Abstract
Abiotic stresses, notably cold stress, significantly influence various aspects of plant development and reproduction. Various approaches have been proposed to counteract the adverse impacts of cold stress on plant productivity. The unique properties of nanoparticles contribute to an enhanced tolerance of plants to challenging conditions. This study explores the impact of titanium dioxide nanoparticles (TiO2 NPs) on cold-stress tolerance in fenugreek, as well as genes expression involved in the diosgenin biosynthesis pathway. Varied concentrations of TiO2 NPs (0, 2, 5, and 10 ppm) were sprayed on fenugreek plants subjected to cold stress at 10 °C during 6, 24, and 48 h. Our findings revealed that the utilization of 2 and 5 ppm of TiO2 NPs, positively influenced pigments biosynthesis and enzymatic and non-enzymatic antioxidant activities. It also effectively reduced electrolyte leakage and malondialdehyde content, mitigating the adverse effects of cold stress. The study also highlighted TiO2 NPs’ affirmative impact on defense signaling pathways, including abscisic acid, nitric oxide, and auxin, in fenugreek. Moreover, TiO2 NPs significantly influenced the expression of genes related to diosgenin biosynthesis. Simultaneous exposure to cold stress and TiO2 NPs led to a substantial increase in diosgenin content, with the upregulation of SEP, SQS, CAS, and SSR genes compared to control conditions. This research indicated that TiO2 NPs application could effectively stimulate fenugreek biosynthesis of primary and secondary metabolites, consequently enhancing plant tolerance to cold stress. The study’s outcomes hold promise for potential applications in the metabolic engineering of diosgenin in fenugreek.
Similar content being viewed by others
Introduction
The escalating global population and unfavorable environmental conditions leading to reduced agricultural production have sparked concerns worldwide. The adverse impact of environmental stresses, particularly on plant growth and yield, underscores the need for in-depth investigations into plant responses to these challenges1. Abiotic stresses, notably cold stress (CS), significantly influence various aspects of plant development and reproduction2. These stresses induce changes in plants’ morphological, physiological, biochemical, and molecular characteristics, ultimately leading to adaptations to stressful conditions. CS induces molecular changes in plants, varying with the intensity and duration of exposure. In the short term, cold triggers membrane rigidification, increased reactive oxygen species (ROS), and activation of cold-responsive (COR) genes via transcription factors like CBF/DREB, leading to the production of protective proteins, osmolytes, and antioxidants. Medium-term exposure enhances these responses, with COR proteins stabilizing cellular structures, membranes undergoing lipid desaturation to maintain fluidity, and metabolic shifts prioritizing survival over growth. Prolonged cold or freezing leads to epigenetic modifications, increased accumulation of cryoprotective metabolites, and structural adaptations such as thicker cell walls and extracellular ice nucleation to prevent intracellular freezing. With persistent exposure, plants may enter dormancy to minimize metabolic activity. However, in cold-sensitive species, extreme or extended cold can overwhelm these defenses, causing irreversible cellular damage. These mechanisms illustrate plants’ dynamic strategies for cold acclimation and freezing tolerance2,3,4.
Various approaches have been proposed to counteract the adverse impacts of CS on plant productivity. One effective method involves the use of nanoparticles (NPs) to alleviate the harmful consequences of abiotic stresses5. Titanium dioxide nanoparticles (TiO2 NPs) have garnered significant attention and found widespread use across industries. Numerous studies substantiate the positive effects of TiO2 NPs in enhancing plant tolerance to abiotic stresses, providing compelling evidence of their efficacy5,6. A notable example demonstrating the efficacy of TiO2 NPs treatment in mitigating the impact of cold stress can be observed in chickpea plants, resulting in distinct gene expression patterns related to cell signaling, cell defense, chromatin modification, and transcription regulation4. Furthermore, significant improvements were observed in photosynthesis efficiency, energy metabolism, and hydrogen peroxide (H2O2) content of chickpea plants, attributed to the positive regulation of genes involved in the biosynthesis of phosphoenolpyruvate carboxylase (PEPC), both subunits of RUBISCO, and chlorophyll a/b binding proteins upon the application of TiO2 NPs. Notably, TiO2 NPs stand out among other particles due to their high chemical resistance, non-toxicity, long lifespan, widespread availability, and cost-effectiveness7. Recent research has highlighted the significant role of TiO2 NPs in effectively reducing the accumulation of malondialdehyde (MDA) through the induction of antioxidant systems6. Earlier studies have also attested that TiO2 NPs can prevent oxidative stress and decrease membrane damage under CS conditions in plants8.
Trigonella foenum-graecum (fenugreek), an annual plant of the Fabaceae family, is native to India and North Africa. With a rich history in culinary use and a common presence in herbal medicine, fenugreek stands out for its precious source of diosgenin9. Diosgenin, a biologically active steroid sapogenin, proves highly effective in treating various ailments, including hyperlipidemia, diabetes, cardiovascular disease, cancer, oxidative stress, and inflammation10. The application of stimulants and elicitors represents a highly successful strategy for enhancing the production of secondary metabolites, particularly those produced at low concentrations or absent under normal conditions11,14. Stimulants and elicitors play a crucial role in augmenting the biosynthesis of secondary metabolites by directly and indirectly inducing the expression of genes associated with their production15.
The selection of the ideal planting date emerges as a pivotal factor in achieving maximum crop yield16. In Iran, fenugreek offers flexibility in planting, allowing for sowing in either autumn or spring. Autumn crops generally yield higher due to the extended growing season. However, delays in autumn planting may expose the plant to damage from CS17. To mitigate this challenge, the development of cold-tolerance varieties or the application of elicitors could be effective. The current study endeavors to evaluate the effectiveness of TiO2 NPs in enhancing fenugreek plant tolerance to CS and to examine their impact on diosgenin content. Additionally, the study seeks to identify the optimal concentration of TiO2 NPs for maximizing diosgenin production in fenugreek.
Results
Pigments content
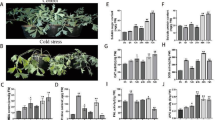
Exposure to cold stress for 48 h resulted in a significant reduction in chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents, with decreases of 61%, 72%, 67%, and 58%, respectively (p < 0.01), compared to normal conditions without the application of TiO2 NPs. However, when different levels of TiO2 NPs were applied in the absence of cold stress, particularly at 2 and 5 ppm, there was a notable increase in chlorophylls and carotenoid contents compared to normal conditions (Table 1). The highest contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid were recorded at 8.50, 3.34, 11.84, and 3.43 mg g− 1 FW, respectively, after 48 h of using 5 ppm TiO2 NPs without cold stress. Interestingly, the application of different levels of TiO2 NPs combined with cold stress not only prevented the reduction of chlorophyll and carotenoid contents but also led to their increase compared to cold treatment without TiO2 NPs application. The results demonstrated that treating fenugreek plants subjected to 48 h of cold stress with 5 ppm TiO2 NPs effectively boosted chlorophyll a (by 145%), chlorophyll b (by 200%), total chlorophyll (by 165%), and carotenoid (by 105%) contents when compared to cold stress alone (Table 1).
Electrolyte leakage and MDA content
The percentage of EC and MDA content exhibited a significant increase (2-fold, p < 0.01) with the prolongation of cold stress duration from 6 to 48 h, compared to normal temperature conditions. However, the treatment of plants subjected to cold stress, as well as plants at normal temperature, with different levels of TiO2 NPs (especially 2 and 5 ppm) resulted in a significant reduction in EC percentage and MDA content. Interestingly, the highest values for these two indicators (MDA: 27 µmol g− 1 FW and EC: 36%) were observed in the cold stress group treated with 10 ppm TiO2 NPs, while the lowest values (MDA: 8.67 µmol g− 1 FW and EC: 11%) were recorded with the application of 5 ppm TiO2 NPs (Table 1). This suggests that TiO2 NPs, particularly at lower concentrations, effectively mitigate the adverse effects of cold stress on cell membrane integrity and lipid peroxidation in fenugreek plants.
Soluble protein content and antioxidant activities
Cold stress, particularly after 48 h, resulted in a significant increase in soluble protein content (p < 0.01) compared to normal conditions (Table 2). Interestingly, the application of 5 ppm TiO2 NPs, 24 and 48 h after application under non-stress conditions, further enhanced the soluble protein content by 33% and 48%, respectively, compared to normal conditions. Additionally, subjecting the plants to cold stress along with TiO2 NPs resulted in a significant elevation in soluble protein content compared to cold stress treatment alone (without TiO2 NPs application). The highest soluble protein content (118.33 mg g− 1 FW protein) was observed after 48 h of cold stress and 5 ppm TiO2 NPs treatment, representing an 85% increase compared to the control (Table 2).
The activity of APX exhibited a notable increase upon lowering the temperature from 23 °C to 10 °C (p < 0.01). While extending the sampling time from 6 to 48 h and applying 2 and 5 ppm TiO2 NPs resulted in a significant elevation in APX activity, the application of 10 ppm TiO2 NPs nearly halved the activity of this enzyme. Additionally, the highest recorded APX activity (41 nmol oxidized ascorbate min− 1 mg− 1 protein) was observed during 48 h of cold stress with 5 ppm TiO2 NPs (Table 2).
In comparison to normal temperature, the CAT activity exhibited a sharp increase in response to applied cold stress. The utilization of 2 and 5 ppm of TiO2 NPs significantly influenced this enzyme, with the activity doubling (100%) 48 h after the application of 5 ppm TiO2 NPs compared to the control (without TiO2 NPs and normal temperature). The simultaneous application of various levels of TiO2 NPs and cold stress significantly heightened the activity of this enzyme. The highest CAT activity was recorded at 49 nmol of H2O2 decomposed min− 1 mg− 1 protein with the application of 5 ppm TiO2 NPs and after 48 h of cold stress. However, applying 10 ppm TiO2 NPs had an adverse effect on CAT activity and lowered it to 10 and 12 nmol of H2O2 decomposed min− 1 mg− 1 protein at 23 °C and 10 °C, respectively (Table 2).
