Abstract
Tick-borne infections are the most common vector-borne diseases in the USA. Ticks harbor and transmit several infections with Lyme disease being the most common tickborne infection in the US and Europe. Lack of awareness about tick populations, specific diagnostic tests, and overlapping signs and symptoms of tick-borne infections can often lead to misdiagnosis affecting treatment and the prevalence data reported especially for non-Lyme tick-borne infections. The diagnostic tests currently available for tick-borne diseases are severely limited in their ability to provide accurate results and cannot detect multiple pathogens in a single run. The multiplex protein microarray developed at Vibrant was designed to detect multiple serological antibodies thereby detecting exposure to multiple pathogens simultaneously. Our microarray in its present form can accommodate 400 antigens (molecules that can bind to specific antibodies) and can multiplex across antigen types, whole cell lysates, recombinant proteins, and peptides. A designed array containing multiple antigens of several microbes including Borrelia burgdorferi, the Lyme disease spirochete, was manufactured and evaluated. The immunoglobulin M (IgM) and G (IgG) responses against several tick-borne microbes and other infectious agents were analyzed for analytical and clinical performance. The microarray improved IgM and IgG sensitivities and specificities of individual microbes when compared with the respective gold standards. The testing was also performed in a single run in comparison to multiple runs needed for comparable testing standards. In summary, our study presents a flexible multiplex microarray platform that can provide quick results with high sensitivity and specificity for evaluating exposure to varied infectious agents especially tick-borne pathogens.
Similar content being viewed by others
Introduction
Most vector-borne infections in the USA can be attributed to pathogens transmitted via tick bites. Of all tick-borne infections identified to date, Lyme disease is the most prevalent infection1. Lyme disease is a potentially serious bacterial infection transmitted by ticks and was first reported in the mid-1970s in the USA. The etiological agent was identified later as Borrelia burgdorferi2,3,4. Several studies have reported the presence of co-infections along with B. burgdorferi5 including Babesia spp.6, Bartonella spp.7, Ehrlichia spp.8, Anaplasma phagocytophilum8, Powassan virus (POWV)9, Toxoplasma gondii10, Rickettsia spp.11, tick-borne encephalitis virus (TBEV)12, and West Nile virus (WNV)13. Additionally, prolonged exposure to B. burgdorferi and other tick-borne pathogens could potentially weaken the patient’s immune system increasing the risk of infections by Epstein Barr virus (EBV)14, cytomegalovirus (CMV)14, parvovirus B19 (B19V)5, coxsackie B virus15, herpes simplex virus 1 (HSV-1)16, herpes simplex virus 2 (HSV-2)16, and human herpes virus 6 (HHV-6)14.
Ticks have been shown to transmit more than one infectious agent in a single bite. For instance, a study by Wormser et al. showed that there was a risk of getting infected with (A) phagocytophilum (30%) and (B) microti (24%) along with B. burgdorferi17. Currently, multi-tiered testing is carried out for diagnosing tick-borne infections18 (https://tinyurl.com/33564b8z). In this method, the infectious agents are tested sequentially, starting with B. burgdorferi. This method is time-consuming and can often lead to delayed diagnosis, accompanied with high cost to the patient18,19 (https://tinyurl.com/33564b8z). Testing for multiple infections in a single run can help physicians arrive at an accurate diagnosis especially since Lyme disease shares symptoms with other vector-borne co-infections20. The existing diagnostic assays possess various limitations that restrict their applicability in the diagnosis of these infections. The diagnosis of Lyme disease and other infectious diseases using several blot-based and single-plex ELISA tests remain rudimentary in terms of arriving at a diagnostic conclusion21. Additionally, blot-based assays may have overlapping proteins with similar mass requiring additional testing to tease out the specific antigen to which the antibody is bound. A multiplex system can detect the biomarkers of Lyme disease, potential co-infections, and other infections in a single run. A serology-based multiplexing system may be preferred to a PCR multiplex system mainly due to its accessibility, for instance using dried blood spots (DBS)22. Due to the transient nature of the organisms, the timeline in which the patients are bacteremic/viremic/parasitemic is short making it difficult to obtain genetic material of the pathogens for nucleic acid-based diagnosis. However, this limitation is overcome by using serology23. A serology-based system is also ideal for population screening and surveillance since it can indicate past exposure to a pathogen.
