Abstract
This study investigated the relationship between peroneal muscle echogenicity and balance function in individuals with chronic ankle instability (CAI). While prior research has examined peroneal muscle activity, reaction time, and balance, the impact of echogenicity—an indicator of myosteatosis/fibrosis—remained underexplored. Cross-sectional study. Sixty-two adults with CAI were included. Peroneal muscle size, echogenicity, and stiffness were assessed using ultrasound. Dynamic balance was evaluated via the Y balance test (YBT), and static postural control was evaluated during lateral step-down (LSDT) and single-leg stance test (SLST). Eversion strength was assessed with a dynamometer. The relationship between muscle characteristics and balance was assessed using canonical correlation and stepwise linear regression. Individuals with increased peroneal muscle echogenicity had reduced muscle size, poorer eversion strength, and poorer balance. Eversion strength is positively associated with YBT scores across all echogenicity levels and negatively associated with posture parameters during the LSDT in moderate echogenicity. Peroneal longus stiffness was positively associated with YBT in severe echogenicity and posture parameters during the SLST. Increased peroneal muscle echogenicity is associated with poorer eversion strength and stiffness, resulting in poorer balance performance. Improving the peroneal muscle quality may enhance functions in the CAI condition.
Similar content being viewed by others
Introduction
Lateral ankle sprains (LAS) are a common soft tissue injury that can lead to Chronic Ankle Instability (CAI)1,2. Up to 40% of individuals with an acute LAS progress to CAI due to injury severity, structural deformity, and inadequate rehabilitation3,4. The prevalence of CAI constitutes 25%, ranging between 7% and 53% in different populations5,6. CAI is characterised by recurrent ankle sprains, perception of the ankle giving way, persistent pain, muscle weakness, and impaired postural control, restricting exercise and impacting the quality of life7,8,9. Therefore, an adequate and early treatment is essential to avoid the condition’s progression.
The function of the peroneal muscles, which play a crucial role in maintaining ankle joint stability, is among the key factors in influencing CAI progression. Peroneal muscles contribute to eversion strength and are pivotal in maintaining ankle joint stability, especially in the sagittal and frontal planes after a LAS event. They are the main peri-ankle structure that compensates for the impaired functions of the various lateral ligaments10. However, the changes in the peroneal muscle quality (including the presence of myosteatosis and fibrosis) remain understudied and may influence eversion strength10,11. According to previous literature, post-LAS events may change the peroneal muscle architecture where increased adipose tissue correlates with an increased frequency of ankle sprains and exacerbate balance issues12,13. However, only two studies have investigated the presence of myosteatosis/ fibrosis in the muscles in a small sample size of individuals experiencing an acute LAS. In other types of musculoskeletal injuries, such as ACL injuries and rotator cuff tears, it has been observed that a decrease in muscle size, along with the formation of fibrotic tissue and fatty tissue deposition, limits force generation as less area of the muscle is occupied by contractile components14,15,16,17,18. The increase in fibrotic tissue and adipose tissue deposition in the skeletal muscle could alter the pennation angle and fascial length and decrease force production19. Consequently, this affects the physical functions18,20. In addition, an inverse relationship between the central activation ratio during an isometric contraction and intramuscular fat/fibrosis tissue accumulation in skeletal muscle was identified in other population groups that have high muscle echogenicity (e.g. elderly population)21. It has been highlighted that the increase in IMAT in the quadricep muscle is associated with delayed or incomplete motor unit recruitment, hence the muscles may not activate efficiently22. The decreased ability of muscle activation may impair an individual’s ability to recover from a balance perturbation21. Hence, altering the amount of adipose tissue/fibrosis in the muscle is associated with both improving neuromuscular activation and altering the proprioception through mechanical changes23. This could reduce posture control sway and balance function23.
In addition, an increased passive stiffness in CAI is observed to stabilise the ankle and foot, possibly due to an increase in peroneal longus activity after an ankle inversion, resulting in an eccentric strain injury24,25,26,27,28. Muscle stiffness reflects the ability of the structures around the joints to resist passive displacement of the joint, with either inadequate passive stiffness or excessive passive stiffness leading to poor clinical outcomes29,30. Therefore, investigating the changes in passive stiffness during post-injury can aid clinicians in monitoring the capsuloligamentous tissue alterations effectively. Currently, limited evidence exists regarding the relationship between muscle quality and stability among the CAI population; hence, evaluating whether poorer peroneal muscle quality affects balance functions may provide insights for future targeted treatment of the muscle31,32,33.