GPX activity in plants subjected to cold stress was twice as high as in plants at normal temperature, particularly after 48 h of cold stress. Moreover, the application of 2 and 5 ppm of TiO2 NPs in both temperature groups elevated the activity of this enzyme, with the highest GPX activity (43 nmol guaiacol min− 1 mg− 1 protein) observed under the application of 5 ppm TiO2 NPs after 48 h of cold stress (Table 2). It is noteworthy that the application of 10 ppm TiO2 NPs under both normal and cold conditions reduced the activity of this enzyme by 35% and 53%, respectively, compared to the control (without TiO2 NPs application).
The application of cold stress significantly enhanced the activity of the SOD enzyme compared to the normal conditions (p < 0.01). Also, with the application of TiO2 NPs and increasing the sampling time, the activity of this enzyme increased remarkably, with the highest amount observed in pots treated with 5 ppm of TiO2 NPs after 48 h (43 nmol min− 1 mg− 1 protein). The concomitant application of 5 ppm TiO2 NPs and 48 h of cold treatment enhanced SOD activity by 40% compared to cold stress alone (without TiO2 NPs). At the same time, the effect of 10 ppm TiO2 NPs and increasing the sampling time reduced the activity of this enzyme to some extent (Table 2).
Total phenol and proline content
Total phenol and proline content exhibited a significant increase (p < 0.01) with prolonged exposure to cold stress compared to the control. Moreover, using TiO2 NPs without cold stress elevated these two traits compared to normal conditions. The application of 5 ppm TiO2 NPs after 48 h under control temperature increased the total phenol and proline content by 67% and 90%, respectively, compared to the TiO2 NPs-free condition. The application of various levels of TiO2 NPs in cold-treated plants resulted in an elevation of these traits compared to cold stress conditions alone. Exposure to 48 h of cold stress along with the application of 5 ppm TiO2 NPs raised the total phenol and proline contents by 200 and 205%, respectively, compared to the TiO2 NPs-free and no cold-stress conditions. However, these compounds significantly decreased with the increase of TiO2 NPs to 10 ppm (Fig. 1).
Phytohormones content
The impact of various durations (from 6 to 48 h) of cold stress on auxin content revealed a significant decline (p < 0.01) compared to normal conditions (Fig. 1). Under cold stress alone, auxin content decreased by 32% and 51% after 24 and 48 h, respectively, compared to normal conditions. The combined application of TiO2 NPs and cold stress significantly enhanced auxin content compared to cold treatment alone. The highest auxin content, reaching 48.26 ng g− 1 FW, was observed with the application of 5 ppm TiO2 NPs after 48 h of cold stress (Fig. 1).
The mean comparison of the effects of TiO2 NPs concentrations and different harvesting times at 23 ºC and 10 ºC on the content of total phenol, proline, auxin, and abscisic acid. T0, T2, T5, and T10 indicate the levels of 0, 2, 5, and 10 ppm of TiO2 NPs, respectively. H6, H24, and H48 represent different harvest times (6, 24, and 48 h) after the applied treatments, respectively. The mean values are compared using Duncan’s method at a 1% probability level. The same letters represent insignificant differences.
Moreover, cold stress led to a time-dependent increase (p < 0.01) in the ABA content compared to normal conditions. Cold stress raised ABA content by 60% after 48 h compared to the normal temperature. Additionally, the application of TiO2 NPs without cold stress increased ABA content compared to control conditions. The simultaneous application of different levels of TiO2 NPs and cold stress, especially 5 ppm after 48 h, caused a significant 37% increase in ABA content compared to cold stress treatment alone (without TiO2 NPs application) (Fig. 1).
H2O2 content
The application of cold stress induced a significant increase (p < 0.01) in the H2O2 content compared to normal temperature conditions (Fig. 2). Specifically, after 24 and 48 h of cold stress, the H2O2 content increased by more than 2 and 3 times, respectively, compared to the control conditions. Interestingly, the addition of different levels of TiO2 NPs to the pots experiencing 48 h of cold stress had a mitigating effect on the H2O2 content. In comparison to conditions without TiO2 NPs, the addition of TiO2 NPs led to a reduction in H2O2 content by 24%, 75%, and 21%, respectively (Fig. 2).
The comparison effects of TiO2 NPs concentrations and different harvesting times at 23 ºC and 10 ºC on hydrogen peroxide and nitric oxide content. T0, T2, T5, and T10 indicate different concentrations of TiO2 NPs (0, 2, 5, and 10 ppm, respectively) .H6, H24, and H48 represent different harvest times after the applied treatments (6, 24, and 48 h, respectively).The mean values are compared using Duncan’s method at a 1% probability level. The same letters represent insignificant differences.
NO content
The NO content in plants subjected to cold stress exhibited significant growth (p < 0.01), especially 48 h after cold stress, compared to normal conditions (Fig. 2). The application of various levels of TiO2 NPs, particularly at 5 ppm under both normal and cold treatments, led to a notable increase in this trait compared to normal conditions (without TiO2 NPs application). The simultaneous application of different levels of TiO2 NPs and cold stress significantly elevated NO levels. However, the highest level of NO was recorded after 48 h of using 5 ppm TiO2 (38 nmol g− 1 FW) under cold stress (Fig. 2).
Gene expression analysis
The expression level of the SQS gene under normal temperature, with the application of 2, 5, and 10 ppm of TiO2 NPs (24 h), increased by 4.7, 4.4, and 0.8-fold, respectively, compared to the control conditions (non-TiO2 NPs) (Fig. 3). Treating plants exposed to cold stress with TiO2 NPs significantly induced the expression of the SQS gene (in some treatments, not necessarily in all of them). Notably, the highest expression level of the SQS gene, reaching 32-fold over the control (normal temperature and without TiO2 NPs application), was recorded when applying 24 h cold stress with the addition of 5 ppm of TiO2 NPs (Fig. 3).
The mean comparison of the effects of TiO2 NPs concentrations and different harvesting times at 23 ºC and 10 ºC on the gene expression levels of SQS, SEP, CAS, and SMT. T0, T2, T5, and T10 indicate the levels of 0, 2, 5, and 10 ppm of TiO2 NPs, respectively. H6, H24, and H48 represent different harvest times (6, 24, and 48 h) after the applied treatments, respectively. The mean values are compared using Duncan’s method at a 1% probability level. The same letters represent insignificant differences.
The results of this study indicated that the expression pattern of the SEP gene is significantly affected by the interaction between TiO2 NPs concentrations and temperature (Fig. 3). Applying 2 ppm TiO2 NPs under normal temperature and 48 h after sampling resulted in around a 13-fold increase in the expression of this gene compared to the control (without TiO2 NPs). At normal temperature, by using 5 and 10 ppm TiO2 NPs (24 h), the expression of this gene increased by 6 and 11 times, respectively, compared to normal conditions (without the application of TiO2 NPs). The application of 2 ppm TiO2 NPs at 6, 24, and 48 h after cold stress resulted in a 4, 7, and 8-fold increase in the expression pattern of the SEP gene compared to normal temperature (without TiO2 NPs application) (Fig. 3).
The CAS gene expression considerably fell at 23 °C following the application of 2 and 5 ppm TiO2 NPs compared to normal temperature without TiO2 NPs application, while at 10 ppm, the expression level of CAS rose after 6 and 24 h, 20 and 8 times, respectively, compared to the control (Fig. 3). The expression of the CAS gene under cold stress without the application of TiO2 NPs changes insignificantly compared to normal temperature and the non-application of TiO2 NPs. The combined application of 6 h cold stress and 10 ppm TiO2 NPs enhanced the expression of the CAS gene 15-fold compared to the control (23 °C and non-TiO2 NPs application) (Fig. 3).
The study’s findings unveiled a general decrease in the expression of the SMT gene in TiO2 NPs-treated samples, both under normal and cold stress conditions (Fig. 3). Particularly noteworthy was the highest expression of the SMT gene observed when applying 2 ppm of TiO2 NPs under normal temperature, resulting in a 1.4-fold increase compared to the control. However, the results indicated that exposing fenugreek plants to a combination of cold stress and TiO2 NPs detrimentally affected the expression of the SMT gene, leading to a reduction in its value compared to the control at 23 °C without TiO2 NPs application (Fig. 3).
Additionally, the study’s findings suggest that the expression pattern of the SSR gene can be significantly influenced by the interaction of TiO2 NPs concentrations and temperature treatments (Fig. 4). The application of 5 ppm of TiO2 NPs (6, 24, and 48 h) under normal conditions increased the expression of the SSR gene by 3.5, 7.5, and 2 times, respectively, compared to the control. Under cold stress, the application of 5 ppm TiO2 NPs (6, 24, and 48 h) resulted in a 4.7, 7.7, and 14-fold increase, respectively, compared to the control (Fig. 4).
The mean comparison effects of TiO2 NPs concentrations and different harvesting times at 23 ºC and 10 ºC on the SSR gene expression, and diosgenin content. T0, T2, T5, and T10 indicate the levels of 0, 2, 5, and 10 ppm of TiO2 NPs, respectively. H6, H24, and H48 represent different harvest times (6, 24, and 48 h) after the applied treatments, respectively. The mean values are compared using Duncan’s method at a 1% probability level. The same letters represent insignificant differences.
Diosgenin content
Diosgenin content of samples was positively influenced by the application of various levels of TiO2 NPs under normal temperature conditions, resulting in an approximately 2 to 3 times increase on average compared to the control (without the application of TiO2 NPs) (Fig. 4). Furthermore, the combined application of cold stress and TiO2 NPs significantly enhanced the diosgenin content in fenugreek compared to normal conditions. Specifically, subjecting plants treated with concentrations of 2, 5, and 10 ppm of TiO2 NPs to cold stress, especially for 24 h, led to a sharp increase in diosgenin content by 195%, 430%, and 180%, respectively, compared to the control (no cold stress and no application of TiO2 NPs). The highest recorded amount of diosgenin (90.33 mg g− 1 FW) was observed in the treatment involving 24 h of cold stress and the application of 5 ppm of TiO2 NPs (Fig. 4).