Our customisable protein microarray design includes antigens physically separated by design unlike blot assays and can multiplex across species. Multiplexing can also be done across antigen types such as recombinant proteins, peptides, and lysates simultaneously. This method can lower test costs since all the manufacturing is automated using bio customised semiconductor processes similar to how electronic chips are made. The multiplex microarray has three main advantages over the existing technologies. It has an ultra-high-density array surface with high reproducibility and better throughput. It can detect a large number of antibodies against varied infectious agents at the same time. Detection of antibodies can be performed using low sample volumes with low cost and a fast turnaround time21. Given the flexible nature of the multiplex platform, we aimed to provide a multiplexed testing solution for Lyme disease, its co-infections, and other possible infections of interest.
Materials and methods
Patients sera
The sera were collected from 2990 (843 samples and 2147 controls) individuals after seeking appropriate Institutional Review Board (IRB) approvals under respective collaborators (Supplementary Table 1). The study was conducted under the ethical principles that have their origins in the Declaration of Helsinki. The Western Institutional Review Board, WIRB (work order #1-1574995-1) waived the need for informed consent requirement. Table 1 lists the provided samples for Lyme disease, co-infections, and other infections along with the counts, respective collaborators and methods used to ascertain the clinical diagnosis by the physician. Supplementary Table 2 provides information regarding the vendors and strains of the pathogens used in the study. These reference sera were tested at Vibrant America Clinical Labs (Clinical Laboratory Improvement (CLIA) and College of American Pathologists (CAP) accredited facility) by laboratory personnel in a blinded manner. The sera from healthy patients were considered negative and were used to set the cut-off values and were investigated under IRB (work order #1-1574995-1) determined by WIRB to employ de-linked and de-identified human specimens and medical data for research findings. The negative sera were collected from across the US including endemic and nonendemic regions for these infections.
Processing of wafers
Wafers were functionalized as described previously21,24 (https://tinyurl.com/mr9ctppy). Briefly, silicon wafers were exposed to an environment of pure oxygen for 2 h followed by washing (deionized water) and coating (1% (vol/vol) with 3-aminopropyltriethoxysilane (APTES) in N-methylpyrrolidone (NMP). Curing was carried out at 120 °C for 60 min under an N2 atmosphere and humidity-controlled environment. Coating and incubation of the wafer with a co-polymer solution of poly (L-lysine) and poly (lactic acid) for 24 h were carried out to increase the binding efficiency of the surface on to which the antigens were immobilized via passive adsorption/hydrophobic interactions with the copolymers [Fig. 1].
Wafer Processing, Antigen Immobilisation, Pillar Plate Assembly. A poly (lactic acid) and poly (L-lysine) copolymer solution is coated onto the silicon wafers and further immobilized with protein probes [steps 1–3]. The wafers are then diced into microchips using a stealth dicing process [step 4]. A standard die sorting system is used to pick and place the microchips onto carrier plates [step 5]. The carrier tapes are loaded onto a high throughput surface mount technology (SMT) component placement system and individual microchips are placed onto 24-pillar plates. Each pillar consists of 87 microchips [step 6]. Created using Figma, Version 116.15 (https://www.figma.com/).
Immobilization of antigens
The antigens included in the assay are listed in Table 2. Pathogens transmitted by ticks and their respective antigens for potential future additions are listed in Fig. 225,26,27,28,29,30 (https://tinyurl.com/mwz2u6kf). The recombinant antigens were expressed in E. coli bacteria using full-length cDNA coding for the respective antigens fused with a hexa histidine purification tag. The whole cell lysate was obtained from organisms cultured according to ATCC protocols prior to lysing them which yielded a cocktail of the cell membrane, cell wall, and cytosolic proteins. Peptide antigens were synthesized by photolithography as shown in our previous publications31,32. The capture antigens including the recombinant antigens that mimic the natural pathogen and the whole-cell lysate were incubated on the wafer at a concentration of 1.0 µg/ml and reacted for 24 h at 4 °C. The unbound antigens were removed by washing with aqueous phosphate buffer and the unreacted substrate was quenched with a blocking solution containing bovine serum albumin (BSA) and glycine. The immobilized antigens were classified with unique identifiers assigned to each wafer. In this study, we employed the microarray to detect Lyme disease, co-infections, and other agents of interest including, B. microti, B. henselae, A. phagocytophilum, E. chaffeensis, R. typhi, R. rickettsii, POWV, TBEV, WNV, coxsackie B virus, CMV, EBV, B19V, T. gondii, HSV-1, HSV-2, and HHV-6 [Fig. 2].
Pillar plate assembly
Individual wafers were stealth diced into 0.70 × 0.70mm2 microchips for each antigen. A standard die-sorting system was used to pick and place these wafers onto individual carrier tapes. The carrier tapes were then placed onto a high-throughput surface mount technology (SMT) component placement system. Finally, microchips were mounted onto 24 pillar plates and each pillar contains 87 microchips with each chip designated for one antigen – recombinant protein, peptide or whole cell lysates [Fig. 1].