The current conservative treatment effect remains heterogeneous as the training may not specifically improve muscle quality as the effectiveness of the conservative treatment varies and does not address the full continuum of impairment and disability34,35,36,37,38,39,40. For instance, the improvement of muscle quality (e.g. muscle echogenicity) can be hindered by the significant loading from the strengthening rehabilitation programme41. The resistance band training performed with a band tied in a neutral position may not mechanically strengthen the peroneal longus muscle as the muscle is associated with eversion in ankle plantarflexion42. This is further supported by a systematic review conducted by Luan et al.36 which found that the rigidity and unvaried strengthening rehabilitation is not effective in mitigating symptoms associated with the CAI condition. Concurrently, systematic reviews have highlighted that balance training may not be effective in restoring static balance38,39. Therefore, evaluating the quality status of the peroneal muscles may be essential to prevent the development of CAI.
This cross-sectional study aimed to investigate if increasing the peroneal muscle echogenicity has smaller peroneal muscle size, higher passive stiffness, poorer strength, and balance function. In addition, to explore the relationship between muscle strength and stiffness with various balance assessments according to the echogenicity gradings. We hypothesised that higher peroneal muscle echogenicity is associated with weaker muscle strength, smaller peroneal muscle cross-sectional area, higher passive stiffness and poorer balance functions.
Methods
Study design
This cross-sectional study was conducted in compliance with the Declaration of Helsinki and was approved by The Joint Chinese University of Hong Kong- New Territories East Cluster Clinical Reseach Ethics Committee (Ethics approval number: 2022.263). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) initiative was adopted. Patients with CAI were recruited from a local hospital between June 2023 and January 2024.
Inclusion and exclusion criteria
Clinical diagnoses were made by the Foot and Ankle surgeons per the International Ankle Consortium24. The recruited patients were aged between 18 and 50 years old with a history of at least one significant inversion ankle sprain, in which the initial sprain must have occurred at least 12 months before study enrolment. The sprain had to be associated with inflammatory symptoms and created at least one interrupted day of desired physical activity, with the recent sprain occurring more than 3 months before study enrolment. Participants should report at least two episodes of giving way in the 6 months before study enrolment. Furthermore, patients were screened with the self-reported ankle instability, Cumberland Ankle Instability Tool (CAIT) < 20.5 (Cantonese)43, which had been validated in the Hong Kong population with a high sensitivity of 0.900 and high specificity of 0.860. Lastly, the surgeon had to evaluate the patients with a positive anterior drawer test. Patients were excluded if they had reported a history of fractures, dislocations, or surgeries of the lower extremities, any lower extremity neuromusculoskeletal abnormalities, or neurological disorders. X-rays and ultrasounds were arranged by the surgeon for the patients to ascertain the absence of fractures or any abnormalities in their lower extremities. Written informed consent was obtained from all participants. G * Power3.1.9.2 statistical software (NeuIsenburg, Aichach, Germany)44 calculated the sample as 62, based on the multiple linear regression model using the calculated effect sizes from our study between 0.21 and 0.54, β of 0.2 and α of 0.05.
Measures
CAIT was used to measure perceived instability, consisting of nine questions including a question on ankle pain, and questions on feeling ankle instability. The score ranged from 0 to 30 with a greater score indicating higher stability43,45.
The YBT (FMS Y balance) evaluated the dynamic balance46,47,48,49. Participants began with their non-injured limbs first before progressing to their injured limbs50. The relative length (cm) of the lower limb was measured (anterior superior iliac crest-medial malleolus). The formula for the normalized maximal reach distance is the longest distance in each direction divided by the participant’s leg length multiplied by 10051.
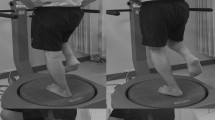
Tekscan Matscan ® pressure mat model 3150 was used to evaluate postural control52,53. The lateral step-down test (LSDT) stimulates stair-like movement where the participants step down from the 30 cm high block and balance unilaterally for 20 s on the pressure mat54. However, the data obtained from the Tekscan pressure mat was initiated after the beginning of the study, resulting in an incomplete baseline data set for this variable.