Discussion
In the present study, a decrease in chlorophylls and carotenoid content was observed under cold stress conditions (Table 1). This decline in chlorophyll biosynthesis might be attributed to the shared pathways of chlorophyll and proline biosynthesis, particularly through the common precursor glutamate. The increased accumulation of proline under cold stress could potentially disrupt chlorophyll biosynthesis, leading to a reduction in chlorophyll content18. Interestingly, in plants treated with TiO2 NPs under cold stress, there was a significant increase in chlorophylls and carotenoid content. TiO2 NPs, by diminishing the accumulation of H2O2 in fenugreek plants, appears to prevent the degradation of chlorophylls and carotenoids and stimulates their biosynthesis, especially at levels of 2 and 5 ppm after 6 and 24 h. The application of TiO2 NPs has been reported to enhance the activity of various proteins involved in photosynthesis, including fructose 1–6 bisphosphatase in the Calvin cycle, gluconeogenesis enzymes, and enzymes related to the pentose phosphate cycle, which plays crucial roles in carbohydrate metabolism19.
Under abiotic stress conditions, the production of ROS poses a risk to the stability of photosynthetic pigments. However, empirical evidence supports the efficacy of applying TiO2 NPs in mitigating the negative impacts of abiotic stresses. This mitigation is achieved by reducing H2O2 accumulation, thereby providing protection and enhancing the resilience of photosynthetic pigments against degradation20. Consistent with these findings, Parvin et al.21 reported an increased abundance of photosynthetic pigments in Linum usitatissimum L. (Linaceae) when exposed to TiO2 NPs. These results underscore the potential of TiO2 NPs in safeguarding photosynthetic pigments and promoting their stability, even under stressful conditions.
Nanoparticles have the potential to influence various aspects of photosynthesis, impacting light absorption efficiency, electron transport, and carbon assimilation. They can alter both the structure and function of chloroplasts, affecting chlorophyll content, chloroplast membrane integrity, and the activity of photosynthetic enzymes. These modifications may lead to changes in photosynthetic rates, stomatal conductance, and assimilate generation, ultimately influencing plant growth and stress tolerance22,24. TiO2 NPs, in particular, exhibit a dual role. Firstly, they enhance light energy absorption by regulating the expression of LHCII-b in the thylakoid membrane, facilitating its transfer from Photosystem I (PSI) to Photosystem II (PSII). Simultaneously, they expedite processes such as light energy conversion, electron transfer, photolysis of water, and oxygen evolution25. The application of NPs has been shown to augment the activity of the Rubisco enzyme by regulating the expression of miRNA51 and enhancing the activity of ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO)23,26.
In general, NPs have the potential to mitigate the adverse effects of abiotic stresses by improving chlorophyll fluorescence parameters and amplifying Photosystem II (PSII) efficiency. NPs can interact with various components of plant cells, including cell membranes, organelles, and enzymes, influencing their structure and function. These interactions actively contribute to shaping crucial processes such as photosynthesis, respiration, water and nutrient absorption, and hormone signaling. These processes are essential for plants to effectively adapt to abiotic stresses22,23,26.
In the present study, it was observed that under cold stress conditions, the MDA content and EC percentage significantly increased (Table 1). However, the application of different levels of TiO2 NPs demonstrated a notable reduction in the percentage of EC and MDA content under cold stress conditions. This reduction indicates a decrease in the peroxidation of membrane fatty acids, suggesting that TiO2 NPs can modulate the effects of cold stress on plant cells. While all concentrations of TiO2 NPs effectively prevented the increase in these two indices, the concentration of 5 ppm proved to be particularly effective for both indices and significantly alleviated the negative effects of cold stress. The impact of TiO2 NPs on reducing MDA content is highly dependent on the transmission of signaling pathways, ultimately regulating metabolic changes against oxidative stress in response to cold stress27. A related study suggested that low concentrations of TiO2 NPs (5 ppm) during cold stress in chickpea genotypes resulted in a decrease in MDA content EC percentage28.
Distinct and discernible patterns in H2O2 levels and antioxidant responses were observed in fenugreek plants subjected to various concentrations of TiO2 NPs (2, 5, and 10 ppm) during different durations (6, 24, and 48 h) of cold stress. The results indicate an increase in total protein content under cold stress conditions, likely attributed to the upregulation of genes associated with primary metabolism, osmotic regulation, structural changes, as well as the increased expression of LEA (Late Embryogenesis Abundant) genes29. These genetic responses play a crucial role in adapting to and mitigating the effects of cold stress on fenugreek plants. Additionally, it was observed that the total protein content of fenugreek plants treated with 2 and 5 ppm TiO2 NPs significantly increased under cold stress conditions. The augmentation in protein content can be attributed to the modulation of key genes regulating vital physiological and biochemical processes by TiO2 NPs, consequently playing a role in enhancing plant metabolism30.
Under cold stress conditions (48 h), the activity of antioxidant enzymes exhibited significant increases, including APX (160%), CAT (95%), GPX (100%), and SOD (105%), compared to normal conditions (p < 0.01) (Table 2). This study examined the activity of antioxidant enzymes, including CAT, SOD, GPX, and APX, which play an important role in clearing ROS and free radicals, and maintaining cell homeostasis and cell membrane stability. The results demonstrated that the application of TiO2 NPs, particularly at concentrations of 2 and 5 ppm, significantly increased the activity of these enzymes in both temperature conditions (23 °C and 10 °C). The increased activity of these enzymes suggests that TiO2 NPs have the potential to enhance the detoxification of H2O2 in plants by stabilizing cellular compounds and improving the physical properties of cell membranes31. This is in line with previous research conducted by Liu et al.32, which revealed that the application of TiO2 NPs under drought stress can enhance antioxidant enzyme activity, reduce lipid peroxidation, and improve membrane integrity in plants.
The activity of the antioxidant defense system in plants exposed to NPs is impacted by the augmentation of antioxidant gene expression, and biosynthesis of amino acids, nutrients, and osmolytes. Moreover, NPs prompt antioxidant activity in plants by significantly elevating the accumulation of fructose, glucose, trehalose, and sucrose33. Yang et al.34 proposed that the enhancement of light absorption by TiO2 NPs contributes to the improved conversion and transfer of energy from light, inhibiting chloroplast aging and ultimately enhancing the photosynthetic activity of chloroplasts. This could be attributed to the heightened activity of antioxidant enzymes like superoxide dismutase, peroxidase, and catalase, resulting from the intervention of TiO2 NPs that serve to counteract excessive light exposure to the chloroplast. The utilization of TiO2 NPs was noted to alleviate oxidative stress, diminish membrane damage, and enhance plant performance in cold conditions35,36. According to the findings of the present experiment, a concentration of 10 ppm of TiO2 NPs exerted a detrimental impact on the activity of antioxidant enzymes (Table 2). Non-optimal concentrations of TiO2 NPs could result in the deactivation of the antioxidant defense system, ultimately diminishing plant efficiency due to potential toxicity. Hence, it is imperative to pinpoint the optimal concentration of TiO2 NPs for leveraging its role as a biosynthesis stimulator for primary and secondary metabolites, thereby allowing for the modulation of its impact on cold effects.
NPs have the capacity to interact with plant DNA, RNA, and proteins, resulting in modifications to gene expression and the activation of stress-responsive genes. These alterations can affect the synthesis of stress-related proteins, osmolytes, and other molecules crucial for stress tolerance23,37. Another noteworthy outcome of the current study is the increased total phenol content observed under cold stress, particularly during the 48 h, and with the application of TiO2 NPs, especially at 5 ppm, in comparison to the control. Additionally, the application of TiO2 NPs (2 and 5 ppm) under normal temperature conditions led to a higher total phenol content than the control (Fig. 1). These results align with the findings of Karimizadeh et al.38, who reported an increase in phenol accumulation in plants following the application of TiO2 NPs. It is important to note that in response to oxidative stress, the activity of biosynthetic enzymes and the degradation of phenolic compounds change, leading to the accumulation of phenolic compounds in plants6. Numerous studies have documented the heightened activity of the enzyme phenylalanine ammonia-lyase (PAL), considered a catalyst for the phenol biosynthesis pathway39,40. In tobacco plants subjected to drought stress, the application of putrescine resulted in an increased activity of the PAL enzyme41. Phenols neutralize ROS by their capacity to donate oxygen atoms to free radicals42,43.
According to our findings, the proline content significantly increased under cold stress, particularly after 48 h, compared to the normal conditions. Furthermore, various levels of TiO2 NPs, including 2 and 5 ppm in each temperature group, elevated the proline content (Fig. 1). The amino acid proline accumulates extensively in plants in response to diverse stresses such as cold, salinity, and drought44. During stress conditions, proline collaborates with antioxidant enzymes to prevent the denaturation of cell membrane proteins, thereby safeguarding the structure and activity of proteins against damage caused by increased ROS and MDA production45. The utilization of NPs has been observed to enhance the proline content in plants, suggesting that these substances potentially enhance the activity of proteins and enzymes responsible for proline biosynthesis. As a result, they contribute to maintaining the photosynthetic cycle and regulating nutrient levels in plants6. In line with these findings, Mustafa et al.46 reported that the exogenous application of TiO2 NPs (40 mg/l) in two wheat varieties under salinity stress at concentrations of 100 and 150 mM acted as an osmotic protector against salt stress. This protective effect was demonstrated through the stabilization of cell membranes and preservation of enzyme integrity, ultimately leading to improved accumulation of soluble sugars and proline content in plants. The increase in proline accumulation in plants following TiO2 NPs application is facilitated by the regulation of the P5CS1 gene, which encodes d1-pyrroline-5-carboxylate. This gene plays a role in the synthesis of proline and sugar, contributing to the enhanced functionality of the cell membrane, proteins, and enzymes. Ultimately, this mechanism promotes the plant’s tolerance under stress conditions6,24,46.