Immunochip assay and antibody detection
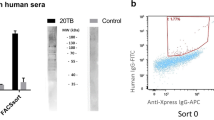
Serum samples were probed using 1:20 dilution on the pillar plate and incubated for 1 h at room temperature followed by alternate washing and incubation as described previously21. The plate was then incubated for an hour with the secondary antibody (1:2000 dilution of Goat Anti-Human IgG HRP and Goat Anti-Human IgM HRP individually) and washed with TBST buffer followed by DI Water. The plates were left for drying preceding the addition of chemiluminescent substrate and the performance of chemiluminescent imaging. An enhanced IgM sensitivity was achieved by pre-reacting the sera with goat anti-human IgG leading to IgG stripping prior to IgM testing.
The detection of multiplex antibodies is based on the chemiluminescent immunoassay and can be performed using < 200 µL of serum. Sample dilution, multi-step incubation, and multi-solution washing are programmed into liquid handlers. The immunochip has the capacity to assay 192 individual specimens in 2 h. Raw chemiluminescent signals for each probe are extracted and converted into intensity plots by an in-house reporter software. This method of automatic antigen detection can dramatically shorten the turnaround time, reduce the cost of labor and instrument, and eliminate the need for manual handling and subjective interpretation of the WB or IB test results when compared to the traditional two-tiered testing recommended by the CDC. All the antibodies are detected in a single run.
Data Analysis
An in-house software extracts the chemiluminescent signals from the generated images which were converted to intensity plots. The average intensity of each antibody was compared with the cut-off values assigned for each antigen to track seropositivity.
Results
Custom protein microarray platform
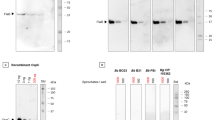
The main components of the Immunochip platform include multiple silicon-based 0.70 × 0.70 mm2 microchips that are laser diced from antigen-immobilized wafers, a customized 24 well compatible plate containing 24 pillars, each containing 87 microchips that are picked and placed into a multiplex microarray assembly, and a high-resolution imager capable of simultaneously detecting chemiluminescent signals from labelled antigen–antibody reactions at each microchip throughout the multiplex microarray (Fig. 1). Each chip can be considered analogous to an individual band in a western blot; however, the proteins are physically separated eliminating cross-reactive issues usually seen in blot-based assays for proteins with similar mass. Figure 1 provides an overview of the microarray manufacturing process. Figure 2 shows the individual chips that are placed in each pillar, a single serum sample will be applied to each pillar thereby assaying the antibodies in serum against all antigens at the same time.
Analysis of serological response
The Vibrant tick-borne disease panel tests for IgG and IgM antibodies for Lyme disease and other infectious agents as mentioned in Table 2; Fig. 2. The IgM and IgG immune responses of serum samples obtained from various laboratories (Table 1) were analysed, and the clinical sensitivities and specificities were tabulated in Table 3. The samples reacted with specific immunoreactive epitopes on the 87 different antigens that were being tested.
Enhanced IgM assay
IgM antibodies are markers of an acute primary infection and are produced during the first two weeks of infection, whereas IgG antibodies are produced few days later and may remain for life. Total IgM antibodies make up only 5–10% of all the circulating antibodies. B. burgdorferi IgM antibodies may persist in Lyme disease patients’ years after the initial infection33. An in-house IgM assay was developed with removal of most IgG antibodies and other non-specific proteins from the serum prior to the IgM immunoassay. This helped to increase the sensitivity and specificity of the assay. Human IgG was removed by incubating the serum with a purified goat anti-human (GAH) IgG Fc fragment.
