Abstract
This study explored the great potential of endophytic fungi associated with red seaweed Gracilaria sp. and brown seaweed Sargassum sp. of the Bay of Bengal, Bangladesh, for the first time. Endophytic fungi were identified taxonomically by morphological features and molecular characterisation (ITS sequence). The identification of six fungal isolates revealed five different fungal species belonging to four genera, namely, Aspergillus subversicolor, A. terreus and Cladosporium halotolerans isolated from Gracilaria sp. and Chaetomium globosum, A. terreus and Curvularia perotidis isolated from Sargassum sp. The ethyl acetate extracts of fungal endophytes were evaluated for antimicrobial activity, DPPH radical scavenging activity, and brine shrimp lethality bioassay. Amongst all the fungal extracts evaluated in this study, four showed mild to moderate inhibitory activity (10–14 mm) against the tested bacterial strains. Exceptionally, Chaetomium globosum exerted significant antibacterial activity with the highest zone of inhibition (21 ± 0.3 mm) against the Gram-negative bacterial strain Pseudomonas aeruginosa and also showed moderate antifungal activity (13 ± 0.9 mm) against A. niger. Sargassum sp.-derived A. terreus exhibited the strongest DPPH radical scavenging activity (IC50 value of 7.88 ± 0.09 µg/mL). All the fungal extracts have significant lethality against brine shrimp nauplii (LC50 value of ≤ 20.39 ± 4.04 µg/mL), while Curvularia perotidis and Cladosporium halotolerans were the most effective (LC50 values of 9.30 ± 2.96 µg/mL and 9.94 ± 3.49 µg/mL, respectively). Gas chromatography-mass spectrometry (GC-MS) analysis of the crude extracts identified the presence of several chemical compounds. These bioactive chemical constituents might contribute to the exhibited bioactivity in this study. The current study’s findings support the beneficial impacts of the fungal endophytes on exerting biological activities and consequently as valuable resources of bioactive compounds.
Similar content being viewed by others
Introduction
Endophytes, including fungi or bacteria, invade and grow inside the plant tissues, typically inducing harmless interactions1. Rather, the endophytic fungi aid the host plant in tolerating environmental stresses and combating pathogenic microbes by synthesizing a plethora of bioactive metabolites2,3. However, some endophytes can occasionally become opportunistic under specific conditions like stress or resource scarcity, potentially causing diseases or loss of plant biomass4,5. In recent times, endophytic fungi have garnered increasing interest as the fact unveiled that they can produce host plant-derived secondary metabolites6,7,8. They are also suitable for large-scale production using innovative cultivation techniques, improving the chances of discovering new drugs from microbial natural products9,10. Unique ecological roles and functional attributes distinguish marine fungi from other microbial groups. Their relevance in marine ecosystems, particularly in terms of their interactions with hosts, diversity, and biotechnological potential, often makes them more attractive for metabolite exploitation than marine bacteria or other microbes11,12,13. In this context, researchers worldwide have primarily investigated the endophytic fungal kingdom for active metabolites, intending to discover novel antibacterial14, antifungal15, cytotoxic16, and anticancer compounds17.
However, the potential of seaweed-associated endophytic fungi, mainly those inhabited in the Bay of Bengal of Bangladesh, has yet to be explored comprehensively. Global changes in geographical conditions, climate, and seawater bring an obvious change in the marine biodiversity of the endophytic fungal community4,18. The primary goals of the current study were to isolate the fungal endophytes associated with native seaweed species in the Bay of Bengal, Bangladesh, evaluate their biological potential, and perform a metabolite profiling of the fungal extracts. Seaweeds, also known as marine macroalgae, are considered commercially valuable and environmentally sustainable marine resources. Since ancient times, numerous seaweed species have been utilized as alternative food sources, in cosmetics, pharmaceuticals, fertilizers, biofuel production, and wastewater treatment19. Along with various positive impacts, seaweeds provide habitats and food for crucial marine ecosystem components, including endophytic fungi, significantly affecting the climate. The Bay of Bengal, Bangladesh, nourishes over 335 seaweed species20. Of these, red and brown seaweeds are most abundantly available in this diverse ecosystem. Previous studies have demonstrated the biological, biochemical, and nutritional analysis of the seaweed species on Bangladesh’s coastline21.
Gracilaria is a genus of red seaweeds (Rhodophyta) in the family Gracilariaceae. It encompasses a diverse array of bioactive compounds, like agarans, phenolic acids, diterpenes, mycosporine-like amino acids, sulfonic acids, lipids, steroids, xanthoproteins, oxylipins, heterosides, and bromophenols22. Sargassum, a genus of brown seaweeds (Phaeophyta) in the family Sargassaceae, contains a wide variety of novel secondary metabolites such as sulfated polysaccharides, sterols, polyphenols, amino acids, flavonoids, phlobatannin, cardiac glycosides, saponins, terpenoids, fucoxanthin and beta-carotene-like pigments23,24,25. Thus, these two types of edible seaweed have many health and medicinal benefits, such as anti-inflammatory, cytotoxic, antitumor, antioxidant, antimicrobial, antiaging, antidiabetic, analgesic, immunomodulatory, heart disease prevention, improved digestion, obesity control, brain tissue formation, maintaining bone health, anticancer, and anticoagulant effects19,22,23,24,25,26. However, the literature needs more evidence regarding the potential of fungal endophytes of these two seaweed species on the Bangladesh coast. Researchers are gradually utilizing naturally grown seaweeds in Bangladesh and their associated endophytic fungi due to their wide variety and abundance of bioactive secondary metabolites.
Thus, it is necessary to assess the effectiveness of endophytic fungi derived from well-known seaweeds. Moreover, the preliminary screening of biological and chemical potentials offers a chance for further investigation into the discovery of novel compounds. The current study represents the isolation of fungal endophytes from the red seaweed Gracilaria sp. and the brown seaweed Sargassum sp. in the Bay of Bengal, Bangladesh, using a well-established cultivation system, followed by bioactivity screening of fungal crude extracts using standardized protocols to assess antimicrobial, antioxidant, and cytotoxic potentials as well as chemical characterisation using gas chromatography-mass spectrometry (GC-MS) analysis.
Methods
Collection, identification, and extraction of seaweed samples
Fresh seaweed samples were collected from the coastline of Saint Martin’s Island, Bangladesh, in November 2021. The sample specimens have been preserved in the Bangladesh National Herbarium. The pigmentation and morphological features primarily lead to the identification of seaweed species. The seaweed samples were properly air-dried first and then oven-dried at 40 ˚C for 24 h before grinding. The powdered samples were extracted with methanol at room temperature. Thus, the seaweed extracts were prepared by filtration and subsequent solvent evaporation.
Isolation and extraction of marine endophytic fungi
Endophytic fungi were isolated from fresh and disease-free seaweed samples. To ensure effective isolation of endophytes, the seaweed samples were surface-sterilized using 70% ethanol followed by 5% sodium hypochlorite solution. After sterilization, the samples were thoroughly rinsed with sterile distilled water to remove any residual chemicals. The seaweed samples were then cut into small pieces, and introduced into the water agar media (16 gm agar in 1000 mL distilled water) supplemented with 0.01% streptomycin27. Simultaneously, imprints of the surface-sterilized seaweed samples were prepared on the same culture medium to check for any superficial fungal contaminants. Following the favorable incubation condition (i.e., at 28 ± 2 ˚C in the dark) for 4–6 weeks, visible fungal hyphae growing from the seaweed inoculates were subcultured onto potato dextrose agar (PDA) media to separate each fungal species. The separated fungal endophytes were then streaked or serially diluted to obtain pure cultures.