Under normal conditions, ROS are not only produced in plants but also play a role in enhancing cell function by participating in cell signaling activities. However, when plants are exposed to biotic and abiotic stresses, the balance between ROS production and consumption is disrupted, leading to irreparable damage to the plant. Consequently, plants have evolved unique defense mechanisms to cope with stress47.The findings of this study indicated that cold stress treatment leads to an increased production of H2O2 as ROS. However, upon the application of different concentrations of TiO2 NPs under normal temperature, the content of H2O2 gradually decreased, suggesting its potential role in mitigating the harmful effects of ROS. Moreover, treating stressed plants with 2 and 5 ppm of TiO2 NPs effectively reduced the H2O2 content compared to cold treatment alone (Fig. 2). When plants face abiotic stresses such as cold and drought, TiO2 NPs eliminate H2O2 by boosting the activity of antioxidants like CAT, SOD, and peroxidase23. In light of the current study’s findings, the use of TiO2 NPs could be considered a practical method to alleviate the adverse effects of cold stress. Our findings align with the research conducted by Weisany et al.48 revealing that elevated H2O2 levels resulting from oxidative reactions in soybeans under salt stress lead to increased enzyme activity of CAT and APX. The heightened activity of antioxidant enzymes in plants treated with TiO2 NPs under stress likely contributes to improved positive interactions and stronger signaling for the activation of additional defense mechanisms. The detoxification of H2O2 by NPs treatment may be linked to the stability of cell composition and the enhancement of the physical properties of the cell membrane49,50,51.
In this study, plants exposed to 23°C and treated with 2 and 5 ppm of TiO₂ NPs exhibited minimal changes in H2O2 levels, while significant alterations in antioxidant enzyme activity were observed under these conditions. This outcome can be attributed to several factors47.
1. Anticipation or preparation to cope with potential stresses: Plants may proactively increase the production of antioxidant enzymes in response to slight environmental changes, such as fluctuations in temperature, light, or nutrient levels, to be ready to cope with potential stressful conditions. In this case, enzymes rise to quickly break down ROS, such as hydrogen peroxide, if they increase36,47.
2. Regulation of internal signaling: antioxidant enzymes, in addition to their role in scavenging ROS, can play a role in cellular signaling processes. The increase of these enzymes may be related to the internal settings of the plant and growth and development processes. For example, an increase in superoxide dismutase or glutathione peroxidase can lead to changes in signals regulating growth and differentiation37,47.
3. Adaptation and development of defense mechanisms: Plants may enhance their defense mechanisms under the influence of mild environmental factors or even as part of the growth cycle. The increase of antioxidant enzymes may be a part of this adaptation process so that the plant can better cope with possible stresses such as water shortage, salinity, or light stresses in the future37,47.
4. Controlling ROS levels at the signaling level: Under normal conditions, plants may want to keep ROS levels low enough to only act as signals to regulate biological processes, rather than reaching harmful levels. Increasing antioxidant enzymes can help maintain this delicate balance. In general, an increase in antioxidant enzymes without a change in H2O2 levels could indicate a preventive or regulatory mechanism in the plant that acts to protect or optimize physiological functions36,47.
NO, serving as a crucial signaling molecule, plays a pivotal role in diverse biological functions, including seed germination, plant maturation and senescence, and the regulation of growth and development in plants52,53. The NO-dependent signaling pathway not only activates enzymatic and non-enzymatic defense systems but also governs the production and elimination of ROS, lipid peroxidation, electrolyte leakage, and boosts the production of osmolytes such as proline31,54. Our research findings indicated that the application of cold stress, particularly after 48 h, and various concentrations of TiO2 NPs, especially at 5 ppm, significantly increased NO (Fig. 2). Other studies have reported the positive effects of cold stress and TiO2 NPs on increasing NO content55. In this study, the application of TiO2 NPs led to an increase in NO content (Fig. 2). Additionally, numerous studies have indicated that elevated levels of NO and TiO2 NPs can significantly enhance the activity of antioxidant enzymes54,55,56,57,58. The application of TiO2 NPs in stressed plants, either by inducing the activity of the nitrate reductase enzyme and subsequently increasing NO content or by directly improving the activity of antioxidant enzymes, plays a role in promoting the stability of plant cells54,56.
Nanoparticles possess the capability to influence the synthesis, metabolism, and signaling of plant hormones, including ABA, salicylic acid (SA), auxin, and jasmonic acid (JA). These hormones play crucial roles in regulating plant responses to various abiotic stresses such as drought, salinity, and heavy metal toxicity. Depending on their properties and concentrations, NPs can either enhance or suppress the production and signaling of these hormones4,7,59.
In this study, cold stress resulted in a substantial reduction in auxin content, consistent with previous research indicating the impact of cold stress on the redirection of auxin signaling60. The application of various concentrations of TiO2 NPs under normal temperature, especially at 5 ppm, led to an increase in auxin content (Fig. 1). Additionally, the auxin content under cold stress conditions, with the application of various concentrations of TiO2 NPs, was significantly higher compared to cold stress conditions without the application of TiO2 NPs. A recent study on soybeans also reported an elevation in auxin content following the application of TiO2 NPs61. Several reports have highlighted the impact of TiO2 NPs on plant growth regulators, specifically auxin, leading to increased root length and regulation of auxin in Arabidopsis62,63. The results of this study suggest that subjecting fenugreek plants to cold stress in the presence of TiO2 NPs could potentially enhance auxin content by upregulating the expression of auxin biosynthesis genes.
The findings of this study also demonstrate a significant increase (p < 0.01) in ABA content under cold stress conditions and with the application of 2 and 5 ppm of TiO2 NPs, compared to the control group (Fig. 3). This observation is consistent with the results of other researchers64,65. The application of TiO2 NPs at normal temperature also increased ABA content, aligning with the findings of Chaca et al.66. Additionally, Syu et al.67 observed that the application of silver NPs in Arabidopsis elevated ABA levels by enhancing the expression of the gene encoding the rate-limiting biosynthetic enzyme NCED3. It is noteworthy that the promoter regions of several genes associated with heavy metal stress contain ABA-responsive elements (ABRE), suggesting a strong correlation between ABA content and the levels of gene transcripts68. The presence of NPs appears to stimulate cellular redox status and phytohormone production, leading to the generation of specific signaling pathways that impact the transcription of nuclear genes. Extensive research has been conducted on the effects of NPs on phytohormones, NO, and gene transcription in various plant species69. NPs can alter DNA methylation patterns, histone modifications, and small RNA expression, leading to changes in gene expression and stress tolerance. Investigating the epigenetic mechanisms underlying NPs-induced stress responses could provide a deeper understanding of their long-term effects on plant physiology and adaptation70,71.
Notably, the combination of 24 h of cold stress and the application of 5 ppm TiO2 exhibited the highest level of SQS gene expression, reaching 31.84 (Fig. 3). Our findings revealed that the application of 2 ppm of TiO2 NPs at normal temperature after 48 h significantly increased the expression of SEP by 13 times compared to the control. When 2 ppm of TiO2 NPs was applied after 6, 24, and 48 h of cold stress, there was a 4, 7, and 8-fold increase in the expression of this gene, respectively, compared to the control (Fig. 3). These findings suggest that the application of TiO2 NPs as a functional elicitor may effectively enhance the expression of this gene. The expression pattern of the CAS gene differed from that of the two previously mentioned genes (SQS and SEP), indicating that under cold stress conditions without the application of TiO2 NPs, the expression of CAS did not change significantly compared to the control. Moreover, the application of 10 ppm TiO2 NPs after 6 h at both temperatures of 23 °C and 10 °C resulted in a 20-fold and 15-fold increase in the expression of this gene, respectively, compared to the control (at 23 °C and without titanium dioxide) (Fig. 3).
In our study, the expression of the SMT gene was reduced in NPs-treated samples under both normal and cold stress conditions. The highest expression of this gene was observed with the application of 2 ppm of TiO2 NPs at normal temperature (1.4 times) (Fig. 3). The results indicated that exposure of fenugreek to cold stress and TiO2 NPs had a negative impact on SMT gene expression, leading to a reduction in its expression compared to the control (at 23 °C without TiO2 NPs). However, the expression patterns of the SSR gene revealed that the interaction between TiO2 NPs and cold stress significantly influenced its expression (Fig. 4). For instance, with the application of cold stress (6, 24, and 48 h) along with 5 ppm of TiO2 NPs, SSR gene expression increased by 4.7, 7.7, and 14-fold, respectively, compared to the control.
It appears that the SSR gene governs the diosgenin biosynthesis pathway, while the SMT gene intervenes in the process, directing the pathway toward the production of plant sterols and similar compounds72,73. Given that the expression of the SMT gene (a rival gene) remained almost unchanged, and the expression of the SSR gene (the main gene) increased with the application of cold stress and TiO2 NPs treatment, it can be concluded that the simultaneous application of TiO2 NPs and cold stress may be an efficient approach to enhance diosgenin content (Fig. 4). This aligns with findings in the thymoquinone biosynthesis pathway in Nigella sativa, where the expression of main genes increased with the induction of TiO2 NPs74. Zhang et al.75 proposed that silver NPs enhanced the production of secondary metabolites in Artemisia annua. With the application of these NPs, the weight of artemisinin increased from 1.67 to 2.86 mg per gram of dry weight76. The findings of Moshirian Farahi et al.77 demonstrated that TiO2 NPs played a crucial role in driving changes in the expression of genes in Vitex plants. The induction of this NP affected the plant’s antioxidant enzymes and altered the expression of genes involved in the terpenoid biosynthesis pathway.