For the single-leg stance test (SLST), participants were instructed to balance on the pressure mat for 20 s with their eyes opened and closed55,56. The sway values were analysed by the Sway Analysis Module (SAM™).
An isometric digital handheld dynamometer (MicroFET2, Manual Muscle Testing) was used to measure eversion strength57. A handheld dynamometer was placed on the lateral 5th metatarsal head and the patient exerted the maximum eversion power for 5 s isometrically. The maximum strength was taken as the participant’s peak strength and was normalised by the body weight (kg). The inter-rater operator measurement had an intraclass correlation coefficient (ICC) of 0.942, and a 95% confidence interval (CI): of 0.806–0.983. The intra-operator measurement between sessions was ICC: 0.917, 95% CI: 0.621–0.981).
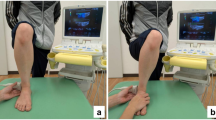
An ultrasound system (Supersonic Imagine’s Aixplorer, Aix-en-Provence, France) with a linear transducer array (5-18 MHz; SuperLinear 18 − 5) was used to measure the cross-sectional area (CSA) of the peroneal muscles, stiffness and echogenicity, Appendix 1. According to the SENIAM recommendations, the probe was placed at the upper ¼ position between the tip of the head of the fibula and the tip of the lateral malleolus of the patient58,59. Markings were made on the measurement points for reproducibility. After applying sufficient gel to the probe, the probe was placed perpendicular to the markings with minimal force60. To minimise the effect of the ultrasound anisotropy, the probe was held in a maximally vertical position to capture the peroneal muscles in a short-axis view60. At the same position, a region of interest with an analysis area of a 5 mm diameter circle was set near the centre at the peroneal longus to measure the passive stiffness, Appendix 261. The transducer was placed for < 10 s to confirm the shear elastic modulus at a stable colour distribution. Since the skeletal muscle is not assumed to be isotropic, the shear modulus is reported as a young modulus divided by 362. For the ultrasound measurements, the inter-operator measurement was conducted for the CSA 25% (ICC: 0.929, 95% CI: 0.669–0.986) and young modulus (ICC: 0.935, 95% CI: 0.677–0.987). The intra-operator between sessions for CSA 25% was reported (ICC: 0.860, 95% CI: 0.383–0.979), and young modulus (ICC: 0.912, 95% CI: 0.609–0.980). Visual assessment using the Modified Heckmatt Scale (MHS)63,64 was used to categorise muscle echogenicity by the trained researcher and a physiotherapist independently to minimize any subjectivity. The four different types of grading65 can be seen in Table 1. The inter-rater was 0.93 (95% CI: 0.89–0.95) and the intra-class correlation was 0.92 (95% CI: 0.89–0.95).
Statistical analysis
The characteristics of the study population were presented as the median(inter-quartile range) or frequency (n)%. The distribution of the quantitative data was assessed by the Kolmogorov-Smirnov test (p > 0.05). Peroneal muscle quality and balance functions were compared by the echogenicity grading using one-way ANOVA, and presented as median (IQR). Posthoc analysis with Bonferroni correction was conducted for the different echogenicity grading. The distribution of the quantitative data was assessed by Kolmogorov-Smirnov test (p > 0.05). Canonical correlation analysis was employed to examine the relationship between peroneal muscle quality and balance functions. Canonical loading was generated to link between the original and canonical variables. Variables with a loading > 0.3 were deemed significant for the variables of interest66. Hence, the total composite score of YBT, area of the centre of pressure (COP) during SLST (eyes opened), and anteroposterior/mediolateral excursion sway during LSDT were selected as a representative for each outcome to be included in the stepwise linear regression, stratified according to the grades of echogenicity. This was to identify the influence of muscle echogenicity on strength and stiffness affecting balance functions with the inclusion of confounding factors such as age, gender, and body mass index (BMI). Multiple imputation by chained equations with five different iterations was conducted to address the missing data for the postural control parameters during LSDT among individuals who were classified in the MHS group 4. No sensitive analysis, but sub-group analysis was conducted instead. Variance inflation factors exceeding 10 were checked to address multicollinearity. Please refer to Appendix Table 2 for the relevant VIF values reported. All statistical tests were conducted using a two-sided 5% significance level using SPSS 29.0 (IBM).