The isolated endophytic fungi were cultivated on PDA media for 21–28 days at 28 ± 2 °C. The fungal cultures were stored at -20 °C for 48 h and then thawed to room temperature to facilitate the separation of the aqueous phase. After thawing, the culture media containing fungal mycelia were extracted using ethyl acetate28. Thus, the fungal crude extracts were prepared by filtration and subsequent solvent evaporation.
Identification of marine endophytic fungi
The isolated endophytic fungi were identified taxonomically based on their morphological and molecular characteristics27. The primary recognition of fungal genera was predicted by a thorough investigation of macroscopic and microscopic features of colony morphology, which was further confirmed by molecular analysis. Freshly cultured fungal tissue was frozen in liquid nitrogen and ground into a fine powder with a mortar and pestle. The mycelial powder was then processed for DNA extraction using the Maxwell™ 16 platform (Maxwell 16 LEV DNA Kit, AS1420, Promega, USA)29. The Internal Transcribed Spacer (ITS) region of the isolated fungal DNA was amplified by PCR using the forward primer ITS 5 (5’GGAAGTAAAAGTCGTAACAAGG3’) and the reverse primer ITS 4 (5’TCCTCCGCTTATTGATATGC3’)30. The amplified ITS sequences of fungal DNA were subjected to nucleotide BLAST (Basic Local Alignment Search Tool) analysis against the NCBI (National Center for Biotechnology Information) GenBank databases. Subsequent identification up to the species level was guided by phylogenetic analysis. The phylogenetic tree was constructed in MEGA-X software (version: 10.1.0.1, https://www.megasoftware.net/) using the Neighbor-Joining method31. The assembled ITS sequences of each isolate were deposited in the NCBI GenBank, and corresponding accession numbers were received in response.
Antimicrobial activity
The fungal crude extracts were subjected to evaluate antimicrobial activity using the disc diffusion method32. Overall, five bacterial strains, i.e., two Gram-positive bacterial strains, Staphylococcus aureus (ATCC 9144) and Bacillus megaterium (ATCC 13578), and three Gram-negative bacterial strains, Escherichia coli (ATCC 11303), Salmonella typhi (ATCC 13311), and Pseudomonas aeruginosa (ATCC 27833), and two fungal strains, Aspergillus niger (ATCC 1004) and A. flavus (UCFT 02), were used to assess the antimicrobial activity. The sample discs were prepared with 100 µg extract per disc, using dichloromethane as the solvent. After overnight diffusion from the discs, the bacterial and fungal cultures were incubated for 16–18 h at 37 ± 2 ˚C and for 44–52 h at 28 ± 2 ˚C, respectively. The inhibitory activity of the fungal extracts was measured in terms of the zone of inhibition (in mm) and compared to that of the standards, kanamycin and ketoconazole (30 µg/disc), respectively.
Antioxidant activity
The antioxidant activity of the fungal crude extracts was evaluated by testing 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity33. This colorimetric method aims to observe how fungal isolates affect the DPPH free radical’s ability to oxidize by measuring absorbance with a spectrophotometer at 517 nm. The sample solutions were prepared with varying concentrations from 200 µg/mL to 0.78 µg/mL obtained by serial dilution using methanol as the solvent. These sample solutions were then treated with a DPPH solution (20 µg/mL) in equal volume and allowed to rest for 30 min in the dark. The DPPH radical scavenging activity (RSA) of the sample extracts was estimated by the formula: RSA (%) = [(Acontrol − Atest sample) / Acontrol] × 100. The IC50 values (the concentration that can inhibit oxidation by 50%, in µg/mL) were determined through logarithmic regression analysis of the % inhibition versus concentration curve. These values were then compared with those of two reference antioxidants, ascorbic acid and trolox.
Cytotoxic activity
The cytotoxic activity of the fungal crude extracts was evaluated using the brine shrimp lethality assay34. This assay utilizes nauplii of brine shrimp Artemia salina to assess the sample’s toxicity by measuring the percentage of living nauplii 24 h after the sample treatment. The sample solutions were prepared with concentrations ranging from 400 µg/mL to 1.59 µg/mL by serial dilution using dimethyl sulfoxide (DMSO) as the solvent. These sample solutions were added to brine water (5 mL) containing ten living shrimp nauplii and rested for 24 h. The brine shrimp lethality of the sample extracts was estimated by determining the LC50 values (the concentration lethal to 50% brine shrimp nauplii, in µg/mL). These values were then compared with those of the reference cytotoxic agent, vincristine sulfate.
Gas chromatography-mass spectrometry (GC-MS) analysis
Gas chromatography-mass spectrometry analyses were performed on ethyl acetate extracts of fungal isolates and methanol extracts of host seaweeds using the Clarus®690 gas chromatograph (PerkinElmer, USA) coupled with a Clarus® SQ 8 C mass spectrophotometer. A sample (1 µL, in splitless mode) was injected at a constant inlet temperature of 280 ˚C. 99.999% pure helium was used as a carrier gas at a 1 mL/min flow rate for a 40–60 min run time using Elite-35 and HP-5MS columns (30 m X 0.25 mm, 0.25 μm film thickness). The column temperature was raised at a rate of 4–5 ˚C/min, starting from 60 ˚C to 240 ˚C, and maintained for 4–15 min35. Electron ionization (EI) mode was applied for sample analysis at an energy of 70 eV. The compounds were identified by comparing their mass spectra with those in the National Institute of Standards and Technology (NIST) database, using a minimum match quality of 90% as the criterion.
Data analysis
Each bioactivity test was conducted in triplicate, and the data were recorded accordingly. The logistic regression method was used to calculate the IC50 and LC50 values. The values are presented as mean ± standard deviation (SD), n = 3. Microsoft Excel was used to prepare the calculations and graphs.
Results
The sterilization method proved highly effective in eliminating superficial contaminants, as evidenced by the absence of fungal growth on the control imprints of surface-sterilized seaweed samples. This ensured the successful isolation of six endophytic fungi from Gracilaria sp. and Sargassum sp. Following purification, the fungal endophytes were preserved at 4 °C for further analysis and maintained by subculturing every two months.
Identification of endophytic fungal isolates
Three endophytic fungi bearing internal strain nos. GE-1, GE-2, and GE-3 were isolated from the red seaweed Gracilaria sp. (Herbarium accession no. DACB-90626), which were later identified as Aspergillus subversicolor36, A. terreus37, and Cladosporium halotolerans38, respectively. Another three fungal endophytes bearing internal strain nos. SE-1, SE-2, and SE-3 were isolated from the brown seaweed Sargassum sp. (Herbarium accession no. DACB-90627), which were later identified as Chaetomium globosum39, A. terreus37, and Curvularia perotidis40,41, respectively. The morphological and molecular characteristics of isolated endophytic fungi and their GenBank accession numbers are presented in (Tables 1 and 2). Figures 1 and 2 show pictures of fungal colony surface, colony reverse, microscopic view, and phylogenetic tree reflecting how closely each isolate is related to its best match.
Antimicrobial activity
Most fungal crude extracts revealed mild-to-moderate zone of inhibition (10–15 mm) against tested bacterial strains. Among the samples tested, Chaetomium globosum (SE-1) exhibited significant activity against the Gram-negative bacterial strain Pseudomonas aeruginosa, with the highest zone of inhibition (21 ± 0.3 mm) (Fig. 3A). The same isolate, SE-1, showed the highest inhibition (13 ± 0.9 mm) against the fungal strain Aspergillus niger, while others were primarily inactive against tested fungal strains. The solvent control (dichloromethane) showed no activity.
Bioactivity screening of the marine endophytic fungi. (A) Antimicrobial activity, compared with standards kanamycin (KAN) and ketoconazole (KET). (B) DPPH radical scavenging activity, compared with standards ascorbic acid (ASA) and trolox (TRX). (C) Brine shrimp lethality bioassay, compared with standard vincristine sulfate (VCS). Values are expressed as mean ± SD, n = 3. Codes on the horizontal axis represent internal strain nos. of the endophytic fungal isolates.