The small size of TiO2 NPs allows them to efficiently and rapidly penetrate plant cells, thereby stimulating gene expression. TiO2 NPs can act as stressor factors, enhancing plant defense activities and thereby triggering the expression of genes responsible for secondary metabolite production in plants78. Karimzadeh et al.38 documented that the foliar application of TiO2 NPs under drought stress increased the expression of the RAS gene. Similarly, it has been noted that the utilization of silver NPs augments the expression of tydc7, DBOX, DIOX2, and bbe1 genes in Papaver somniferum L79.
The behavior of NPs can significantly differ based on their concentration and structure. Lower concentrations of NPs tend to stimulate the biosynthesis of both primary and secondary metabolites, while higher concentrations are more likely to induce toxicity and disrupt cellular processes80. The findings of the current study also affirmed that (for most traits, though not universally), the application of 2 and 5 ppm of TiO2 NPs effectively mitigated the impact of cold stress on the plant and resulted in an increase in the expression of genes related to diosgenin biosynthesis. In contrast, the use of 10 ppm of TiO2 NPs had fewer positive effects and, in some cases, even demonstrated negative effects. The outcomes of diverse studies underscore the impact of NPs on stimulating the biosynthesis of secondary metabolites. In alignment with our findings, it has been documented that selenium NPs are linked to the stimulation of secondary metabolism by inducing the expression of RAS and HPPR genes in Melissa officinalis69. Sheikhi et al.14 reported that subjecting fenugreek plants to heat stress with 24-epibrassinolide (8 µM for 6 h) significantly upregulates the expression of SQS, SEP, CAS, and SMT genes, along with a notable increase in diosgenin content by 8, 2.8, 11, 17, and 6 times, respectively.
The rise in essential oil production observed with TiO2 NPs application in plants is congruent with the enhancement of growth conditions, photosynthesis, the expression of genes involved in secondary metabolite biosynthesis, and defense mechanisms. The improvement in essential oils in TiO2 NPs-treated plants can be attributed to increased access to substrates and essential components, along with specialized biosynthesis enzymes23. Our findings align with the outcomes of Ebadollahi et al.81, who reported that TiO2 NPs positively stimulates the synthesis of secondary metabolites in plants under stress conditions through diverse signaling cascades, including ROS, mitogen-activated protein kinases, calcium flux, gene expression, and the activation of specific metabolic enzymes. NPs elevate the expression of genes associated with the generation of hormonal signals, including GmWRKY27, GmMYB118, and GmMYB174, as well as the synthesis of secondary metabolites and defense mechanisms. Consequently, this influence contributes to the enhancement of plant tolerance under stress conditions82,83.
NPs possess the capacity to interact with various components of signaling pathways in plants, including receptors, kinases, and transcription factors. These interactions can lead to the modulation of stress-related gene expression and the activation of downstream signaling cascades7,84. Understanding the molecular basis of NPs-mediated modulation of signaling pathways is crucial for elucidating the mechanisms underlying NPs-induced stress tolerance in plants. By deciphering the specific signaling events and molecular interactions involved, researchers can gain insights into the intricate network of stress-responsive genes and pathways that are influenced by NPs7,84,86. By manipulating these pathways, it may be possible to improve plant tolerance to various abiotic stresses, leading to increased crop productivity and sustainability. It is important to note that while the field application of TiO2 NPs holds promise, further research is needed to fully understand their long-term effects, potential risks, and optimal application methods. Responsible application and regulation of nanotechnology in agriculture are crucial to ensure its safe and sustainable implementation. Overall, the application TiO2 NPs in agriculture offers a range of mechanisms and potential benefits, including enhanced nutrient uptake, improved water use efficiency, seed germination, hormonal regulation, and environmental remediation. These NPs have the potential to revolutionize agricultural practices and contribute to more sustainable and productive farming systems84,87.
Materials and methods
Plant materials and treatments preparation
The taxonomy of the studied plant was confirmed by a specialist botanist from the Ministry of Agriculture Jihad of Tehran, Iran. The plant material was obtained under the supervision and permission of the Ministry of Agriculture Jihad of Tehran, Iran as well as national guidelines, with all authors complying with all local and national guidelines. For this study, fenugreek seeds sourced from the Boshruyeh genotype were sterilized using a 2% sodium hypochlorite solution for 10 min. Following sterilization, the seeds underwent multiple rinses with sterile water before being planted in pots measuring 20 × 25 × 30 cm. The potting mixture comprised equal parts of peat moss, perlite, and sand. The growth chamber maintained a consistent photoperiod of 16 –8 h, with temperature settings between 23 and 25 °C and humidity levels ranging from 55 to 60%. Fluorescent lamps, with a light intensity of 400 µmol m− 2 s− 1, were employed to ensure adequate illumination for the plants.
For the study, TiO2 NPs with an average size of 20–30 nm, existing in the 99.7% anatase form, were procured from Sigma-Aldrich, USA. TiO2 NP solutions were prepared using double-distilled water at concentrations of 2, 5, and 10 ppm, as established by previous studies4,8,28. These concentrations were chosen based on preliminary experiments. To ensure proper dissolution and achieve a nanoscale solution, ultrasonic treatment was applied using an Elmasonic P model ultrasonic bath for 45 min. Seedlings, measuring 15 cm in height and featuring 5–8 leaves (At 4 weeks old), were subjected to spraying with three different levels of TiO2 NPs solution (2, 5, and 10 ppm). Each concentration of TiO2 NPs was sprayed uniformly on all plants using an automatic sprayer, with approximately 10 cc applied per plant. The pots were randomly divided into three groups; the control group received a spray of double-distilled water. Subsequently, one group remained in standard conditions (23 °C), while the other was placed in a growth chamber set at 10 °C to induce CS. Sampling occurred at 6, 24, and 48 h after the initiation of treatments. Leaf samples were promptly frozen in liquid nitrogen and stored at -80 °C. The middle leaves from the apex of plants in each treatment were selected for property measurements, assuming a similar physiological age for all seedlings4,8,28.
Pigments assay
To quantify chlorophyll (a, b, and total) and carotenoids, 500 mg of shredded leaves were homogenized in 5 ml of 80% acetone. After centrifugation at 3500 rpm for 15 min at 4 °C, the supernatants were carefully transferred to new Falcon tubes, and their volumes were adjusted to 10 ml using 80% acetone88. Finally, a spectrophotometer (UV-1800) was utilized to determine the light absorption at wavelengths of 480, 645, and 663 nm, providing accurate measurements of pigment concentrations. Chlorophyll and carotenoid contents were ultimately calculated using the following formulas:
Chlorophyll a: 11.75 (A663) – 2.35 (A645).
Chlorophyll b: 18.61 (A645) – 3.96 (A663).
Carotenoid: 1000 (A480) – 2.27 Chla– 81.4 Chlb/229.
Where A = Absorbance, Chla = chlorophyll a (mg/L), Chlb = chlorophyll b (mg/L), Carotenoids (mg/L). For converting the concentrations from mg/l to mg/g fresh weight, each value multiplied by (extraction volume/ (sample weight*1000))89.
Electrolyte leakage percentage
To prepare leaf samples for electrolyte leakage (EC) assessment, a punching machine was employed to create 1 cm diameter discs from intact leaves. These discs were subsequently placed in Falcon tubes containing 10 ml of distilled water, ensuring complete water absorption by the samples using a vacuum pump. The Falcon tubes were then positioned in a shaker, and the electrolytic conductivity (Ec1) of the samples was measured with an EC meter from Weilheim, Germany. To determine Ec2, the samples underwent immersion in a hot water bath for one hour. The damage index was calculated using the formula established by Hepburn et al.90:
% EL=(Ec1 / Ec2) * 100. This formula allowed for the accurate quantification of electrolyte leakage as a percentage.
Measurement of malondialdehyde content
The determination of malondialdehyde (MDA) content followed the method introduced by Heath and Packer91. In summary, 2 ml of extraction buffer (0.5% trichloroacetic acid) was homogenized with 250 mg of shredded leaf samples and centrifuged at 3500 rpm for 15 min. Subsequently, 1 ml of the resulting supernatant was combined with 2 ml of a 0.5% thiobarbituric acid solution containing trichloroacetic acid (20% TCA). The mixture was incubated in hot water (90 °C) for 20 min, followed by prompt transfer to an ice bath for the same duration. After centrifugation at 3500 rpm for 15 min, the samples were measured at 532 and 600 nm using a UV-1800 spectrophotometer. Using the following equation, the quantity of MDA was calculated92:
MDA = [(532 –600 nm)/(QD × QF)] × DF. In this formula, QD = Cuvette diameter (1 cm), QF = Extinction coefficient (155 mmol/cm), and DF = Dilution factor (20).
Assessment of total protein and antioxidant enzyme activity
To evaluate the total protein content and the activities of antioxidant enzymes, namely catalase (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD), and guaiacol peroxidase (GPX), an enzyme extract was prepared according to the following protocol. Initially, 0.5 g of ground leaf tissue underwent blending with 2 ml of phosphate buffer (50 mM, pH 7.0) and subsequent centrifugation at 3500 rpm for 15 min at 4 °C. The resulting supernatant was utilized for conducting the antioxidant enzyme activity assay. In accordance with widely accepted methods, the total protein content was determined following Bradford’s protocol92. Simultaneously, the activities of CAT93, APX94, SOD95, and GPX96 were measured using a UV-1800 spectrophotometer.
Total phenol content measurement
1 gram of each dried plant sample was initially mixed with 10 ml of 80% methanol at 40 °C and subjected to shaking for 24 h. Following filtration with filter paper, the resulting extract was combined with 0.5 ml of Folin–Ciocalteu reagent and 4 ml of 1 M Na2CO3 solution. The mixture underwent incubation for 15 min at room temperature. Finally, the absorbance of the samples was measured at 765 nm using a UV-1800 spectrophotometer. For each sample, three replications were performed, and the standard curve of gallic acid (Merck, CAS Number: 149-91-7) at eight concentrations served as the reference to quantify the total phenol content97.