Results
A total number of n = 482 were screened for eligibility between June 2023 to January 2024 in a local hospital by trained research assistants. However, due to the exclusion criteria such as refusal to participate after explaining the procedures (n = 83), individuals who had previous lower limb surgeries (n = 56), acute lower limb injuries (n = 97), being < 18 years old or > 50 years old (n = 120), and having CAIT scores > 20.5 (n = 64) were excluded. Finally, a total of n = 62 participants (40 females, and 22 males) who answered questionnaires on self-demographic factors, medical history, and CAIT scores, and performed clinical outcome measures together with ultrasound imaging were included for analysis (Fig. 1).
The median age of the population is 29 (23, 37) years old, with a median BMI of 22.2 (20.5, 25.4) kg/m2. The median duration of the last ankle sprain experienced was 36.0 (16.5, 60.0) months, with mild pain at the ankle during exercise experienced with a median visual analog scale of 25.0 (10.45, 75). The median score for CAIT for the affected limb among patients with unilateral ankle sprain was 13.5 (11.0, 18.0) meanwhile the self-reported CAIT score for the affected limb among patients with bilateral ankle sprain was 18.0 (13.5, 20.5). More than 50% of patients with CAI experienced ankle instability despite prior rehabilitation treatment (Table 2).
Eversion strength demonstrated a significant difference across various echogenicity grading (p < 0.001) with grade 2 of the echogenicity exhibiting the highest strength (19.89 kg, SD = 6.01), while grade 4 had the lowest (12.48 kg, SD = 3.73) (Fig. 2). A significant difference was observed in the CSA of the peroneal across the echogenicity grading (p < 0.001). Grade 2 had the biggest CSA (8.47 cm², SD = 1.74), while grade 4 had the smallest CSA of the peroneal muscle (6.80 cm², SD = 0.97). No significant difference was identified in peroneal longus stiffness across the echogenicity grading (p = 0.244).
For the LSDT (Fig. 3), a significant difference was observed in the anterior-posterior excursion sway between grades 2 and 4, with a mean difference of -3.52 (95% CI: -4.404, -2.640), p < 0.001). Moreover, a significant difference was observed between grades 3 and 4, with a mean difference of -3.82 (95% CI: -4.77, -2.88), p < 0.001). In addition, a significant difference was found in the mediolateral excursion sway between grades 2 and 4, with a mean difference of -0.40 (95% CI: -0.725, -0.066), p = 0.012). Additionally, a significant difference was observed between grades 3 and 4, with a mean difference of -0.41 (95% CI: -0.706, -0.115), p = 0.003). No significant differences were observed in the area of COP excursion sway during the LSDT. Across the Y balance test measures, a significant difference was found in the posteromedial (%) (p = 0.004), posterolateral (p = 0.013), and composite scores (p = 0.014) between grade 2 and grade 4. No significant differences were found in anterior (%) scores (p = 0.570) and between the sway parameters during SLST (Fig. 4).
The canonical correlation yielded five pairs of canonical variables (Fig. 5). Only the first set had a successful correlation of 0.766 (p < 0.001), explaining 25% of the variance of the peroneal muscle quality and 20.1% of the observed static and dynamic balance variances. According to the canonical loading, we have selected eversion strength (rs = 0.789), peroneal longus stiffness (rs = 0.424), and MHS (rs= -0.365) which contributed to x (independent factors) in peroneal muscle characteristics. Concurrently, we have selected the total composite score of the YBT (rs = 0.660) to represent the dynamic balance, the area of COP (rs = 0.453) to represent SLST, and anteroposterior excursion sway (rs=-0.397), and mediolateral excursion sway (rs= -0.391) to represent LSDT that have contributed to y in balance functions.
Model depicting the first canonical correlation between peroneal muscle quality and balance functions showing the canonical correlation (r) and the canonical loading for each variable are presented adjacent to their arrow. Xs independent variable, Ys dependent variable. The significant contributors to the relationship are highlighted in green. EO eye open, EC eye close, AP anterioposterior, ML mediolateral, LSDT lateral step down test.
According to the stepwise multiple linear regression analysis, the result showed that eversion strength (p < 0.001) was associated with the YBT performance regardless of the muscle echogenicity (Table 3). Peroneal longus stiffness (p < 0.001) was associated with the YBT performance in the highest grade of muscle echogenicity. Meanwhile, eversion strength was inversely associated with anteroposterior (p < 0.001) and mediolateral excursion (p = 0.002) sway in moderate echogenicity during the LSDT. Moreover, peroneal longus stiffness was associated with the area of COP during SLST (eyes opened) across different echogenicity severities (p < 0.05). Age, gender, and BMI were significantly associated with the different balance functions.