Antioxidant activity
The antioxidant screening revealed that five of the six fungal crude extracts could scavenge DPPH radicals (IC50, 7.88–178.32 µg/mL) (Fig. 3B). Exceptionally, very strong antioxidant activity was exhibited by Aspergillus terreus (SE-2), having the lowest IC50 value (7.88 ± 0.09 µg/mL) among all the extracts tested. Cladosporium halotolerans (GE-3) had no scavenging activity at the experimental concentration (IC50 > 250 µg/mL)42. The other fungal extracts had mild to strong antioxidant activity.
Cytotoxic activity
All the fungal crude extracts showed significant cytotoxic activity regarding brine shrimp lethality bioassay (LC50, 9.30–20.39 µg/mL) (Fig. 3C). However, Curvularia perotidis (SE-3) exhibited the lowest LC50 value (9.30 ± 2.96 µg/mL) among all the extracts tested, indicating potent cytotoxicity. Cladosporium halotolerans (GE-3) had similar potency with a lower LC50 value of 9.94 ± 3.49 µg/mL. Aspergillus subversicolor (GE-1), A. terreus (GE-2), Chaetomium globosum (SE-1) and A. terreus (SE-2) all showed significant cytotoxicity with LC50 values of 19.73 ± 4.84 µg/mL, 19.01 ± 1.02 µg/mL, 14.97 ± 4.39 µg/mL, and 20.39 ± 4.04 µg/mL, respectively.
Chemical characterisation
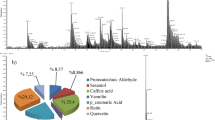
The GC-MS analyses of seaweed extract from Gracilaria sp. (GE) and its associated endophytic fungal extracts (GE-1, GE-2, and GE-3) led to identifying 12, 13, 33, and 21 nos. of compounds, respectively. Similarly, GC-MS analyses on seaweed extract from Sargassum sp. (SE) and its associated endophytic fungal extracts (SE-1, SE-2, and SE-3) identified 12, 3, 15, and 17 nos. of compounds, respectively. Figure S1 (supplementary material) presents the distinct GC-MS chromatograms of the investigated extracts. The identified compounds with their retention time, molecular weight, molecular formula, and peak area percent are presented in (Tables 3 and 4). Based on the percentage, the most abundant component was Dodecanoic acid, 1,2,3-propanetriyl ester (1) in the seaweed extracts GE (92.10%) and SE (95.49%), and fungal extracts GE-1 (77.86%) and GE-3 (76.84%). The fungal extract GE-2 contained the following prominent bioactive components: Erucic acid (2, 11.71%), Hexanedioic acid, bis(2-ethylhexyl) ester (3, 7.28%), 26,27-Di(nor)-cholest-5, 7, 23-trien-22-ol, 3-methoxymethoxy- (4, 6.42%), Glutaric acid, dodec-2-en-1-yl pentafluorophenyl ester (5, 5.66%), and n-Hexadecanoic acid (6, 5.44%). The compound 6 was also present in the fungal extract GE-3 (9.95%). Other compounds identified abundantly were 1-(ethanesulfonyl)-2-(ethylsulfanyl)ethane (7, 99.52%) in the fungal extract SE-1, Methyl-4-deoxy-2-O-methyl-β-L-threo-hex-4-enopyranosid uronate (8, 26.43%), 3-(methylthio)propyl nonanoate (9, 25.63%), and Glycidyl palmitate (10, 6.68%) in the fungal extract SE-2, and once more, compound 10 (87.63%) in the fungal extract SE-3. The structures of these major bioactive chemical compounds of marine algal and fungal origin are presented in (Fig. 4).
Discussion
Bangladesh, a tropical country bordered by the Bay of Bengal to the south, boasts the extended coastline enriched with diverse seaweed resources. This presents a unique opportunity for more comprehensive investigations into the enormous possibilities of seaweed-associated endophytes, including fungi, bacteria, and other microbes. Marine microbes are prolific producers of bioactive molecules, such as antibiotics, cytotoxic agents, and antifungals, with applications in both pharmaceutical and agricultural fields43. Marine fungi remain relatively underexplored compared to terrestrial fungi, offering a vast reservoir of untapped biodiversity and bioactive potential44. This makes their study highly relevant for discovering novel compounds that are often absent or less abundant in other microbial sources. For example, certain exclusively fungal metabolites, such as cytochalasins45, patulin46, citrinin47, sterigmatocystin48, and gliotoxin49, have been reported for their potential pharmacological activities, including anticancer and immunosuppressive properties. Although several studies worldwide have demonstrated the great potential of marine endophytic fungi, there is insufficient information regarding the significance of seaweed-associated endophytic fungal flora in Bangladesh. A deeper understanding of this concept is required, as the bioactivity and chemical composition of fungal endophytes vary depending on geographic location and species4,18. Additionally, evaluating antimicrobial, antioxidant, and cytotoxic activities is a reliable measure of the ability of fungal endophytes to produce bioactive secondary metabolites. Thus, this type of research validates a seaweed species and its endophytic fungus as a leading source and eventually facilitates the discovery of novel bioactive compounds.
This study represents the preliminary evaluation of marine endophytic fungi, isolated for the first time, from two seaweeds, Gracilaria sp. and Sargassum sp., inhabited in the Bay of Bengal, Bangladesh. Among six fungal isolates in this study, Aspergillus was the dominant genus (internal strain nos. GE-1, GE-2, and SE-2), while the others belonged to the genera Cladosporium, Chaetomium, and Curvularia (internal strain nos. GE-3, SE-1, and SE-3, respectively) (Figs. 1 and 2; Tables 1 and 2). These genera are commonly detected across different indoor environments, terrestrial and marine plants50 and are classified under the ascomycota division, a major dominant group within the fungi kingdom. Phylogenetic analysis illustrated the relationships of the fungal isolates with their genetically similar species. Each isolate was found to form a distinct monophyletic clade with its most closely related species. Taxonomically diversified endophytic fungi are widely found on seaweeds, sea sponges, sea snails, tunicates, and other sea organisms, playing vital roles in marine habitats51. A previous study reported the isolation of Chaetomium globosum, Nigrospora magnoliae, Curvularia sp., Curvularia moringae, Aspergillus terreus, and Collariella sp. from the green seaweed Ulva sp. collected from the coastal area of Bangladesh27. Lini et al. identified endophytic fungal isolates from marine weeds of Bangladesh, including a Fusarium sp. and a Penicillium sp. from red seaweed Hypnea musciformis, a Fusarium sp. from brown seaweed S. crassifolium, a Penicillium sp. from green seaweed Dictyota dichotoma and a Aspergillus sp. from green seaweed Caulerpa peltate52.
Antimicrobial screening revealed that extracts of Aspergillus terreus (GE-2), Chaetomium globosum (SE-1), and A. terreus (SE-2) were more promising among all the evaluated extracts (Fig. 3A). Developing new antimicrobial agents is essential to combat the growing threat of drug-resistant microbes. It is quite natural that the crude extracts may exhibit lower antimicrobial activity compared to the standards. However, identifying and analyzing the specific metabolites responsible for this activity can lead to the discovery of novel and potent antimicrobial agents. For example, marine-derived A. terreus has been reported to produce the novel antibacterial metabolite butyrolactone J with a minimal inhibitory concentration of 12.5 µg/mL against Staphylococcus aureus53.
The isolates GE-2 and SE-2, both identified as A. terreus, demonstrated antibacterial activity but did not show antifungal activity. Extract of GE-2 inhibited all five tested bacterial strains with zones of 7–12 mm, while extract of SE-2 inhibited three out of five strains with larger zones of 11–14 mm. Interestingly, another A. terreus strain isolated from a sea worm (Spirorbis sp.) showed no antibacterial activity, with no inhibition zones observed54. In contrast, A. terreus isolated from the leaf of the terrestrial plant Psidium guajava exhibited strong antibacterial activity, with inhibition zones ranging from 18 to 26 mm when tested with 10,000 µg of fungal extract55.
