhinolaryngol. 279, 1943–1950. https://doi.org/10.1007/s00405-021-06996-y (2022).
Kim, J. H., Lee, C. R., Oh, D. Y., Jun, Y. J. & Moon, S. H. Comparison of efficacy between three-Dimensional Printing and Manual-bending implants for Inferomedial Orbital fracture: a retrospective study. Appl. Sci. 11, 7971. https://doi.org/10.3390/app11177971 (2021).
Dal Canto, A. J. & Linberg, J. V. Comparison of orbital fracture repair performed within 14 days versus 15 to 29 days after trauma. Ophthalmic Plast. Reconstr. Surg. 24, 437–443. https://doi.org/10.1097/IOP.0b013e31818aac9b (2008).
Yoo, Y. J., Yang, H. K., Kim, N. & Hwang, J. M. Pediatric orbital wall fractures: prognostic factors of diplopia and ocular motility limitation. PLoS One. 12, e0184945 (2017).
Woodruff, G., O’Reilly, C. & Kraft, S. P. Functional scoring of the field of binocular single vision in patients with diplopia. Ophthalmology 94, 1554–1561. https://doi.org/10.1016/s0161-6420(87)33247-6 (1987).
Hamedani, M., Pournaras, J. A. & Goldblum, D. Diagnosis and management of enophthalmos. Surv. Ophthalmol. 52, 457–473. https://doi.org/10.1016/j.survophthal.2007.06.009 (2007).
Jansen, J. et al. Orbital volume analysis: validation of a semi-automatic software segmentation method. Int. J. Comput. Assist. Radiol. Surg. 11, 11–18. https://doi.org/10.1007/s11548-015-1254-6 (2016).
Regensburg, N. I. et al. A new and validated CT-based method for the calculation of orbital soft tissue volumes. Invest. Ophthalmol. Vis. Sci. 49, 1758–1762. https://doi.org/10.1167/iovs.07-1030 (2008).
Choi, S. H., Kang, D. H. & Gu, J. H. The correlation between the Orbital volume ratio and Enophthalmos in Unoperated Blowout fractures. Arch. Plast. Surg. 43, 518–522. https://doi.org/10.5999/aps.2016.43.6.518 (2016).
Lian, X. et al. Landmark-Independent Method to Determine Midsagittal Plane and its clinical application in Craniomaxillofacial Trauma. J. Oral Maxillofac. Surg. 76 1511.e1–1511.e9 (2018).
Krause, M., Hümpfner-Hierl, H., Kruber, D., Sterker, I. & Hierl, T. Calculation of resected orbital wall areas in the treatment of endocrine orbitopathy. J. Craniomaxillofac. Surg. 45, 485–490. https://doi.org/10.1016/j.jcms.2017.01.015 (2017).
Kim, J. W., Goldberg, R. A. & Shorr, N. The inferomedial orbital strut: an anatomic and radiographic study. Ophthalmic Plast. Reconstr. Surg. 18, 355–364. https://doi.org/10.1097/00002341-200209000-00007 (2002).
René, C. Update on orbital anatomy. Eye 20, 1119–1129. https://doi.org/10.1038/sj.eye.6702376 (2006).
Bozkurt, Y. & Karayel, E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J. Mater. Res. Technol. 14, 1430–1450. https://doi.org/10.1016/j.jmrt.2021.07.050 (2021).
Chepurnyi, Y., Zhukovtseva, O., Kopchak, A. & Kanura, O. Clinical application of automated virtual orbital reconstruction for orbital fracture management with patient-specific implants: a prospective comparative study. J. Craniomaxillofac. Surg. 50, 686–691. https://doi.org/10.1016/j.jcms.2022.05.006 (2022).
Pietzka, S. et al. Comparison of Anatomical Preformed Titanium Implants and patient-specific CAD/CAM Implants in the Primary Reconstruction of isolated Orbital Fractures-A Retrospective Study. J. Pers. Med. 13, 846. https://doi.org/10.3390/jpm13050846 (2023).
Gander, T. et al. Patient specific implants (PSI) in reconstruction of orbital floor and wall fractures. J. Craniomaxillofac. Surg. 43, 126–130. https://doi.org/10.1016/j.jcms.2014.10.024 (2015).
Riedle, H., Seitz, V., Schraudolf, L. & Franke, J. Generation of 3D Silicone Models of Anatomic Soft Tissue Structures - A Comparison of Direct 3D Printing and Molding Techniques. ieeexplore.ieee.org/document/8626687 (2018).