Analytical performance
The analytical performance of the immunochip was evaluated for precision (repeatability/reproducibility), analytical sensitivity, reportable range, linearity, and matrix equivalency studies. Samples for negatives, low or moderate positives, and high positives were run with duplication to determine the analytical performance metrics. The precision study used a panel of 11 samples and was run over a period of 20 days with 2 duplicates per run and 4 runs per day. The results are tabulated as shown in Supplementary Table 3. Lot to Lot reproducibility was also tested to check for variation in the manufacturing of the pillar plates by running a panel of 11 samples with 5 replicates per run, 3 runs per day over a period of 5 days using 3 manufactured lots. The results are tabulated as shown in Supplementary Table 4. Testing of protein-free serum matrix samples and low antibody concentration samples with 2 replicates per run, 2 runs per day over a period of three days was used to determine analytical sensitivity. The limit of blank (LoB) and limit of quantitation (LoQ) was calculated using the mean and standard deviation of the blank and the low antibody concentration samples as shown in Supplementary Table 5. The linearity and reportable range were verified by running samples with varying levels of antibodies and checking assay recovery, the results are tabulated in Supplementary Table 6. Matrix equivalence studies are shown in Supplementary Table 7. The potential interference of specific endogenous and exogenous substances with the immunochip was evaluated by performing an interfering substance study. The interfering substances tested were 60 mg/dl bilirubin, 100 mg/ml cholesterol, 1000 mg/ml triglycerides, 1000 mg/ml hemoglobin, and 6 g/dl albumin. There was no interference between the immunochip and the substances tested at the mentioned levels.
Clinical sensitivity and specificity
Table 3 provides an overview of the IgG and IgM sensitivities and specificities measured by the Vibrant microarray. This is compared with the pperformance of the current gold standard tests for the particular pathogen. The Vibrant microarray was able to achieve high sensitivities and specificities when compared with the gold standards for each pathogen. Supplementary Table 8 provides more details on the gold standard diagnostic tests for the pathogens along with the modes of transmission and their endemic regions.
Evaluating the antigens ofBorrelia burgdorferi.
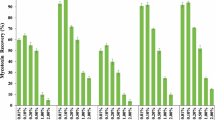
In this study, individual antigens of B. burgdorferi were tested for reactivity with IgG and IgM antibodies (Table 4). The heat map (Fig. 3) shows the performance metrics of the different antigens. Testing for Lyme disease since 1994 has been based on conventional two-tiered testing (CTTT) where an enzyme immunoassay (EIA) is followed by a specific immunoblot for a definitive diagnosis. Recently, CTTT has been replaced with a modified two-tier testing (MTTT) in which an EIA using whole cell lysate is followed by an EIA using C6 peptide. This shows the increasing shift away from blot-based testing to conventional ELISA. MTTT removes the burden of immunoblots which are tedious to run, more expensive and could have subjective interpretation of bands49. Complete replacement of immunoblots can be done using a microarray platform, such as the one described here. The full data set would be available to the physicians to make a nuanced diagnosis instead of a narrow subset of antigens run on ELISAs.
Heat map showing Lyme disease antigen reactivity. The positivity cutoff for each antigen was set at greater than 10 chemiluminescent units (CU) (shown as yellow or red). The color key is as follows: Red - High positive (CU > 20); Yellow, orange - Moderate positive (CU = 10.1–20); White - Negative (CU ≤ 10).
Discussion
Ticks are among the most important sources of vector-borne infections in the US50. The spread of ticks across the US has been steadily increasing over the past decades. In parallel, the discovery of novel pathogens that are spread by ticks has also seen dramatic increases51. Currently, there are 14 major tickborne diseases according to the CDC namely, Lyme disease, babesiosis, ehrlichiosis, Rocky Mountain spotted fever, Southern tick-associated rash illness (STARI), hard tick relapsing fever, soft tick relapsing fever, tularemia, anaplasmosis, Colorado tick fever, bourbon virus, heartland virus, Rickettsia parkeri rickettsiosis, 364D rickettsiosis, and Powassan virus52 (https://www.cdc.gov/ticks/diseases/index.html). Patients are rarely tested for all possible infections that could be transmitted via a tick bite53. The current diagnostic tests are severely limited in distinguishing various tick-borne infections and several studies have revealed that non-Lyme disease tick-borne infections are heavily underdiagnosed54.
Among varied testing options, PCR and serology-based assays are reliable and most widely used. PCR has several advantages as it detects pathogenic DNA/RNA which conclusively proves the organism’s presence. It has high specificity and has a high throughput with assay run times of about 2 h. It can also detect the infection during its early stages55. There are however certain drawbacks to testing using PCR, especially with tickborne infections. Pathogens transmitted by vectors may be transient in the blood resulting in clinically false negative PCR in tick-borne diseases and other infections, namely, B. burgdorferi, R. typhi, T. gondii, HSV-1, EBV, TBEV, and WNV23. PCR testing requires specialised laboratories and equipment for testing. PCR may not detect all strains and variants and is limited to detecting known pathogens56. Multiplexing with PCR is limited due to fixed number of analytes that can be parallelly read using PCR instrumentation.