Proline content assay
The proline content was assessed following the method developed by Bates et al.98. In this procedure, 100 mg of shredded fresh leaf tissue was thoroughly mixed with 10 ml of a 3% sulfosalicylic acid solution, and the resulting mixture was filtered through Whatman filter paper. Subsequently, an equal amount (2 ml) of the obtained extract, pure hydrogen reagent, and pure acetic acid were thoroughly mixed and subjected to a hot water bath at 90 °C for 60 min. The samples were promptly transferred to an ice bath for 10 min, and 4 ml of toluene was added to the solutions. The red phase, containing the amino acid proline, formed at the top. To determine the proline content, the absorbance of the samples was recorded at 520 nm using a UV-1800 spectrophotometer.
Determination of hydrogen peroxide content
The leaf tissue (0.5 g) was finely ground and thoroughly mixed with 5 ml of 0.1% trichloroacetic acid (TCA), immediately before being centrifuged at 35,000 rpm. Next, a mixture, composed of the supernatant (750 µl), 10 mM potassium phosphate buffer (750 µl), and 1 mM potassium iodide (1.5 mL) was accurately prepared and their absorption rate was recorded at 390 nm. It is worth noting that, owing to the sensitivity of H2O2, this experiment was conducted in relative darkness on ice (4 °C)99. Ultimately, the H2O2 content was measured using standard curves (Sigma, USA, CAS Number: 7722-84-1).
Nitric oxide content assay
To measure Nitric Oxide (NO) content, the methodology outlined by Zhou et al.100 was followed. In this process, 3 ml of 50 mM cold acetic acid buffer containing 4% zinc acetate with a pH of 4 was added to 0.6 g of shredded leaf tissue. The samples underwent two-stage centrifugation: initially, centrifugation at 4 °C and 3000 rpm for 15 min, followed by washing the supernatants with 1 ml of extraction buffer and repeating the centrifugation under the same conditions. In the subsequent step, the resulting supernatant was thoroughly mixed with 0.1 g of activated charcoal and filtered using an appropriate filter. The filtered solution (1 ml) was then mixed with 1 ml of grease agent and left for 30 min at room temperature. The absorption rate of the solution was measured at 540 nm. To quantify the NO content, a NaNO2 standard curve was employed (Sigma, USA, CAS Number: 7632-00-0).
Phytohormones content measurement
For the assessment of auxin content, an extract was prepared following the method described by Zhou et al.101. Subsequently, HPLC analysis (Agilent Technologies Inc., USA, 1200 series) was conducted, as per the methodology proposed by Cao et al.102. The auxin content in leaf tissue was determined using HPLC with a flow rate of 0.8 ml/min, employing a C18 column (4.6 μm, 250 mm length, and 5 mm diameter). The HPLC column was maintained at a temperature of 30 °C, and a gradient of 0.45% formic acid and acetonitrile was utilized. The gradient included the following steps: 0–5 min with a ratio of 95:5% (v/v) of formic acid/acetonitrile, 5–6 min transitioning from 95:5% to 0:100% (v/v), and finally, 6–16 min with a ratio of 0:100% (v/v). To determine auxin concentration, a linear regression equation from standard (Sigma, USA, CAS Number: 87-51-4) calibration curves was applied. The peak areas at the maximum wavelength of 260 nm were considered for quantification.
The evaluation of abscisic acid (ABA) content utilized the method introduced by Hubick and Reid103. An HPLC column (Agilent Technologies Inc., USA, 1200 series, C18, 4.2 μm, 250 mm length, and 5 mm diameter) was employed according to standard ABA. Chromatographic separation involved two mobile phases with water/acetonitrile/formic acid in volume ratios of 94.9:5:0.1 (A) and 10:89.9:0.1 (B). The elution program included maintaining 100% A for 5 min, followed by two consecutive linear gradients from 0 to 6% B in 10 min and from 6 to 100% B in 5 min, with a final maintenance of 100% B for another 5 min104. A flow rate of 0.8 ml/min was applied at a wavelength of 254 nm. Quantification was performed based on the specific area of the observed peak concerning the retention time of the standard abscisic acid sample, obtained from Sigma, USA (CAS Number: 21293-29-8).
RNA extraction and cDNA synthesis
RNA extraction was carried out using the RNeasy plant mini kit (Qiagen) following the manufacturer’s instructions. To eliminate DNA contaminants, one microgram of the extracted RNA was treated with DNase (Thermo Fisher Company). Subsequently, the quality and quantity of RNA were assessed using a 1% agarose gel and a NanoDrop spectrophotometer. The RNA concentration for all samples was standardized to 200 ng/µL for cDNA synthesis. For cDNA synthesis, 20 µl of complementary DNA (cDNA) was synthesized using the Reverse Transcriptase kit (Bio-Rad, Hercules, CA, USA), following the manufacturer’s instructions. Primers were designed using the Primer 3 Plus online program, and their accuracy was verified using Oligo Analyzer v.3.1 (http://eu.idtdna.com/calc/analyzer) (Table 3).
Real-time PCR reaction
For the assessment of relative gene expression via real-time PCR, SYBR®Green PCR Master Mix 2X (Ampliqon) was utilized. The real-time PCR reaction volume was 10 µl, and the experiment was three biological and technical replications. It is worth noting that the concentration of the synthesized cDNA was 1000 ng/µL, and the concentration of cDNA used in the real-time PCR reaction was adjusted to 100 ng/µL. The temperature and time conditions consisted of 35 cycles at 95 °C for 20 s and 60 °C for 40 s, followed by a single cycle at 95 °C for 15 min. To ensure the samples were uncontaminated, a negative control was included in the experiment.
Diosgenin content assay
The evaluation of diosgenin content followed the method introduced by Zolfaghari et al.72. Utilizing an HPLC column (Agilent Technologies Inc., USA, 1200 series, C18, 4.2 μm, 250 mm length, 5 mm diameter) at a flow rate of 0.8 ml/min and a wavelength of 210 nm, diosgenin content was measured. The mobile phase comprised acetonitrile and water in a 90:10 ratio, with a flow rate of 0.7 ml and a wavelength of 210 nm105. Various concentrations of diosgenin (Sigma-Aldrich-Germany; CAS Number: 512049), including 100, 150, 300, 500, and 1000 ppm, were prepared by dissolving the compound in acetonitrile and subjecting it to ultra-sonication for 30 min.
Data analysis
The current research was structured as a factorial experiment based on a completely randomized design. Following the completion of an analysis of variance, the Duncan test was employed for mean comparisons using SPSS 26 software (IBM SPSS, Armonk, NY, USA). For the real-time PCR data analysis, the relative standard curve method was applied. The accuracy of the raw data was ensured by examining melting and amplification curves. The analysis followed the formula 2−ΔΔCt, as introduced by Livak and Schmittgen106.
Conclusion
This investigation explored the use of TiO2 NPs to enhance the plant immune system and bolster fenugreek’s resilience to cold stress. The application of these NPs demonstrated several positive effects, including the prevention of a decline in chlorophyll and carotenoid content under cold stress and a significant increase in the levels of these valuable compounds. Additionally, TiO2 NPs boosted the activity of antioxidant enzymes, creating favorable conditions for the breakdown of H2O2 following cold stress. This, in turn, prevented an escalation in fatty acid peroxidation and the subsequent leakage of these compounds from the cell. The study also examined the impact of TiO2 NPs and cold stress on the expression of specific genes in the diosgenin biosynthesis pathway. The results suggested that TiO2 NPs could modify the expression of fenugreek genes by activating signaling pathways. In summary, the findings indicate that TiO2 NPs may serve as effective stimulants in the biosynthesis of both primary and secondary metabolites, particularly diosgenin. Moreover, they have the potential to mitigate the adverse effects of cold stress on fenugreek, highlighting their possible application in agriculture for enhancing plant resilience and productivity.
Data availability
The data generated or analyzed in this study are included in this article. Other materials that support the findings of this study are available from the corresponding author on reasonable request.
References
Olayinka, B. U. et al. Stresses in Plants: Biotic and Abiotic. Book chapter. (2021).
Winfield, M. O., Lu, C., Wilson, I. D., Coghill, J. A. & Edwards, K. J. Plant responses to cold: Transcriptome analysis of wheat. Plant Biotechnol. J. 8(7), 749–771 (2010).
Novak, A. et al. Light-quality and temperature-dependent CBF14 gene expression modulates freezing tolerance in cereals. J. Exp Bot. 67, 1285–1295 (2015).
Amini, S., Maali-Amiri, R., Mohammadi, R. & Kazemi-Shahandashti, S. S. cDNA-AFLP analysis of transcripts induced in chickpea plants by TiO2 nanoparticles during cold stress. Plant Physiol. Biochem. 111, 39–49 (2017).
Shang, Y. et al. Applications of nanotechnology in plant growth and crop protection. A review. Molecules 24, 2558 (2019).
Kardavan Ghabel, V. & Karamian, R. The effects of titanium dioxide nanoparticles and spermine on physiological responses of licorice (Glycyrrhiza glabra L.) to cold stress. J. Plant Physiol. Breed. 10(1), 93–110 (2020).
Šebesta, M. et al. Field application of ZnO and TiO2 nanoparticles on agricultural plants. Agronomy 11(11), 2281 (2021).
Mohammadi, R., Amiri, N. M. & Mantri, L. Effect of TiO2 nanoparticles on oxidative damage and antioxidant defense systems in chickpea seedlings during cold stress. Russ. J. Plant Physiol. 61, 768–775 (2013).
Wu, S. et al. The potential of Diosgenin in treating psoriasis: Studies from HaCaT keratinocytes and imiquimod-induced murine model. Life Sci. 241, 117115 (2020).
Das, S. et al. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. Plos One 7, e46641 (2012).
Arabasadi, M., Ebrahimi, A., Amerian, M. R., Ebrahimibasabi, E. & Azadvari, E. The amelioration of salt stress-induced damage in fenugreek through the application of cold plasma and melatonin. Plant Physiol. Biochem. 207, 108382 (2024).