On the other hand, extract of C. globosum (SE-1) inhibited the growth of three out of five tested bacterial strains, exhibiting the largest zone of inhibition (21 ± 0.3 mm) against the Gram-negative bacterial strain Pseudomonas aeruginosa. This finding aligns with the studies of Kamat et al.56 and Noor et al.27, who reported that marine green macroalgae-derived C. globosum produces diverse secondary metabolites exerting antimicrobial potential with inhibition zones of 13–27 mm. Goda et al.57 also investigated the antibacterial potential of C. globosum strains isolated from medicinal terrestrial plants in Egypt, finding inhibition zones ranging from 10.4 to 25.4 mm with E. coli and P. aeruginosa being the most sensitive strains.
In the current study, extract of Curvularia perotidis (SE-3) was mildly active only against P. aeruginosa, which was the most susceptible bacterial strain (10–21 mm) to the tested extracts. In contrast, bacterial strains, Bacillus megaterium and Salmonella typhi, and fungal strains, A. niger and (A) flavus were less susceptible. Only the extract of isolate GE-2 (A. terreus) inhibited the growth of (B) megaterium and S. typhi mildly and insignificantly, respectively, compared to other isolates. Extracts of other isolates, A. subversicolor (GE-1) and Cladosporium halotolerans (GE-3) showed inactivity against all the test microorganisms. A zone of inhibition of ≤ 7 mm is considered inactive against microbial growth58. The highest antifungal activity with inhibition zone of 13 ± 0.9 mm was observed by the extract of Chaetomium globosum (SE-1) against A. niger. Other isolates were insignificant or inactive against the tested fungal strains. Goda et al.57 also reported that three out of six (C) globosum isolates inhibited Candida albicans with zones of 11.3–25.6 mm.
Most fungal extracts exhibited mild to strong antioxidant activity, with IC50 values ranging from 7.88 to 178.32 µg/mL (Fig. 3B). The strongest DPPH radical scavenging activity was observed by the extract of A. terreus (SE-2), with the lowest IC50 value of 7.88 ± 0.09 µg/mL among all the extracts tested42. However, the extracts of A. terreus (GE-2) and Curvularia perotidis (SE-3) showed strong antioxidant activity, with IC50 values of 15.08 ± 0.44 and 16.82 ± 0.11 µg/mL, respectively. The other extracts of A. subversicolor (GE-1) and Chaetomium globosum (SE-1) exhibited mild antioxidant activity, with IC50 values of 178.32 ± 0.28 and 112.87 ± 5.09 µg/mL, respectively. The findings presented in this study are supported by the concurrence of Ahamed and Murugan59 and Lini et al.52. Their studies reported the antioxidant nature (IC50, 17–20 µg/mL) of Aspergillus sp. derived from marine green macroalgae. Al Mousa et al.60 also documented soil-derived A. terreus showing antioxidant properties with an IC50 value of 470 µg/mL. Segaran et al.61 evaluated the antioxidant potential of Curvularia sp. isolated from the medicinal herb Boerhaavia diffusa L., finding only about 10% inhibition with ethyl acetate extract at 100 µg/mL concentration, while the highest inhibition (approximately 27%) was observed with butanol extract. In contrast, the ethyl acetate extract of C. perotidis (SE-3) in this study showed significantly higher inhibition (83.6%) at 100 µg/mL concentration.
The brine shrimp lethality bioassay serves as a preliminary screening tool for evaluating the cytotoxic activity of fungal crude extracts. Here, an LC50 value of ≤ 30 µg/mL is considered indicative of significant activity34. Thus, from this study, we can claim that all the fungal isolates have significant cytotoxicity, with LC50 values of ≤ 20.39 ± 4.04 µg/mL. Curvularia perotidis (SE-3) and Cladosporium halotolerans (GE-3) exhibited the strongest activity (LC50, 9.30 ± 2.96 µg/mL and 9.94 ± 3.49 µg/mL, respectively) among the samples tested (Fig. 3C). According to Noor et al.27 and Vega-Portalatino et al.62, seaweed-derived Curvularia sp. has a significant impact on cytotoxicity (LC50, 16–18 µg/mL) and provides new insights into the prospects of optimizing its metabolites as anticancer compounds. Sandrawati et al.63 also reported cytotoxic activity of Cladosporium halotolerans isolated from the marine sponge Dactylospongia sp., with an LC50 value of 64.7 µg/mL. These findings highlight the variability in bioactive potential of fungal extracts depending on their source and ecological niche. Table 5 summarizes the overall biological activities observed in this study. Thus, further studies are necessary to explore the biological potential of these extracts more intensely.
Several chemical compounds were identified from the experimental extracts by GC-MS analysis, most of which were different fatty acids or their esters. These identified compounds are thought to be accountable for the exerted bioactivity of the extracts. Previous studies have reported overwhelming pharmacological activities of these fatty acids and their esters, with their usage in cosmetics and biofuel production64. In this study, the most abundant metabolite of marine algal and fungal origin was Dodecanoic acid, 1,2,3-propanetriyl ester (1). Compound 1 is a lauric acid ester (also known as trilaurin) that has been reported for antioxidant, antibacterial, antiviral, hypocholesterolemic, antiarthritic, nematocidal, hepatoprotective and mosquito-repellent activities65,66. The fungal isolates GE-1 and GE-3 produced compound 1 in a significant quantity (77.86% and 76.84%, respectively), similar to the host seaweed (92.10%). A minor compound, Tetradecanoic acid, 10,13-dimethyl-, methyl ester, was also found in all the associated fungal extracts like the host Gracilaria sp., while the other metabolites were unique. The strong cytotoxic and mild antioxidant nature of Aspergillus subversicolor (GE-1) might be due to the abundance of compound 1.
The fungal extract of isolate GE-2 contained Erucic acid (2, also called Z-13-docosenoic acid) in major quantity (11.71%), and its amide derivative, 13-Docosenamide, (Z)- (also called Erucamide), in minor quantity (2.78%). Compound 2 and its amide derivative have been reported for diverse beneficial effects, including antibacterial, antifungal, anti-inflammatory, neuroprotective, cytotoxic, and anticancer activities67,68. Other major bioactive compounds of fungal extract of GE-2 were Hexanedioic acid, bis(2-ethylhexyl) ester (3, 7.28%), 26,27-Di (nor)-cholest-5, 7, 23-trien-22-ol, 3-methoxymethoxy- (4, 6.42%), Glutaric acid, dodec-2-en-1-yl pentafluorophenyl ester (5, 5.66%), n-Hexadecanoic acid (6, 5.44%). Hexanedioic acid, bis(2-ethylhexyl) ester (3) showed antidiabetic, anti-inflammatory, and anticancer activity in the previous studies, and also has minor uses as a plasticizer69. Sekaran et al. evaluated the potential chemoprotective activity of 26,27-Di (nor)-cholest-5, 7, 23-trien-22-ol, 3-methoxymethoxy- (4) against hepatocellular carcinoma by In-silico study70. Glutaric acid, dodec-2-en-1-yl pentafluorophenyl ester (5) has no reported pharmacological activity. On the other hand, n-Hexadecanoic acid (6), also called palmitic acid, has been reported to have various bioactivities such as antioxidant, anti-inflammatory, antibacterial, anticancer, 5-alpha reductase inhibitor, hemolytic, antiandrogenic flavor, pesticide, nematicide, hypocholesterolemic, potent mosquito larvicide71,72,73,74,75. A. terreus (GE-2) revealed a potent antioxidant and cytotoxic nature with mild antibacterial activity (Table 5), possibly due to the existence of its aforementioned major components. Abdel-Wahab et al. reported 13-Docosenamide, (Z)- and palmitic acid (6) as major components of some marine microbes (including yeast and thraustochytid isolates)76.