Discussion
In our study, patients with CAI exhibiting higher muscle echogenicity showed a reduced peroneal muscle size, weaker eversion strength, diminished dynamic stability, and increased postural sway during LSDT compared to those with lower echogenicity. We found that eversion strength significantly predicted YBT performance throughout all echogenicity levels and the sway parameters during the LSDT only in moderate echogenicity. Additionally, higher passive stiffness was positively associated with an area of COP sway in SLST in all grades of echogenicity.
Currently, there are no direct studies on evaluating peroneal muscle echogenicity and balance functions in individuals with CAI. However, Arima et al.13 have found an association between an increase in peroneal muscle echogenicity and an increase in sprain frequency in this specific population. According to Mcvey et al.67 and Palmieri-Smith et al.68, the attributing factors to an increase in adipose tissue within the muscle constitute both reflex inhibition and decreased muscle activity after ankle sprains. Furthermore, the increased muscle adipose tissue could also contribute to poor eversion strength.
Our findings corroborated with a prior study that demonstrated a higher muscle echogenicity and reduced peroneal muscle strength on the affected side among patients with a history of ankle sprains11. Apart from diminishing neural activation, the intramuscular adipose tissue or fibrosis could also elicit a systemic effect within the muscles where the decreased inhibitory signal cells from the muscle cells could differentiate the mesenchymal stem cells into fat cells within the muscles11,69. The presence of fibro-adipogenic progenitors during muscle injury was suggested as one of the main mechanisms that play a role in the regeneration environment from compensatory to pathologic70. The pathologic condition contributes to fatty deposition and fibrotic formation, which affects the muscle quality negatively and physical functions. Furthermore, the increase in intramuscular adipose tissue may affect the electrical signals required for muscle contraction and the decreased ability to fully activate the muscle may impair the patient’s ability to recover from a balance disturbance22.
According to Zhu et al.71., intramuscular adipose tissue induces various physiological processes within the skeletal muscle. Intramuscular adipose tissue could affect mitochondrial function, induce muscle inflammation, facilitate fatty infiltration-induced insulin resistance, cause damage to the skeletal muscle fibers and modulate intercellular signalling pathways within the skeletal muscle that regulate myofiber growth and apoptosis71. Consequently, this may alter the muscle structure by impairing protein synthesis and decreasing contractile elements in the muscles, resulting in muscle atrophy and decreased neuromuscular activation71. In particular, the denervation of motor neurons and muscle fibers72,73 coupled with a large reduction in type II muscle fiber, may result in a lower capacity to produce force and impact on neuromuscular activation22. Thus, this disruption may lead to a delay in peroneal reaction time, which can influence balance functions and predispose to future ankle sprains. Furthermore, the increase in intramuscular adipose tissue could alter the proprioception through mechanical changes to the muscles and balance functions, such as increasing mechanical impedance and making it harder for the proprioceptors to detect the muscle length and tension74,75. Hence, this could impair the sensory feedback pathway and further impair the balance functions76.
In addition, our results were also aligned with the findings from other population groups, intramuscular adiposity is associated with accelerated functional impairments77,78,79,80. For instance, an increase in muscle echogenicity was associated with poor postural control among the elderly, where an accumulation of intramuscular adipose tissue may affect the muscle activation speed and efficiency for maintaining balance81. Furthermore, the fat infiltration could trigger muscular inflammation through cell differentiation82,83,84,85,86,87. Thus, this inhibits the ability for muscle repair and regeneration, thereby impairing muscle mechanics and contributing to function deficits.
Our results showed a positive association between eversion strength and the overall YBT performance regardless of echogenicity severity, indicating that a strong eversion strength is required to maintain ankle stability, allowing for more confident and extended reach in all directions of YBT88. Our results were aligned with previous studies that have identified the importance of eversion strength lies in its significant effect in stabilising both medial and lateral components of the YBT reach directions89,90,91. These directions may create postural control perturbation. A plausible mechanism could be due to enhanced proprioception required during YBT, where the peroneal muscle received sensory feedback required for increased precision of muscle control around the ankle9. Studies comparing the effects of rehabilitation training demonstrated that an increase in eversion strength has better YBT performance, suggesting that changes in muscle echogenicity through training are associated with the change in eversion strength92,93,94,95. Interestingly, our results did not align with Ko et al.96. This could be possibly due to the differences in the characteristics of the patients who require surgical procedures where severe mechanical deficits may have a greater effect on dynamic balance compared to eversion strength97. Hence, this may suggest that if prolonged duration of symptoms and muscle disuse were left unattended, this may result in further complications that require non-conservative treatment.