Mohamadi Esboei, M., Ebrahimi, A., Amerian, M. R. & Alipour, H. Melatonin confers fenugreek tolerance to salinity stress by stimulating the biosynthesis processes of enzymatic, non-enzymatic antioxidants, and diosgenin content. Front. Plant Sci 13, 890613 (2022).
Ebrahimibasabi, E., Ebrahimi, A., Momeni, M. & Amerian, M. R. Elevated expression of diosgenin-related genes and stimulation of the defense system in Trigonella foenum-graecum (Fenugreek) by cold plasma treatment. Sci. Hortic. 271, 109494 (2020).
Sheikhi, S. et al. Exogenous 24-epibrassinolide ameliorates tolerance to high-temperature by adjusting the biosynthesis of pigments, enzymatic, non-enzymatic antioxidants, and diosgenin content in fenugreek. Sci. Rep. 13(1), 6661 (2023).
Ivănescu, B., Burlec, A., Crivoi, F., Rosu, C. & Corciovă, A. Secondary metabolites from artemisia genus as biopesticides. Molecules 26, 3061 (2021).
Sacks, W. J., Delphine, D., Jonathan, A. & Navin, R. Crop planting dates: An analysis of global patterns. Global Ecol. Biogeogr. 19, 607–620 (2010).
Mirmiran, S. M. et al. Selection of fenugreek (Trigonella foenum-graecum L.) landraces for fall planting and freezing tolerance. Iran Agric. Res. 40(1), 71–82 (2021).
Khalid, K. A., Silva, J. A. T. & Cai, W. Water deficit and polyethylene glycol 6000 affects morphological and biochemical characters of Pelargonium odoratissimum L. Sci. Hort. 125, 159–166 (2010).
Ahmad, B., Shabbir, A., Jaleel, H., Khan, M. M. A. & Sadiq, Y. Efficacy of titanium dioxide nanoparticles in modulating photosynthesis, peltate glandular trichomes and essential oil production and quality in Mentha piperita. L. Plant Biol 13, 6–15 (2018).
Abdel Latef, A. A. H., Srivastava, A. K., El-sadek, M. S. A., Kordrostami, M. & Tran, L. S. P. Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad. Dev 29, 1065–1073 (2018).
Parvin, S. et al. Effects of single and multiple species Inocula of Arbuscular Mycorrhizal fungi on the salinity tolerance of a Bangladeshi rice (Oryza sativa L.) cultivar. Mycorrhiza 30, 431–444 (2020).
Mohammadi, H., Esmailpour, M. & Gheranpaye, A. Effects of TiO2 nanoparticles and water-deficit stress on morpho-physiological characteristics of dragonhead (Dracocephalum moldavica L.) plants. Acta Agric. Slovenica 107(2), 385–396 (2016).
Gohari, G. et al. Titanium dioxide nanoparticles (TiO2 Nps) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum Moldavica. Sci. Rep 10, 912 (2020).
Satti, S. H. et al. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. Plos one 16(2), e0246880 (2021).
Alabdallah, N. M. et al. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants 10, 1730 (2021).
Zhang, Y., Liu, N., Wang, W., Sun, J. & Zhu, L. Photosynthesis and related metabolic mechanism of promoted rice (Oryza sativa L.) growth by TiO2 nanoparticles. Front. Environ. Sci. Engin. 14, 1–12 (2020).
Yadav, S. K. Cold stress tolerance mechanisms in plants. A review. Agron. Sustain Dev. 30(3), 515–527 (2010).
Kazemi Shahandashti, S. S., Maali Amiri, R., Zeinali, H. & Ramezanpour, S. S. Change in membrane fatty acid compositions and cold induced responses in chickpea. Mol. Biol. Rep. 40, 893–903 (2013).
Roychoudhury, A., Roy, C. & Sengupta, D. N. Transgenic tobacco plants over expressing the heterologous lea gene Rab16A from rice during high salt and water deficit display enhanced tolerance to salinity stress. Plant Cell Rep. 26(10), 1839–1859 (2007).
Tumburu, L., Andersen, C. P., Rygiewicz, P. T. & Reichman, J. R. Phenotypic and genomic responses to titanium dioxide and cerium oxide nanoparticles in Arabidopsis germinant. Environ. Toxicol. Chem 34, 70–83 (2015).
Khan, M. Nano-titanium dioxide (nano-TiO2) mitigates NaCl stress by enhancing antioxidative enzymes and accumulation of compatible solutes in tomato (Lycopersicon esculentum Mill.). J. Plant Sci 11, 1–11 (2016).
Liu, C. T., Wang, W., Mao, B. G. & Chu, C. C. Cold stress tolerance in rice: Physiological changes, molecular mechanism, and future prospects. Chuan Hereditas 40(3), 171–185 (2018).
Heikal, Y. M., El-Esawi, M. A., El-Ballat, EM. & Abdel-Aziz, H. M. Applications of nanoparticles for mitigating salinity and drought stress in plants: an overview on the physiological, biochemical, and molecular genetic aspects. New Z. J. Crop Hortic. Sci. 1–31 (2020).
Yang, X., Cao, C. L., Erickson, K., Hohn Maghirang, R. & Klabunde, K. Synthesis of visible-light-active TiO2-based photocatalysts by carbon and nitrogen doping. J. Catal. 260, 128–133 (2008).
Sun, L. et al. Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Arch. Agron. Soil Sc 67, 245–259 (2021).
Ghani, M. et al. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 289, 133202 (2022).
Rikabad, M. M., Pourakbar, L., Moghaddam, S. S. & Popović-Djordjević, J. Agrobiological, chemical and antioxidant properties of saffron (Crocus sativus L.) exposed to TiO2 nanoparticles and ultraviolet-B stress. Ind. Crops Prod. 137, 137–143 (2019).
Karimzadeh, G., Moieni-Korbekandi, Z. & Sharifi, M. Cold-induced changes of proline, malondialdehyde and chlorophyll in spring canola cultivars. J. Plant Physiol. Breed 4, 1–11 (2014).
Scott, I. M., Clarke, S. M., Wood, J. E. & Mur, L. A. J. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol. 135, 1040–1049 (2004).
Wada, K. C., Yamada, M. & Takeno, K. Stress-induced flowering in pharbitis—A review. Amer. J. Plant Sci. 4, 74–79 (2013).
Hajiboland, R. & Ebrahimi, N. Growth, photosynthesis and phenolic metabolism in tobacco plants under salinity and application of polyamines. J. Plant Biol. 8, 13–26 (2011).
Bose, S. K. & Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 176, 104063 (2020).
Arnao, M. B. & Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 121(2), 195–207 (2018).
Meena, M. et al. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 5, e02952 (2019).
Nasrollahi, V., Mirzaie-asl, A., Piri, K., Nazeri, S. & Mehrabi, R. The effect of drought stress on the expression of key genes involved in the biosynthesis of triterpenoid saponins in licorice (Glycyrrhiza glabra). Phytochemistry 103, 32–37 (2014).
Mustafa, N. et al. Exogenous application of green titanium dioxide nanoparticles (TiO2 NPs) to improve the germination, physiochemical, and yield parameters of wheat plants under salinity stress. Molecules 27, 4884 (2022).
Lee, K., Choi, G. H. & Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J. Pineal Res. 63(4), e12441 (2017).
Weisany, W., Sohrabi, Y., Heidari, G., Siosemardeh, A. & Ghassemi-Golezani, K. Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max.). Plant Omics 5(2), 60–67 (2012).
Zahedi, S. M., Hosseini, M. S., Meybodi, N. D. H. & Silva, J. Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. malase saveh) fruit yield and quality. South Afri. J. Bot. 124, 350–358 (2019).
Zahedi, S. M., Karimi, M., Teixeira, D. & Silva, J. A. The use of nanotechnology to increase quality and yield of fruit crops. J. Sci. Food Agric. 100, 25–31 (2020).
Zahedi, S. M., Hosseini, M. S., Daneshvar Hakimi Meybodi, N. & Peijnenburg, W. Mitigation of the effect of drought on growth and yield of pomegranates by foliar spraying of different sizes of selenium nanoparticles. J. Sci. Food Agric. 101, 5202–5213 (2021).
Signorelli, S. & Considine, M. J. Nitric oxide enables germination by a four-pronged attack on ABA-induced seed dormancy. Front. Plant Sci. 9, 296 (2018).
Kushwaha, B. K. et al. New adventitious root formation and primary root biomass accumulation are regulated by nitric oxide and reactive oxygen species in rice seedlings under arsenate stress. J. Hazard Mater. 361, 134–140 (2019).
Khan, M. N., Mobin, M., Zahid, K. A., Al-Mutairi, K. A. & Siddiqui, Z. H. Role of nano materials in plants under challenging environments. Plant. Physiol. Biochem. 110, 194–209 (2017).
Santa-Cruz, D. M. et al. Nitric oxide synthase-like dependent NO production enhances heme oxygenase up-regulation in ultraviolet-B-irradiated soybean plants. Phytochemistry 71, 1700–1707 (2017).
Cao, X. et al. Nitric oxide synthase mediated early nitric oxide burst alleviates water stress-induced oxidative damage in ammonium-supplied rice roots. BMC Plant Biol. 19, 108 (2019).
Praveen, A., Pandey, A. & Gupta, M. Nitric oxide alters nitrogen metabolism and PIN gene expressions by playing protective role in arsenic challenged Brassica juncea L. Ecotoxicol. Environ. Saf 176, 95–107 (2019).
Siddiqui, M. H. et al. Exogenous nitric oxide alleviates sulfur deficiency-induced oxidative damage in tomato seedlings. Nitric Oxide 94, 95–107 (2020).
Sadique, S., Nisar, S., Dharmadasa, R. M. & Jilani, M. I. Effect of nano-fertilizer and growth hormones on different plants. IJCBS 11, 113–119 (2017).
Bielach, A., Hrtyan, M. & Tognetti, V. B. Plants under stress: Involvement of auxin and cytokinin. Mol. Sci. 18(7), 1427 (2017).