Serology-based testing has several advantages when it comes to tick-borne infection testing. It has the ability to comprehensively assess immune responses and simultaneously detect exposure to multiple pathogens including previous and unresolved infections. Testing two times with a time interval in between can also help diagnose active infections based on altered serum antibody profiles. Simultaneous detection of antibodies against multiple tick-borne pathogens and other related pathogens using a single sample and providing a comprehensive view of the patient’s immune response is a key advantage of serology-based multiplex testing23. The testing can also be done in resource poor settings with collection using DBS as described for Covid-1922. Serological testing can diagnose tick-borne diseases even in the later stages when pathogen detection through molecular methods becomes more challenging23,57. It also reduces the risk of false negatives57. Serological multiplex testing being cost-effective can also contribute to surveillance and epidemiological studies by providing valuable data on the prevalence and distribution of tick-borne diseases, enhancing our understanding of disease dynamics58. Patients can be asymptomatic during the initial stages or may not remember the tick-bite. Serology is useful to detect the infection in such cases. However, serological studies have their own limitations. Serological testing may not be able to detect early/recent infections. It relies heavily on the timing of sample collection and the host’s immune responses. In certain cases, molecular testing may be needed to confirm serological testing23. Despite all this, the benefits of serology testing outweigh its limitations which is why it is recommended by the CDC as the standard of testing for Lyme disease.
Apart from PCR and ELISA serology tests, IFA and culture methods have also been suggested for diagnosing tick-borne infections. Testing using IFA is limited due to a lack of standardized antigenic targets, the subjective establishment of positive thresholds, and cross reactivity. These factors can result in varying accuracy of IFA results across laboratories23. Furthermore, bacterial or viral cultures are not recommended for the diagnosis of tick-borne infections. This is due to the time-consuming nature of the test, the need for special media, and procedures that are only performed at specific laboratories59,60.
This study employed a serology-based microarray developed at Vibrant to multiplex Lyme disease and other tick-borne infections along with a few other infections of interest. The uniqueness of the microarray lies in the application of the immunodominant antigens that eliminate nonspecific binding with high sensitivity needed for accurate diagnosis. Antigens could be evaluated in a multiplex setting to gauge their performance with clinical samples to pick the ideal set of antigens for any infection. To the best of our knowledge, this is the first report on a broad panel of antigens for Lyme disease, co-infections, and other related infections in such a flexible format. The structure of the Vibrant pillar plate is designed to encompass 400 probe chips at each pillar facilitating the detection of an array of co-infections in a single run, saving cost, labor, and time. Further compaction of the chip allows improved performance by enhancing multiplexing and widening its clinical applications. The microarray platform is advantageous over other existing gold standards for tick-borne diseases and was able to overcome several of their limitations. Average time for multitier testing for several tickborne pathogens could take several months whereas the microarray technology takes only about a day to perform21. The microarray detected 17 tick-borne and other infections along with Lyme disease with sensitivities and specificities listed in Table 3.
In conclusion, the protein microarray with a multiplex of antigens was validated for Lyme disease, co-infections, and other related infections. Simultaneous testing for Lyme disease, co-infections, and other related infections makes the diagnosis and treatment easier and quicker. This approach caters to the diagnostic needs of patients owing to its high sensitivity and specificity, affordable cost, quick availability of results, and low sample volume requirement. Measures for syndromic surveillance, diagnostic preparedness in disease outbreak investigations, personal protection, and education of clinical health professionals and patients could play a role in controlling tick-borne infections. As the known repertoire of antigens increases, this flexible microarray format can be customised to include the new antigens. Future editions could also include other infections/agents namely Colorado tick fever, heartland virus, R. parkeri, tick-borne relapsing fever, and tularemia which can be tested in parallel. Novel antigens for pathogens which may include whole cell lysates, recombinant proteins or peptide epitopes can be added as the science progresses leading to continuous improvement in diagnostic technology for detecting tick-borne infections.
Data availability
The data used to support the findings of this study can be acquired from Karthik Krishna (karthik@vibrantsci.com).
Change history
04 July 2025
The original online version of this Article was revised: The original version of this Article omitted an affiliation for Daniel Růžek. The article has been corrected.
References
Rochlin, I. & Toledo, A. Emerging tick-borne pathogens of public health importance: a mini-review. J. Med. Microbiol. 69(6), 781–791 (2020).
Burgdorfer, W. et al. Lyme disease—a tick-borne spirochetosis? Science 216(4552), 1317–1319 (1982).
Johnson, R. C., Schmid, G. P., Hyde, F. W., Steigerwalt, A. G. & Brenner, D. J. Borrelia burgdorferi sp. nov.: etiologic agent of Lyme disease. Int. J. Syst. Evol. MicroBiol. 34(4), 496–497 (1984).