Our findings have demonstrated a negative relationship between eversion strength and anteroposterior and mediolateral sway demonstrated during LSDT, especially among CAI patients who have moderate echogenicity in peroneal muscles. It was suggested that poor eversion strength may impede proper foot positioning during lateral movement and the weight-bearing phase of this test98. Hence, this results in postural perturbance and potentially causes an increased medial knee displacement or loss of balance99. A plausible suggestion that the association was observed only in the moderate echogenicity of the peroneal muscle denotes a critical threshold where eversion strength may be compromised and impaired postural control is observed. Conversely, the absence of associations in other echogenicity levels indicates a complex interplay of factors influencing eversion strength and postural control, potentially involving proprioceptive and neuromuscular adaptations. For instance, patients with heightened echo intensity may experience severe muscle inhibition and joint instability, which result in adapting to a compensatory mechanism, leading to a compensatory mechanism that may overshadow the role of eversion strength100. In contrast, the lack of association between eversion strength and postural sway during LSDT among patients with mild muscle echogenicity suggests that muscle composition is not severely altered and neuromuscular functions are well-preserved65. Currently, our study is the first that has evaluated the relationship between peroneal muscle quality and postural sway displacement during LSDT. Hence, we were not able to compare our findings with other previous works. Previous studies mainly evaluated the quality evaluation of the LSDT in both healthy subjects and CAI individuals101,102,103,104. Only one study has investigated the postural control parameters during LSDT. However, the relationship between peroneal muscle quality and postural control parameters during LSDT was not evaluated54. Furthermore, the authors did not identify any significant results on the displacement of postural sway parameters between CAI and the healthy control group54. Therefore, future studies should explore the COP displacement related to movement quality and clinical outcomes in the context of LSDT. This approach may elucidate the underlying mechanisms for poor postural control during unilateral weight-bearing activities.
In addition, our findings demonstrated that an increase in peroneal muscle stiffness is associated with (1) a longer reach distance in the overall total composite score of the YBT in the highest grade of echogenicity, (2) an increased area of COP excursion sway during SLST irrespective of muscle echogenicity among CAI patients. Our findings are consistent with previous studies that assessed passive stiffness in individuals with CAI and ankle sprains using myotonometry27,105.The observed increase in passive stiffness of the peroneal muscles is a compensatory mechanism for the reduced stiffness of the ankle joint following lateral ankle sprains (LAS)106,107,108. This increased stiffness is primarily attributed to muscle dysfunction resulting from elevated fat infiltration or fibrosis, which replaces contractile tissue and disrupts fiber alignment109.
The force contribution of the peroneal muscles may reach a saturation point at low to moderate levels of motor unit recruitment, potentially hindered by fat infiltration27,110,111,112,113,114. Therefore, enhanced passive stiffness has been identified as an effective medium for transmitting movement-generated mechanical information and facilitating tension transfer among interconnected tissues115. However, individuals with heightened muscle stiffness may need to compensate through muscle overactivation to maintain stability in a unipodal stance, which can impair coordinated motor actions and increase the risk of coordination deficits and injury116.
According to Theret et al.117, muscle fibrosis resulting from sprains may hinder muscle regeneration by promoting the differentiation of fibro-adipogenic progenitors into fibroblasts and adipocytes instead of myogenic cells. This process can lead to increased muscle stiffness and reduced flexibility. Elevated stiffness may also impair neuromuscular control by restricting the range of motion and the dynamics of muscle contractions117. Furthermore, evidence from healthy populations suggests that increased passive stiffness may result in fascial rigidity, which can hyperstimulate muscle spindles and decrease muscle compliance, thus impeding range of motion and overall mobility118 This limits the body’s ability to adapt to sudden changes and perturbations, and the inability to balance during dynamic activities. Furthermore, increasing peroneal muscle stiffness may reduce the body’s ability to absorb and dissipate energy during sudden movement, resulting in a less adaptative postural control strategy119. Therefore, our results may shed some light on how intramuscular adipose tissue is associated with the changes in the mechanics of the skeletal muscle and impacts the balance functions. However, given that the stiffness measurements are restricted to the probe region and stiffness is not uniform across the entire muscle, the implication may be limited120. Future studies should investigate various clinical methods to evaluate passive stiffness.