Hussain, S. et al. Titanium application increases phosphorus uptake through changes in auxin content and root architecture in soybean (Glycine Max L.). Front. Plant Sci 2, 743618 (2021).
Wei, J., Zou, Y., Li, P. & Yuan, X. Titanium dioxide nanoparticles promote root growth by interfering with auxin pathways in Arabidopsis thaliana. Phyton 89, 883–891 (2020).
Hussain, S. et al. Changes in morphology, chlorophyll fluorescence performance and Rubisco activity of soybean in response to foliar application of ionic titanium under normal light and shade environment. Sci. Total Environ. 658, 626–637 (2019).
Fujii, H. et al. In vitro reconstitution of an abscisic acid signaling pathway. Nature 462, 660–664 (2009).
Kim, Y. H., Choi, K. I., Khan, A. L., Waqas, M. & Lee, I. J. Exogenous application of abscisic acid regulates endogenous gibberellins homeostasis and enhances resistance of oriental melon against low temperature. Sci. Hortic. 207, 41–47 (2016).
Chaca, M. V. P. et al. Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max L.. Physiol. Plant 36, 2815–2826 (2014).
Syu, Y. Y., Hung, J. H., Chen, J. C. & Chuang, H. W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 83, 57–64 (2014).
Shukla, D., Krishnamurthy, S. & Sahi, S. V. Genome wide transcriptome analysis reveals ABA mediated response in Arabidopsis during gold treatment. Front. Plant Sci. 5, 652 (2014).
Babajani, A., Iranbakhsh, A., Ardebili, Z. O. & Eslami, B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 26(24), 24430–24444 (2019).
Frazier, T. P., Burklew, C. E. & Zhang, B. Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana tabacum). Funct. Integr. Genom. 14, 75–83 (2014).
Singh, S. et al. Understanding the plant and nanoparticle interface at transcriptomic and proteomic level: A concentric overview. Plant Gene 11, 265–272 (2017).
Zolfaghari, F., Rashidi-Monfared, S., Moieni, A., Abedini, D. & Ebrahimi, A. Improving diosgenin production and its biosynthesis in Trigonella foenum-graecum L. hairy root cultures. Ind. Crops Prod. 145, 112075 (2020).
Mohammadi, M., Mashayekh, T., Rashidi-Monfared, S., Ebrahimi, A. & Abedini, D. New insights into diosgenin biosynthesis pathway and its regulation in Trigonella foenum-graecum L.. Phytochem. Anal. 31(2), 1–13 (2019).
Kahila, M. M. H., Najy, A. M., Rahaie, M. & Mir-Derikvand, M. Effect of nanoparticle treatment on expression of a key gene involved in thymoquinone biosynthetic pathway in (Nigella sativa L.). Nat. Prod. Res. 32(15), 1858–1862 (2018).
Zhang, B., Zheng, L. P., Yi Li, W. & Wen Wang, J. Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr. Nanosci. 9(3), 363–370 (2013).
Zhang, J., Davies, W. J., Gollan, T. & Schurr, U. Control of gas exchange: Evidence for root-shoot communication on drying soil. Annales des sciences forestières INRA/EDP Sciences 46, 292–400 (1989).
Moshirian Farahi, S. M., Iranbakhsh, A. R., Mahmoodzadeh, H. & Ebadi, M. The effect of titanium dioxide nanoparticle on the relative expression of catalase, P450. SOD, diTDS and WRKY genes of Itex agnus-castus L.. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 49(4), 12292–12292 (2021).
Xiao, Y. L., Vijver, M. G., Chen, G. C. & Peijnenburg, W. J. Toxicity and accumulation of Cu and Zn O nanoparticles in Daphnia magna. Environ. Sci. Technol 49, 4657–4664 (2015).
Khodayari, M., Omidi, M., Shahnejat Booshehri, A. A., Yazdani, D. & Naghavi, M. R. Gene expression involved in sanguinarine biosynthesis is affected by nano elicitors in Papaver somniferum L.. J. Med. Plants 14(54), 41–54 (2015).
Liu, Y. et al. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol. Biochem. 100, 113–129 (2016).
Ebadollahi, R., Jafarirad, S., Kosari-Nasab, M. & Mahjouri, S. Effect of explant source, perlite nanoparticles and TiO2/perlite nanocomposites on phytochemical composition of metabolites in callus cultures of Hypericum perforatum. Sci. Rep. 9, 1–15 (2019).
Linh, T. M. et al. Metal-based nanoparticles enhance drought tolerance in soybean. J. Nanomat. 1, 4056563 (2020).
Rushton, P. J., Somssich, I. E., Ringler, P. & Shen, Q. J. WRKY transcription factors. Trends Plant Sci. 15, 247–258 (2010).
Irshad, M. A. et al. Synthesis, characterization and advanced sustainable applications of titanium dioxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 212, 111978 (2021).
Tan, W., Peralta-Videa, J. R. & Gardea-Torresdey, J. L. Interaction of titanium dioxide nanoparticles with soil components and plants: current knowledge and future research needs–a critical review. Environ. Sci. Nano 5(2), 257–278 (2018).
Farooqui, A. et al. Role of nanoparticles in growth and development of plants: A review. Int. J. Pharma Bio Sci. 7(4), 22–37 (2016).
Cox, A., Venkatachalam, P., Sahi, S. & Sharma, N. Silver and titanium dioxide nanoparticle toxicity in plants: A review of current research. Plant Physiol. Biochem. 107, 147–163 (2016).
Arnon, A. N. Method of extraction of chlorophyll in the plants. Agron. J. 23(1), 112–121 (1967).
Lichtenthaler, H. K. & Wellburn, A. R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 11, 591–592 (1983).
Hepburn, H. A., Naylor, R. L. & Stokes, D. T. Electrolyte leakage from winter barley tissue as indicator of winter hardiness. Ann. Appl. Biol. 108, 164–165 (1986).
Heath, R. L. & Packer, L. Photo peroxidation in isolated chloroplasts kinetics and stoichiometry of fatty acid peroxidation. Biochem. Biophys. 125, 189–198 (1986).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Biochemistry 72, 248–254 (1976).
Aebi, H. Catalase in vitro. In Methods in Enzymology (ed. Packer, L.) 121–126 (Academic Press, 1984).
Madhusudhan, R., Ishikawa, T., Sawa, Y., Shigeoka, S. & Shibata, H. Characterization of an ascorbate peroxidase in plastids of tobacco BY-2 cells. J. Plant Physiol. 117(4), 550–557 (2003).
Acar, O., Türkan, I. & Özdemir, F. Superoxide dismutase and peroxidase activities in drought sensitive and resistant barley (Hordeum vulgare L.) varieties. Acta Physiol. Plant. 23(3), 351–356 (2001).
Azevedo Neto, A. D., Prisco, J. T., Enéas-Filho, J., de Abreu, C. E. B. & Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 56(1), 87–94 (2006).
McDonald, W. I. et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann. Neurol. 5, 121–127 (2001).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Springer Plant Soil 39(1), 205–207 (1973).
Loreto, F. & Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products and reduces lipid peroxidation of cellular membranes. Plant Physiol. 127, 1781–1787 (2001).
Zhou, R. et al. The role of biquitination in Drosophila innate immunity. J. Biol. Chem 280(40), 34048–34055 (2005).
Zhou, L. et al. The effect of vegetation on surface temperature: A statistical analysis of NDVI and climate data. Geophys. Res. Lett. 30(22), 2147 (2003).
Cao, J., Murch, S. J., O’Brien, R. & Saxena, P. K. Rapid method for accurate analysis of melatonin, serotonin and auxin in plant samples using liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 1134(1–2), 333–337 (2006).
Hubick, K. T. & Reid, D. M. A rapid method for the extraction and analysis of abscisic acid from plant tissue. Plant Physiol. 65(3), 523–525 (1980).
Dobrev, P. I. & Kaminek, M. Fast and efficient separation of cytokinin from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J. Chromatogr. 950, 21–29 (2002).
Zhou, N., Guo, J. F., Yang, L. Y., Xu, P. & Jiang, B. Determination of diosgenin in Paris polyphylla var. yunnanensis at different collecting time by HPLC. Chin. J. Exp. Trad. Med. Formul. 18, 54 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4), 402–408 (2001).
Acknowledgements
We thank the Shahrood University of Technology for financially supporting and providing the required facilities.
Author information
Authors and Affiliations
Contributions
MJB. Investigation and Methodology; AE. Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Validation; Visualization, Writing-Original Draft, Writing -Review & Editing; PH. Supervision, Validation, Review & Editing; EA. Formal analysis and Review & Editing; ZCH. Formal analysis, Validation; Visualization and Review & Editing. SHGH. Formal analysis, Validation, and Review & Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not involve any studies conducted on human participants or animals by any of the authors. Commercial, or advanced breeding genotypes were used in the study, and no wild material was used.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Babaei, M.J., Ebrahimi, A., Heidari, P. et al. Titanium dioxide -mediated regulation of enzymatic and non-enzymatic antioxidants, pigments, and diosgenin content promotes cold stress tolerance in Trigonella foenum-graecum L.. Sci Rep 15, 1837 (2025). https://doi.org/10.1038/s41598-024-84472-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-84472-3
Keywords
This article is cited by
-
Systemic role of melatonin in enhancing temperature stress tolerance in fenugreek: coordination of antioxidant defense, hormonal regulation, energy status, sulfur metabolism, and diosgenin pathway genes
BMC Plant Biology (2025)
-
Enhancing salt tolerance in Mentha × gracilis through foliar applications of titanium and nano-titanium
BMC Plant Biology (2025)
-
Green synthesis of curcumin nanoparticles, characterization, and their role in alleviating arsenic-induced oxidative stress by enhancing antioxidant defense, photosynthetic pigments, and agronomic traits in wheat
Physiology and Molecular Biology of Plants (2025)
-
Nanoparticle-assisted elicitation of therapeutically important secondary metabolites in plants
Plant Growth Regulation (2025)