Steere, A. C. et al. Lyme borreliosis. Nat. Reviews Disease Primers. 2(1), 1–19 (2016).
Berghoff, W. Chronic lyme disease and co-infections: differential diagnosis. Open. Neurol. J. 6(Suppl 1), 158–178 (2012).
Knapp, K. L. & Rice, N. A. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J. Parasitol. Res. 16(3), 385–391 (2015).
Angelakis, E., Billeter, S. A., Breitschwerdt, E. B., Chomel, B. B. & Raoult, D. Potential for tick-borne bartonelloses. Emerg. Infect. Dis. 16(3), 385–391 (2010).
Ismail, N., Bloch, K. C. & McBride, J. W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 30(1), 261–292 (2010).
Hart, C. E., Middleton, F. A. & Thangamani, S. Infection with Borrelia burgdorferi increases the replication and dissemination of coinfecting Powassan Virus in Ixodes scapularis ticks. Viruses 14(7), 1584 (2022).
Ben-Harari, R. R. Tick transmission of toxoplasmosis. Expert Rev. Anti-infective Therapy. 17(11), 911–917 (2019).
Koetsveld, J., Tijsse-Klasen, E., Herremans, T., Hovius, J. W. R. & Sprong, H. Serological and molecular evidence for spotted fever group Rickettsia and Borrelia burgdorferi Sensu Lato co-infections in the Netherlands. Ticks Tick. Borne Dis. 7(2), 371–377 (2016).
Gustafson, R., Svenungsson, B., Gardulf, A., Stiernstedt, G. & Forsgren, M. Prevalence of tick-borne encephalitis and Lyme borreliosis in a defined Swedish population. Scand. J. Infect. Dis. 22(3), 297–306 (1990).
Lawrie, C. H., Uzcátegui, N. Y., Gould, E. A. & Nuttall, P. A. Ixodid and argasid tick species and West Nile virus. (2004).
Smith, A., Oertle, J., Warren, D. & Prato, D. Chronic Lyme Disease Complex and its commonly undiagnosed primary and secondary co-infections. Open. J. Med. Microbiol., 5(3). (2015).
Freundt, E. C., Beatty, D. C., Stegall-Faulk, T. & Wright, S. M. Possible Tick-Borne Human Enterovirus resulting in aseptic meningitis. J. Clin. Microbiol. 43(7), 3471–3473 (2005).
Gylfe, A., Wahlgren, M., Fahlén, L. & Bergström, S. Activation of latent Lyme borreliosis concurrent with a herpes simplex virus type 1 infection. Scand. J. Infect. Dis. 34(12), 922–924 (2002).
Wormser, G. P. et al. S. J. Co-infections in persons with early Lyme disease, New York, USA. Emerging infectious diseases, 25(4), 748. (2019).
Quest diagnostics. Tick-borne Diseases (Quest Diagnostics, 2018).
Drew, D. & Hewitt, H. A qualitative approach to understanding patients’ diagnosis of Lyme disease. Public Health Nurs. 23(1), 20–26 (2006).
Beck, S. et al. A protein microarray-based respiratory viral antigen testing platform for COVID-19 surveillance. Biomedicines 10(9), 2238 (2022).
Jayaraman, V. et al. An ultra-high-density protein microarray for high throughput single-tier serological detection of Lyme disease. Sci. Rep. 10, 18085 (2020).
Grossberg, A. N. et al. A multiplex chemiluminescent immunoassay for serological profiling of COVID-19-positive symptomatic and asymptomatic patients. Nature communications, 12(740). (2021).
Tokarz, R. et al. A multiplex serologic platform for diagnosis of tick-borne diseases. Sci. Rep. 8, 3158 (2018).
Rajasekaran, J. J., Jayaraman, V., Wang, T., Bei, K. & Krishnamurthy, H. K. Methods, systems, and arrays for biomolecular analysis. Patent No. 9417236B2. United States. (2016).
Centers for Disease Control and Prevention. Tickborne Diseases of the United States. Centers for Disease Control and Prevention. Retrieved September 8, 2023. (2022), August 5.
Alhassan, A. et al. Rickettsia rickettsii whole-cell antigens offer protection against rocky mountain spotted fever in the canine host. Infect. Immun. 87(2), e00628–e00618 (2019).
Blanc, G. et al. Molecular evolution of rickettsia surface antigens: evidence of positive selection. Mol. Biol. Evol. 22(10), 2073–2083 (2005).