Similarly, the very strong antioxidant property, strong cytotoxicity, and mild to moderate antibacterial activity of the extract of A. terreus (SE-2) might refer to the contribution of its major constituents. 3-(methylthio)propyl nonanoate (9, 25.63%) is a nonanoic acid ester, one of the major compounds in the fungal extract SE-2. Nonanoic acid, also called pelargonic acid, has antifungal potential against Candida albicans77 and cytotoxicity against the Vero cell line78. Nonanoic acid and some of its esters are also known as odour-active compounds79. Additionally, Methyl-4-deoxy-2-O-methyl-β-L-threo-hex-4-enopyranosid uronate (8, 26.43%) is an ester of hexenuronic acid (HexA) and possesses no known benefits80. HexA has been reported to have antioxidant property81 and possibly a hypoglycemic effect82. Glycidyl palmitate (10), another fatty acid ester, can be used to prepare lysophosphatidic acids, inhibiting apoptosis revealed in an earlier study83.
Compound 10 was found to be a major component in the fungal extract of SE-3 (87.63%). Hashem et al.84 and Pandurangan et al.85 reported Glycidyl palmitate (10) as a fungal metabolite. Another bioactive compound of the isolate SE-3 was Cyclononasiloxane, octadecamethyl (11, 3.2%), a common fungal metabolite5,86 that exhibited antimicrobial and cancer preventive activity87,88. These compounds might contribute to the very strong cytotoxicity, strong antioxidant property, and mild antibacterial activity of Curvularia perotidis (SE-3). A minor component, 13-octadecenoic acid, methyl ester, was also found in the fungal extracts SE-2 and SE-3, similar to the host Sargassum sp. The presence of 1-(ethanesulfonyl)-2-(ethylsulfanyl)ethane (7, 99.52%), a prevalent compound of the extract of Chaetomium globosum (SE-1), could be accountable for its strong cytotoxicity and mild antioxidant activity with mild to strong antimicrobial activity. A literature survey could not reveal any previous report of compound 7, but its similar compounds are well-known as odorants89. The bioactive compounds 1 (76.84%) and 6 (9.95%) might be responsible for the very strong cytotoxicity exhibited by the extract of Cladosporium halotolerans (GE-3). Li et al.90 and Wang et al.91 revealed significant cytotoxicity of some pyrone derivatives isolated from the marine-derived fungus C. halotolerans.
The seaweed extract GE also contained a bioactive compound, squalene (12, 2.11%). Squalene (12) is a triterpene widely found in different seaweed species6,92 and is recognised as a potent radical scavenger, especially in inhibiting lipid peroxidation. It is also indicated for anti-inflammatory activities93, chemopreventive effects by reducing cancer risk94, and significantly high UV protective effects with the least toxicity in cosmetics95. However, further chemical analyses are necessary to investigate the widespread metabolite profiling of each of these extracts.
This research focuses on isolating, identifying, and evaluating potential fungal endophytes associated with two seaweeds, Gracilaria sp. and Sargassum sp., collected from Bangladesh’s Bay of Bengal. This study proves that fungal endophytes derived from these seaweeds are a valuable resource for biologically active compounds and may fulfill the emerging necessity for potent molecules in therapeutics.
Conclusion
Three fungal endophytes were isolated from the red seaweed Gracilaria sp. and another three from the brown seaweed Sargassum sp., inhabited in the Bay of Bengal of Bangladesh for the first time. The present study has disclosed the morphological and molecular characteristics of fungal endophytes. The isolated fungal endophytes revealed notable antimicrobial, antioxidant, and cytotoxic activities. GC-MS-based chemical analysis revealed the presence of 91 different compounds in total among the crude extracts of fungal isolates and their hosts. These identified compounds, especially the major ones, have been previously reported to possess various pharmacological activities, which could be responsible for the observed bioactivity in this study. Further studies should focus on isolating these bioactive compounds and their mechanistic analysis, providing scientific evidence of relevant bioactivity.
Data availability
The datasets generated during the current study are available in the NCBI GenBank repository, under accession numbers OR335219, OR335235, OR338335, OR338812, OR336320, and OR335556.
References
Fanele, A. & Ndlovu, S. I. Endophytic fungal species Nigrospora oryzae and Alternaria alternata exhibit antimicrobial activity against gram-positive and gram-negative multi-drug resistant clinical bacterial isolates. BMC Complement. Med. Ther. 23, 323. https://doi.org/10.1186/s12906-023-04157-8 (2023).
Kaaniche, F. et al. Bioactive secondary metabolites from new endophytic fungus Curvularia. Sp isolated from Rauwolfia macrophylla. PLoS One. 14 (6), e0217627. https://doi.org/10.1371/journal.pone.0217627 (2019).
Jia, M. et al. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front. Microbiol. 7, 906. https://doi.org/10.3389/fmicb.2016.00906 (2016).
Graca, D., Arias-Real, R., Fernandes, I., Cássio, F. & Pascoal, C. Fungal identity mediates the impacts of multiple stressors on freshwater ecosystems. Sci. Total Environ. 937, 173466. https://doi.org/10.1016/j.scitotenv.2024.173466 (2024).
Mahmud, S. M. N. et al. Cytotoxicity, antioxidant, antimicrobial studies and phytochemical screening of endophytic fungi isolated from Justicia Gendarussa. Ann. Agric. Sci. 65, 225–232. https://doi.org/10.1016/j.aoas.2020.12.003 (2020).
Abdalla, M. A. et al. Isolation of endophytic fungi from South African plants, and screening for their antimicrobial and extracellular enzymatic activities and presence of type I polyketide synthases. South. Afr. J. Bot. 134, 336–342. https://doi.org/10.1016/j.sajb.2020.03.021 (2020).
Lutfia, A., Munir, E., Yurnaliza, Y. & Basyuni, M. Chemical analysis and anticancer activity of sesterterpenoid from an endophytic fungus Hypomontagnella monticulosa Zg15SU and its host Zingiber Griffithii Baker. Heliyon 7 (2), e06292. https://doi.org/10.1016/j.heliyon.2021.e06292 (2021).
Teixeira, T. R. et al. Characterisation of the lipid profile of Antarctic brown seaweeds and their endophytic fungi by gas chromatography–mass spectrometry (GC–MS). Polar Biol. 42, 1431–1444. https://doi.org/10.1007/s00300-019-02529-w (2019).
Singh, A., Singh, D. K., Kharwar, R. N., White, J. F. & Gond, S. K. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: insights, avenues, and challenges. Microorganisms 9 (1), 197. https://doi.org/10.3390/microorganisms9010197 (2021).
Tyśkiewicz, K. et al. Characterisation of bioactive compounds in the biomass of black locust, Poplar and Willow. Trees 33, 1235–1263. https://doi.org/10.1007/s00468-019-01837-2 (2019).
Shukla, S. & Kim, M. Marine natural flora: a potent source of anticancer metabolites. Indian J. Geo Mar. Sci. 45 (11), 1412–1421 (2016).
Ahmed, N. T., Noor, S., Rahman, M. M. & Mazid, M. A. Cytotoxic compounds derived from marine algicolous and spongicolous endophytic fungi: a review. Dhaka Univ. J. Pharm. Sci. 20 (2), 247–265. https://doi.org/10.3329/dujps.v20i2.57175 (2021).
Hridoy, M. et al. Putative anticancer compounds from plant-derived endophytic fungi: a review. Molecules 27 (1), 296. https://doi.org/10.3390/molecules27010296 (2022).