Singh, D., Singh, R. & Boparai, K. S. Development and surface improvement of FDM pattern based investment casting of biomedical implants: a state of art review. J. Manuf. Processes. 31, 80–95. https://doi.org/10.1016/j.jmapro.2017.10.026 (2018).
Schlittler, F. et al. What are the limitations of the non-patient-specific implant in titanium reconstruction of the orbit? Br. J. Oral Maxillofac. Surg. 58, e80–e85 (2020).
Singh, A. K., Khanal, N., Chaulagain, R., Sharma, N. & Thieringer, F. M. Is the Pre-shaping of an Orbital Implant on a patient-specific 3D-Printed model advantageous compared to Conventional Free-Hand Shaping? A systematic review and Meta-analysis. J. Clin. Med. 12, 3426 (2023).
Lim, N. K., Kang, D. H., Oh, S. A. & Gu, J. H. Orbital Wall restoring surgery for Inferomedial Blowout fracture. J. Craniofac. Surg. 26, e761–e765 (2015).
Hsu, C. K., Hsieh, M. W., Chang, H. C., Tai, M. C. & Chien, K. H. Anatomic factors Predicting Postoperative Strabismus in Orbital Wall Fracture Repair. Sci. Rep. 9, 14785. https://doi.org/10.1038/s41598-019-51127-7 (2019).
Ordon, A. J., Kozakiewicz, M., Wilczynski, M. & Loba, P. The influence of concomitant medial wall fracture on the results of orbital floor reconstruction. J. Craniomaxillofac. Surg. 46, 573–577. https://doi.org/10.1016/j.jcms.2018.01.005 (2018).
Yamanaka, Y., Watanabe, A., Rajak, S. N., Nakayama, T. & Sotozono, C. The trend of recovery period on postoperative eye movement in orbital blowout fractures. J. Craniomaxillofac. Surg. 49, 688–693. https://doi.org/10.1016/j.jcms.2021.02.005 (2021).
Oh, S. A., Aum, J. H., Kang, D. H. & Gu, J. H. Change of the orbital volume ratio in pure blow-out fractures depending on fracture location. J. Craniofac. Surg. 24, 1083–1087. https://doi.org/10.1097/SCS.0b013e31828b6c2d (2013).
Lim, N. K., Kang, D. H., Oh, S. A. & Gu, J. H. Orbital wall restoring surgery in pure blowout fractures. Arch. Plast. Surg. 41, 686–692. https://doi.org/10.5999/aps.2014.41.6.686 (2014).
de Gomes, P., Perry da Camara, C. & Valejo Coelho, P. Intra- and interreader variability of orbital volume quantification using 3D computed tomography for reconstructed orbital fractures. J. Craniomaxillofac. Surg. 47, 1060–1064. https://doi.org/10.1016/j.jcms.2019.04.010 (2019).
Davies, J. C. et al. Orbital Floor Reconstruction: 3-Dimensional analysis shows comparable morphology of Scapular and Iliac Crest Bone grafts. J. Oral Maxillofac. Surg. 76, 2011–2018. https://doi.org/10.1016/j.joms.2018.03.034 (2018).
Fan, B. et al. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefes Arch. Clin. Exp. Ophthalmol. 255, 2051–2057. https://doi.org/10.1007/s00417-017-3766-y (2017).
Murray-Douglass, A., Snoswell, C., Winter, C. & Harris, R. Three-dimensional (3D) printing for post-traumatic orbital reconstruction, a systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 60, 1176–1183. https://doi.org/10.1016/j.bjoms.2022.07.001 (2022).
Funding
This study was supported by a grant (2020IT0013) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Author information
Authors and Affiliations
Contributions
M.K.Y. collected the data and wrote the paper. H-S.S. designed the study. S.J.H., G.J.K., H-S.S. recruited the subjects. J.O., N.K. processed 3D-modelling and analyzed the data. H-S.S. revised the manuscript and approved the final version of manuscript. All authors attest that they meet the current ICMJE criteria for authorship.
Corresponding author
Ethics declarations
Conflict of interest
The authors indicate no financial support or conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, M.K., Ha, S.J., Kim, G.J. et al. Efficacy of 3D-printed patient specific implant for orbital wall fracture repair in a series of 40 patients. Sci Rep 15, 4087 (2025). https://doi.org/10.1038/s41598-024-84166-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84166-w