Pornwiroon, W., Bourchookarn, A., Paddock, C. D. & Macaluso, K. R. Proteomic analysis of Rickettsia parkeri strain portsmouth. Infect. Immun. 77(12), 5262–5271 (2009).
Kubelkova, K. & Macela, A. Francisella Antibodies Microorganisms, 9(10), 2136. (2021).
Zhu, Y. et al. The Postfusion structure of the heartland virus gc glycoprotein supports taxonomic separation of the bunyaviral families Phenuiviridae and Hantaviridae. J. Virol. 92(1), e01558–e01517 (2017).
Marietta, E. V. et al. Determination of B-cell epitopes in patients with celiac dsease: peptide microarrays. PLoS One. 11(1), e0147777 (2016).
Rostamkolaei, S. K. et al. Synthetic neoepitopes of the transglutaminase-deamidated gliadin complex as biomarkers for diagnosing and monitoring celiac disease. Gastroenterology 156(3), 582–591e1 (2019).
Kalish, R. A. et al. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin. Infect. Dis. 33(6), 780–785 (2001).
Baarsma, M. E. et al. Diagnostic parameters of modified two-tier testing in European patients with early Lyme disease. Eur. J. Clin. Microbiol. Infect. Diseases: Official Publication Eur. Soc. Clin. Microbiol. 39(11), 2143–2152. https://doi.org/10.1007/s10096-020-03946-0 (2020).
Ortiz, J. F. et al. Babesiosis: appreciating the pathophysiology and diverse sequela of the infection. Cureus, 12(10). (2020).
Allizond, V., Costa, C., Sidoti, F., Scutera, S., Bianco, G., Sparti, R., … Musso,T. (2019). Serological and molecular detection of Bartonella henselae in specimens from patients with suspected cat scratch disease in Italy: A comparative study. PloS one, 14(2), e0211945.
Reller, M. E. & Dumler, J. S. Development and clinical validation of a multiplex real-time quantitative PCR assay for human infection by Anaplasma phagocytophilum and Ehrlichia chaffeensis. Trop. Med. Infect. Disease. 3(1), 14 (2018).
Stewart, A. G. & Stewart, A. G. An update on the laboratory diagnosis of Rickettsia spp. infection. Pathogens 10(10), 1319 (2021).
Thomm, A. M., Schotthoefer, A. M., Dupuis, A. P., Kramer, L. D., Frost, H. M., Fritsche,T. R., … Kehl, S. C. (2018). Development and validation of a serologic test panel for detection of Powassan virus infection in US patients residing in regions where Lyme disease is endemic. Msphere, 3(1), e00467-17.
Reusken, C., Boonstra, M., Rugebregt, S., Scherbeijn, S., Chandler, F., Avšič-Županc,T., … GeurtsvanKessel, C. H. (2019). An evaluation of serological methods to diagnose tick-borne encephalitis from serum and cerebrospinal fluid. Journal of Clinical Virology, 120, 78–83.
Girl, P. et al. Comparison of five serological methods for the detection of West Nile Virus antibodies. Viruses 16(5), 788 (2024).
Bryant, P. A., Tingay, D., Dargaville, P. A., Starr, M. & Curtis, N. Neonatal coxsackie B virus infection—a treatable disease? Eur. J. Pediatrics. 163, 223–228 (2004).
Ross, A., Novak, S., Pati, Z., Boppana, B. & S., &, S Overview of the diagnosis of cytomegalovirus infection. Infect. Disorders-Drug Targets (Formerly Curr. Drug Targets-Infectious Disorders). 11(5), 466–474 (2011).
Jenson, H. B. Virologic diagnosis, viral monitoring, and treatment of Epstein-Barr virus infectious mononucleosis. Curr. Infect. Dis. Rep. 6, 200–207 (2004).
Manaresi, E. et al. Diagnosis and quantitative evaluation of parvovirus B19 infections by real-time PCR in the clinical laboratory. J. Med. Virol. 67(2), 275–281 (2002).
Souza, I. M. F. N. B. D., Siqueira, V. D. S., Ribeiro, I. D. C., Moraes, L. S. P.,Prado, D. P. G. D., Rezende, S. R., … Rezende, H. H. A. (2023). Molecular and serological diagnosis of toxoplasmosis: a systematic review and meta-analysis. Revista do Instituto de Medicina Tropical de São Paulo, 65, e19.
Singh, A., Preiksaitis, J. & Romanowski, B. The laboratory diagnosis of herpes simplex virus infections. Can. J. Infect. Dis. Med. Microbiol. 16(2), 92–98 (2005).