Li, H., Hu, P., Wang, Y., Pan, Y. & Liu, G. Enhancing the production of cephalosporin C through modulating the autophagic process of Acremonium chrysogenum. Microb. Cell. Fact. 17 (1), 175. https://doi.org/10.1186/s12934-018-1021-9 (2018).
Kuhnert, E. et al. Enfumafungin synthase represents a novel lineage of fungal triterpene cyclases. Environ. Microbiol. 20 (9), 3325–3342. https://doi.org/10.1111/1462-2920.14333 (2018).
Fang, W. et al. Asperpyrone-type bis-naphtho-γ-pyrones with COX-2–inhibitory activities from marine-derived fungus Aspergillus Niger. Molecules 21 (7), 941. https://doi.org/10.3390/molecules21070941 (2016).
Zhang, P., Li, X-M., Wang, J-N. & Wang, B-G. Oxepine-containing Diketopiperazine alkaloids from the algal-derived endophytic fungus Paecilomyces variotii EN-291. Helv. Chim. Acta. 98 (6), 800–804. https://doi.org/10.1002/hlca.201400328 (2015).
Pan, Y., Zhang, W., Xu, Z., Zuo, Z. & Yuan, T. Fungal community shows more variations by season and particle size than bacteria. Sci. Total Environ. 925, 171584. https://doi.org/10.1016/j.scitotenv.2024.171584 (2024).
Sobuj, M. K. A. et al. Effect of solvents on bioactive compounds and antioxidant activity of Padina tetrastromatica and Gracilaria tenuistipitata seaweeds collected from Bangladesh. Sci. Rep. 11, 19082. https://doi.org/10.1038/s41598-021-98461-3 (2021).
Chowdhury, M. S. N., Hossain, M. S., Uddin, S. A., Alamgir, M. & Sharifuzzaman, S. M. Seaweed aquaculture in Bangladesh: present status, challenges and future prospects. Ocean. Coast Manag. 228, 106309. https://doi.org/10.1016/j.ocecoaman.2022.106309 (2022).
Hossain, M. T. et al. Nutritional value, phytochemical profile, antioxidant property and agar yielding potential of macroalgae from Coasts of Cox’s Bazar and St. Martin’s Island of Bangladesh. J. Aquat. Food Prod. Technol. 30 (2), 217–227. https://doi.org/10.1080/10498850.2020.1869876 (2021).
Rani, A., Saini, K. C., Fartyal, M., Jaitak, V. & Bast, F. A concise review on the bioactive potential of the genus Gracilaria (Rhodophyta). Nucleus 2024, 1–17. https://doi.org/10.1007/s13237-024-00471-9 (2024).
Afreen, A. B., Rasool, F. & Fatima, M. Bioactive properties of brown seaweed, Sargassum Wightii and its nutritional, therapeutic potential and health benefits: a review. J. Environ. Biol. 44, 146–158. https://doi.org/10.22438/jeb/44/2/MRN-5081 (2023).
Sobuj, M. K. A. et al. Qualitative and quantitative phytochemical analysis of brown seaweed Sargassum polycystum collected from Bangladesh with its antioxidant activity determination. Food Chem. Adv. 4, 100565. https://doi.org/10.1016/j.focha.2023.100565 (2024).
Wang, X. et al. Bioactivities of the popular edible brown seaweed Sargassum fusiforme: a review. J. Agric. Food Chem. 71 (44), 16452–16468. https://doi.org/10.1021/acs.jafc.3c05135 (2023).
Hossain, M. S., Alamgir, M., Uddin, S. A. & Chowdhury, M. S. N. Seaweeds for Blue Economy in Bangladeshp. 5–73 (Food and Agriculture Organization of the United Nations, 2020).
Noor, S. et al. Bioactivity and chemical screening of endophytic fungi associated with the seaweed Ulva Sp. of the Bay of Bengal, Bangladesh. Bot. Mar. 67 (2), 115–129. https://doi.org/10.1515/bot-2023-0040 (2024).
Hoque, N. et al. Antimicrobial, antioxidant, and cytotoxic properties of endophytic fungi isolated from Thysanolaena maxima Roxb., Dracaena spicata Roxb. and Aglaonema hookerianum Schott. BMC Complement Med. Ther. 3, 347 https://doi.org/10.1186/s12906-023-04185-4 (2023).
Dalla-Costa, L. M. et al. Comparison of DNA extraction methods used to detect bacterial and yeast DNA from spiked whole blood by real-time PCR. J. Microbiol. Methods. 140, 61–66. https://doi.org/10.1016/j.mimet.2017.06.020 (2017).
Martin, K. J. & Rygiewicz, P. T. Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol. 5, 28. https://doi.org/10.1186/1471-2180-5-28 (2005).
Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35 (6), 1547–1549. https://doi.org/10.1093/molbev/msy096 (2018).
Bauer, A. W., Kirby, W. M. M., Sherris, J. C. & Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45 (4), 493–496 (1966). https://pubmed.ncbi.nlm.nih.gov/5325707/
Brand-Williams, W., Cuvelier, M. E. & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28 (1), 25–30. https://doi.org/10.1016/S0023-6438(95)80008-5 (1995).
Meyer, B. N. et al. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 45 (5), 31–34. https://doi.org/10.1055/s-2007-971236 (1982).
Murshid, G. M. M., Sohrab, M. H., Masud, M. M., Mazid, M. A. & Identification GC-MS analysis and antibacterial activity of endophytic fungi isolated from Trigonella foenum-graecum leaf. Plant. Sci. Today 11 (3). https://doi.org/10.14719/pst.2735 (2024).
Jurjevic, Z., Peterson, S. W. & Horn, B. W. Aspergillus section Versicolores: nine new species and multilocus DNA sequence based phylogeny. IMA Fungus. 3 (1), 59–79. https://doi.org/10.5598/imafungus.2012.03.01.07 (2012).
Walsh, T. J., Hayden, R. T. & Larone, D. H. Larone’s Medically Important Fungi: a Guide To Identification 6th edn (ASM, 2018).
Bensch, K. et al. Cladosporium species in indoor environments. Stud. Mycol. 89, 177–301. https://doi.org/10.1016/j.simyco.2018.03.002 (2018).
Wang, X. W. et al. Diversity and taxonomy of Chaetomium and Chaetomium-like fungi from indoor environments. Stud. Mycol. 84, 145–224. https://doi.org/10.1016/j.simyco.2016.11.005 (2016).
Manamgoda, D. S., Rossman, A. Y., Castlebury, L. A., Chukeatirote, E. & Hyde, K. D. A taxonomic and phylogenetic re-appraisal of the genus Curvularia (Pleosporaceae): human and plant pathogens. Phytotaxa 212 (3), 175–198. https://doi.org/10.11646/phytotaxa.212.3.1 (2015).
Mehrabi-Koushki, M., Pooladi, P., Eisvand, P. & Babaahmadi, G. Curvularia ahvazensis and C. rouhanii spp. Nov. Iran. Mycosphere. 9 (6), 1173–1186. https://doi.org/10.5943/mycosphere/9/6/7 (2018).
Phongpaichit, S. et al. Biological activities of extracts from endophytic fungi isolated from Garcinia plants. FEMS Immunol. Med. Microbiol. 51 (3), 517–525 (2007). https://academic.oup.com/femspd/article/51/3/517/631743
Malve, H. Exploring the ocean for new drug developments: marine Pharmacology. J. Pharm. Bioall Sci. 8, 83–91. https://doi.org/10.4103/0975-7406.171700 (2016).
Eghtedari, M., Porzani, S. J. & Nowruzi, B. Anticancer potential of natural peptides from terrestrial and marine environments: a review. Phytochem Lett. 42, 87–103. https://doi.org/10.1016/j.phytol.2021.02.008 (2021).