Norton, R. A. et al. Detection of human herpesvirus 6 by reverse transcription-PCR. J. Clin. Microbiol. 37(11), 3672–3675 (1999).
Lipsett, S. C., Branda, J. A. & Nigrovica, L. E. Evaluation of the modified two-tiered testing method for diagnosis of Lyme disease in children. J. Clin. Microbiol. 57(10), e00547–e00519 (2019).
Eisen, R. J., Kugeler, K. J., Eisen, L., Beard, C. B. & Paddock, C. D. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 58(3), 319–335 (2017).
Paddock, C. D. et al. CHANGING PARADIGMS FOR TICK-BORNE DISEASES IN THE AMERICAS. In: Forum on Microbial Threats; Board on Global Health; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine. Global Health Impacts of Vector-Borne Diseases: Workshop Summary. Washington (DC): National Academies Press (US). (2016).
About Ticks and Tickborne Centres for Disease Control and Prevention & Diseases | Ticks. CDC. Retrieved June 20, 2024. (2024), May 15.
Brown Marusiak, A. et al. Patterns testing for Tick-Borne diseases and implications for Surveillance in the Southeastern US. JAMA Netw. open. 5(5), e2212334 (2022).
Institute of Medicine (US) Committee on Lyme Disease and Other Tick-Borne Diseases: The State of the Science. Diagnostics and diagnosis. In Critical Needs and Gaps in Understanding Prevention, Amelioration, and Resolution of Lyme and Other Tick-Borne Diseases: The Short-Term and Long-Term Outcomes: Workshop Report. (National Academies Press (US), 2011).
Elnifro, E. M., Ashshi, A. M., Cooper, R. J. & Klapper, P. E. Multiplex PCR: optimization and application in diagnostic virology. Clin. Microbiol. Rev. 13(4), 559–570 (2000).
Liu, H. Y. et al. Polymerase chain reaction and its application in the diagnosis of infectious keratitis. Med. Hypothesis Discovery Innov. Ophthalmol. 8(3), 152 (2019).
Leeflang, M. M. et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect. Dis. 16, 140 (2016).
Hilton, E. et al. Seroprevalence and seroconversion for tick-borne diseases in a high-risk population in the northeast United States. Am. J. Med. 106(4), 404–409 (1999).
Aguero-Rosenfeld, M. E., Wang, G., Schwartz, I. & Wormser, G. P. Diagnosis of lyme borreliosis. Clin. Microbiol. Rev. 18(3), 484–509 (2005).
Theel, E. S., Aguero-Rosenfeld, M. E., Pritt, B., Adem, P. V. & Wormser, G. P. Limitations and confusing aspects of diagnostic testing for neurologic Lyme disease in the in the United States. J. Clin. Microbiol. 57(1), e01406–e01418 (2019).
Acknowledgements
Vibrant Sciences LLC developed the microarray technology showcased in the publication. All IP associated with the microarray manufacture and diagnostics belongs to Vibrant Sciences. The specific roles of authors are stated in the author contribution section.
Funding
Vibrant America provided funding for this study in the form of salaries for authors [CS, SM, KK, VJ, TW, KB, HKK, JJR]. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conception and study design: HKK, JJR, VJ. Performing experiments: KK, TW. Analysis and interpretation: KB, KK. Writing-original draft: HKK, CC, SM. Review and editing: HKK, CC, AJR, RWF, AP, LSB, AC, AF, DR, GKN, LJA, DWA, MAA, DL, CF, PD, MF, MSV, KH, DC, AP, EM, and AN. Sample resources: RWF, AP, LSB, AC, AF, DR, GKN, LJA, DWA, MAA, DL, CF, PD, MF, MSV, KH, DC, EM, AN, AP, and AE. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have read the journal’s policy and the authors of this manuscript have the following competing interests: CC and SM are paid employees of Vibrant America LLC. KK, VJ, TW, KB, HKK, and JJR are paid employees of Vibrant Sciences LLC. RWF, AP, LSB, AC, AF, DR, GKN, LJA, DWA, MAA, DL, CF, PD, MF, MSV, KH, DC, EM, AN, AP, and AE are academic collaborators who provided samples and assisted in the review and editing of the manuscript. AJR is a paid consultant of Vibrant America LLC. Vibrant America offers commercial testing for Lyme disease and other infectious diseases and could benefit from increased testing.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Krishnamurthy, H.K., Jayaraman, V., Krishna, K. et al. A customizable multiplex protein microarray for antibody testing and its application for tick-borne and other infectious diseases. Sci Rep 15, 2527 (2025). https://doi.org/10.1038/s41598-024-84467-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-024-84467-0