Garcia, K. Y. et al. Antiproliferative and cytotoxic cytochalasins from Sparticola triseptata inhibit actin polymerization and aggregation. J. Fungi. 8 (6), 560. https://doi.org/10.3390/jof8060560 (2022).
Bata-Vidács, I. et al. Molecular and chemical evaluation of patulin production of Aspergillus and Penicillium-like species isolated from Hungarian apples. Food Addit. Contam. Part. A. 41 (8), 990–1002. https://doi.org/10.1080/19440049.2024.2364364 (2024).
Sabdaningsih, A. et al. A new citrinin derivative from the Indonesian marine sponge-associated fungus penicillium citrinum. Mar. Drugs. 18 (4), 227. https://doi.org/10.3390/md18040227 (2020).
Mahata, P. K., Dass, R. S., Gunti, L. & Thorat, P. A. First report on the metabolic characterization of sterigmatocystin production by select Aspergillus species from the nidulantes section in Foeniculum vulgare. Front. Microbiol. 13, 958424. https://doi.org/10.3389/fmicb.2022.958424 (2022).
Nguyen, V. T. et al. Gliotoxin isolated from marine fungus Aspergillus Sp. induces apoptosis of human cervical cancer and chondrosarcoma cells. Mar. Drugs. 12 (1), 69–87. https://doi.org/10.3390/md12010069 (2014).
Madsen, A. M. et al. Airborne bacterial and fungal species in workstations of salmon processing plants. Sci. Total Environ. 951, 175471. https://doi.org/10.1016/j.scitotenv.2024.175471 (2024).
Shukla, P. & Xiao, X. Marine natural products as anticancer agents. IOSR J. Pharm. Biol. Sci. 9 (2), 60–64 (2014).
Lini, I. F. et al. Identification and bioactive potential of endophytic fungi from marine weeds available in the coastal area of Bangladesh. Int. J. Pharm. Sci. Res. 11 (3), 1249–1257. https://doi.org/10.13040/IJPSR.0975-8232.11 (2020).
Zhang, X. et al. A Butyrolactone derivative from marine-derived fungal strain Aspergillus terreus BTBU20211037. Nat. Prod. Res. 38, 1–7. https://doi.org/10.1080/14786419.2024.2422515 (2024).
Uras, I. S., Karsli, B., Konuklugil, B., Ocsoy, I. & Demirbas, A. Organic–inorganic nanocomposites of Aspergillus terreus extract and its compounds with antimicrobial properties. Sustainability 15 (5), 4638. https://doi.org/10.3390/su15054638 (2023).
Shehabeldine, A. M. et al. Antimicrobial characteristics of endophytic Aspergillus terreus and acute oral toxicity analysis. Electron. J. Biotechnol. 72, 1–11. https://doi.org/10.1016/j.ejbt.2024.07.003 (2024).
Kamat, S., Kumari, M., Taritla, S. & Jayabaskaran, C. Endophytic fungi of marine Alga from Konkan Coast, India—a rich source of bioactive material. Front. Mar. Sci. 7, 31. https://doi.org/10.3389/fmars.2020.00031 (2020).
Goda, M. S. et al. Antimicrobial potential of different isolates of Chaetomium globosum combined with liquid chromatography tandem mass spectrometry chemical profiling. Biomolecules 13 (12), 1683. https://doi.org/10.3390/biom13121683 (2023).
Rob, T., Rahman, M. R., Mosihuzzaman, M., Rahman, K. M. & Sultana, N. A new compound from penicillium Thiomii, an endophytic fungus, isolated from the root of Terminalia chebula Retz (Haritaki). J. Bangladesh Chem. Soc. 18, 64–69 (2005).
Ahamed, F. & Murugan, M. Isolation and characterisation of marine endophytic fungi from seaweeds, and bioactivity of their crude extracts. J. Pure Appl. Microbiol. 13 (3), 1451–1460. https://doi.org/10.22207/JPAM.13.3.15 (2019).
Al Mousa, A. A. et al. Anti-staphylococcal, anti-candida, and free-radical scavenging potential of soil fungal metabolites: a study supported by phenolic characterization and molecular Docking analysis. Curr. Issues Mol. Biol. 46 (1), 221–243. https://doi.org/10.3390/cimb46010016 (2024).
Segaran, G. & Anitha Uma, Sathiavelu, M. Evaluation of antioxidant, total phenol and phytoconstituents of Culvularia Sp., a fungal endophyte of Boerhaavia diffusa L. Res. J. Biotechnol. 15 (6), 117–122 (2020).
Vega-Portalatino, E. J. et al. Antimicrobial and production of hydrolytic enzymes potentials of bacteria and fungi associated with macroalgae and their applications: a review. Front. Mar. Sci. 10, 1174569. https://doi.org/10.3389/fmars.2023.1174569 (2023).
Sandrawati, N. et al. Antimicrobial and cytotoxic activities of marine Sponge-derived fungal extracts isolated from Dactylospongia Sp. J. Appl. Pharm. Sci. 10 (4), 28–33 (2020).
Kumari, R., Mishra, R. C., Sheoran, R. & Yadav, J. P. Fractionation of antimicrobial compounds from Acacia nilotica twig extract against oral pathogens. Biointerface Res. Appl. Chem. 10 (6), 7097–7105. https://doi.org/10.33263/BRIAC106.70977105 (2020).
Kumar, M. H. et al. Gas chromatography/mass spectrometry analysis of one ayurvedic skin oil, Eladi Kera Thailam. Drug Invent. Today. 11 (10), 2657–2660 (2019).
Sujatha, S., Sara, S. C., Gayathiri, M., Roselin, I. R. & Ruby, R. G. D. Analysis of bioactive compounds present in methanolic extract of Phymatosorus scolopendria (Burm. F.) Pic. Serm. through gas chromatography and mass spectroscopy. Int. J. Pharm. Sci. Res. 11 (7), 3294–3299 https://doi.org/10.13040/IJPSR.0975-8232.11(7) (2020).
Fan, J., Du, X., Zhao, H. & Yao, W. Allelochemicals-mediated interaction between algae and bacteria: direct and indirect contact. Bioresour Technol. 398, 130525. https://doi.org/10.1016/j.biortech.2024.130525 (2024).
Galanty, A., Grudzińska, M., Paździora, W. & Paśko, P. Erucic acid-both sides of the story: a concise review on its beneficial and toxic properties. Molecules 28 (4), 1924. https://doi.org/10.3390/molecules28041924 (2023).
Wulandari, A. P. et al. Metabolite profiling of potential bioactive fractions from ethanol extract of Boehmeria nivea flowers by GC–MS/MS analysis. Phytomed Plus. 4 (2), 100557. https://doi.org/10.1016/j.phyplu.2024.100557 (2024).
Sekaran, G., James, J. V. & Li, X-P. Study of hepatoprotective activity from Acanthus ilicifolius leaves-the combined use of in vitro, in vivo and in silico analysis. Int. J. Phar. Biol. Sci. 10 (4), 118 –128 https://doi.org/10.21276/ijpbs.2020.10.4.20 (2020).
Abubakar, M. N. & Majinda, R. R. GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC). Medicines 3 (1), 3. https://doi.org/10.3390/medicines3010003 (2016).
Araki, H., Hagihara, H., Takigawa, H., Tsujino, Y. & Ozaki, K. Novel genes encoding hexadecanoic acid δ6-desaturase activity in a Rhodococcus Sp. Curr. Microbiol. 73, 646–651. https://doi.org/10.1007/s00284016-1106-9 (2016).
Cartron, M. L. et al. Bactericidal activity of the human skin fatty acid cis-6-hexadecanoic acid on Staphylococcus aureus. Antimicrob. Agents Chemother. 58, 3599–3609. https://doi.org/10.1128/AAC.01043-13 (2014).
Hameed, I. H., Hussein, H. J., Kareem, M. A. & Hamad, N. S. Identification of five newly described bioactive chemical compounds in methanolic extract of Mentha viridis by using gas chromatography - mass spectrometry (GC-MS). J. Pharmacogn Phytother. 7 (7), 107–125. https://doi.org/10.5897/JPP2015.0349 (2015).
Ravi, L. & Krishnan, K. Cytotoxic potential of N-hexadecanoic acid extracted from Kigelia pinnata leaves. Asian J. Cell. Biol. 12, 20–27. https://doi.org/10.3923/ajcb.2017.20.27 (2017).
Abdel-Wahab, M. A., Elgorban, A. M. & Bahkali, A. H. Single cell oil of oleaginous marine microbes from Saudi Arabian mangroves as a potential feedstock for biodiesel production. J. King Saud Univ. Sci. 35 (4), 102615. https://doi.org/10.1016/j.jksus.2023.102615 (2023).
Lee, J-H., Kim, Y-G., Khadke, S. K. & Lee, J. Antibiofilm and antifungal activities of medium-chain fatty acids against Candida albicans via mimicking of the quorum-sensing molecule Farnesol. Microb. Biotechnol. 14 (4), 1353–1366. https://doi.org/10.1111/1751-7915.13710 (2021).
Chong, Z. Y., Sandanamsamy, S., Ismail, N. N., Mohamad, S. & Hanafiah, K. M. Bio-guided fractionation of oil palm (Elaeis guineensis) fruit and interactions of compounds with first-line antituberculosis drugs against Mycobacterium tuberculosis H37Ra. Separations 8, 19. https://doi.org/10.3390/separations8020019 (2021).
Chen, E. et al. Analysis of aroma components from sugarcane to non-centrifugal cane sugar using GC-O-MS. RSC Adv. 10, 32276–32289. https://doi.org/10.1039/d0ra05963c (2020).
Adorjan, I., Jaaskelainen, A-S. & Vuorinen, T. Synthesis and characterisation of the hexenuronic acid model Methyl 4-deoxy-b-LL-threo-hex-4-enopyranosiduronic acid. Carbohydr. Res. 341, 2439–2443. https://doi.org/10.1016/j.carres.2006.06.012 (2006).
Valls, C. & Roncero, M. B. Antioxidant property of TCF pulp with a high hexenuronic acid (HexA) content. Holzforschung 67 (3), 257–263. https://doi.org/10.1515/hf-2012-0114 (2013).
Wu, J. et al. Hypoglycemic effect and mechanism of a pectic polysaccharide with hexenuronic acid from the fruits of Ficus pumila L. in C57BL/KsJ Db/db mice. Carbohydr. Polym. 178, 209–220. https://doi.org/10.1016/j.carbpol.2017.09.050 (2017).
Yakubu, O. E., Otitoju, O. & Onwuka, J. Gas chromatography-mass spectrometry (GC-MS) analysis of aqueous extract of Daniellia oliveri stem bark. Pharm. Anal. Acta. 8, 568. https://doi.org/10.4172/2153-2435.1000568 (2017).
Hashem, A. H. et al. Bioactive compounds and biomedical applications of endophytic fungi: a recent review. Microb. Cell. Fact. 22, 107. https://doi.org/10.1186/s12934-023-02118-x (2023).
Pandurangan, P. et al. Evaluation of antioxidants, antidiabetic, antiinflammatory active compounds from Leptogium rivurale through In-Vitro and In-Silico studies. Lett. Appl. NanoBioSci. 11 (4), 4192 –4200 https://doi.org/10.33263/LIANBS114.41924200 (2022).
Prasher, I. B. & Dhanda, R. K. GC-MS analysis of secondary metabolites of endophytic Nigrospora sphaerica isolated from Parthenium hysterophorus. Int. J. Pharm. Sci. Rev. Res. 44 (1), 217–223 (2017).
Alghamdi, A. I. & Ababutain, I. M. Phytochemical screening and antibacterial activity of Eucalyptus camaldulensisʼs leaves and bark extracts. Asian J. Sci. Res. 12 (2), 202–210. https://doi.org/10.3923/ajsr.2019.202.210 (2019).
Deka, D. & Jha, D. K. Endophytic fungi associated with Brucea mollis wall. Ex Kurz.: a hidden source of antimicrobial and antioxidant metabolites. Biotechnol. Genet. Eng. Rev. 40 (4), 4825–4848. https://doi.org/10.1080/02648725.2023.2216967 (2024).
Li, J-X., Schieberle, P. & Steinhaus, M. Characterisation of the major odor-active compounds in Thai Durian (Durio zibethinus L. ‘Monthong’) by aroma extract Dilution analysis and headspace gas chromatography – olfactometry. J. Agric. Food Chem. 60, 11253–11262. https://doi.org/10.1021/jf303881k (2012).
Li, X. et al. Cytotoxic pyrone derivatives from the deep-sea-derived fungus Cladosporium halotolerans FS702. Nat. Prod. Res. 38 (4), 594–600. https://doi.org/10.1080/14786419.2023.2187794 (2023).
Wang, C. N. et al. Cytotoxic benzopyranone and Xanthone derivatives from a coral symbiotic fungus Cladosporium halotolerans GXIMD 02502. Nat. Prod. Res. 35 (24), 5596–5603. https://doi.org/10.1080/14786419.2020.1799363 (2020).
El-Din, S. M. M. & Alagawany, N. I. Phytochemical constituents and anticoagulation property of marine algae Gelidium crinale, Sargassum hornschuchii and Ulva Linza. Thalassas 35, 381–397. https://doi.org/10.1007/s41208-019-00142-6 (2019).
Latief, M., Muhaimin, M., Amanda, H., Prahandika, G. & Tarigan, I. L. Anti-inflammatory activities of squalene compound of methanol extract of Abroma Augusta L. J. Technol. Lab. 9 (2), 176–185. https://doi.org/10.29238/teknolabjournal.v9i2.228 (2020).
Kumar, S. S. et al. Chemical composition, nutraceuticals characterisation, NMR confirmation of squalene and antioxidant activities of Basella rubra L. seed oil. RSC Adv. 10, 31863–31873. https://doi.org/10.1039/d0ra06048h (2020).
Fernando, I. P. S. et al. Squalene isolated from marine macroalgae Caulerpa racemose and its potent antioxidant and anti-inflammatory activities. J. Food Biochem. 42 (5), e12628. https://doi.org/10.1111/jfbc.12628 (2018).
Acknowledgements
The authors are thankful to the Pharmaceutical Sciences Research Division, BCSIR Dhaka Laboratories, Bangladesh Council of Scientific and Industrial Research (BCSIR), for providing the necessary instrumental facilities and to the Department of Pharmaceutical Chemistry, University of Dhaka, for allocating the required solvent supports to conduct the research work.
Funding
Sadia Noor is highly grateful to the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh for financial support through the Bangabandhu Science and Technology Fellowship. This study was part of her PhD research.
Author information
Authors and Affiliations
Contributions
All authors conceived the research idea. S.N. carried out the methodology and data analysis. S.R.R. and A.A.C. provided necessary resources and logistic support. M.H.S. and M.A.M. supervised the whole study. M.N.B. guided the isolation and identification of fungi. S.N. drafted the original manuscript. M.H.S. and M.A.M. reviewed and edited it. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All the experimental procedures were conducted following the standard protocols.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Noor, S., Begum, M.N., Rony, S.R. et al. Bioactivity and chemical screening of endophytic fungi associated with seaweeds Gracilaria sp. and Sargassum sp. of the Bay of Bengal, Bangladesh. Sci Rep 15, 16121 (2025). https://doi.org/10.1038/s41598-025-00099-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00099-y
Keywords
This article is cited by
-
Aquatic Mycobiomes as Emerging Bioresources of Novel Bioactive Compounds for Drug Discovery
National Academy Science Letters (2025)