Our study had several limitations. Firstly, due to the cross-sectional design, the sequence changes for the balance function deficit or the presence of poor peroneal muscle quality remain unclear, without being able to establish causality. Longitudinal studies should be conducted to establish causality. Secondly, the convenient sampling method from the hospital could lack the representativeness of the population and may not be generalised to other population groups such as among the elderly and athletes, especially when both population groups have a higher exposure to repeated ankle sprains. As such, studies should be stratified in future recruitment to reduce sampling bias. Thirdly, the study also recruited a greater proportion of female participants compared to males. However, since CAI was suggested to be significantly higher in females than males121, our results were likely to represent the general population and provide a better understanding. Future studies should investigate the potential moderating effect of gender. Lastly, the modified Heckmatt scale to evaluate muscle echogenicity is a qualitative assessment. In our study, the usage of the modified Heckmatt scale was conducted independently by a registered physiotherapist and the researcher to reduce subjectivity. Future studies should complement an objective measurement such as magnetic resonance imaging or muscle biopsy which is more invasive to measure muscle echogenicity.
Conclusion
In conclusion, participants with increased peroneal muscle echogenicity exhibited poorer outcomes, including peroneal muscle atrophy, diminished eversion strength, and compromised balance functions in patients with CAI. Future rehabilitation programmes should concentrate on enhancing peroneal muscle architecture as a potential therapeutic strategy. In particular, biophysical interventions such as neuromuscular electrical stimulation, pulse-electromagnetic field therapy, or whole-body vibration therapy etc. aimed at improving peroneal muscle activity to restore the architecture and quality of the peroneal muscle and may potentially aid in the enhancement of balance functions.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- CAI:
-
Chronic ankle instability
- CAIT:
-
Cumberland ankle instability tool
- COP:
-
Centre of pressure
- CSA:
-
Cross-sectional area
- SLST:
-
Single leg stance test
- LAS:
-
Lateral ankle sprain
- LSDT:
-
Lateral step down test
- YBT:
-
Y balance test
References
Raeder, C., Tennler, J., Praetorius, A., Ohmann, T. & Schoepp, C. Delayed functional therapy after acute lateral ankle sprain increases subjective ankle instability - the later, the worse: a retrospective analysis. BMC Sports Sci. Med. Rehabil. 13, 86. https://doi.org/10.1186/s13102-021-00308-x (2021).
Fong, D. T., Hong, Y. & Chan, L. A systematic review on ankle injury and ankle sprain in sports. Sport Med. 37. https://doi.org/10.2165/00007256-200737010-00006 (2007).
Doherty, C. et al. Recovery from a First-Time lateral ankle sprain and the predictors of chronic ankle instability: A prospective cohort analysis. Am. J. Sports Med. 44, 995–1003. https://doi.org/10.1177/0363546516628870 (2016).
Bonnel, F., Toullec, E., Mabit, C. & Tourné, Y. Chronic ankle instability: biomechanics and pathomechanics of ligaments injury and associated lesions. Orthop. Traumatol. Surg. Res. 96, 424–432. https://doi.org/10.1016/j.otsr.2010.04.003 (2010).
Lin, C. I., Houtenbos, S., Lu, Y. H., Mayer, F. & Wippert, P. M. The epidemiology of chronic ankle instability with perceived ankle instability- a systematic review. J. Foot Ankle Res. 14. https://doi.org/10.1186/s13047-021-00480-w (2021).
Mckay, G. D., Payne, G. P. & Oakes, W. R. Ankle injuries in basketball- injury rate and risk factors. Br. J. Sports Med. 35, 103–108 (2001).
Han, S., Lee, H., Son, S. J. & Hopkins, J. T. Effect of varied dorsiflexion range of motion on landing biomechanics in chronic ankle instability. Scand. J. Med. Sci. Sports. 33, 1125–1134. https://doi.org/10.1111/sms.14339 (2023).
Basnett, C. R. et al. Ankle dorsiflexion range of motion influences dynamic balance in individuals with chronic ankle instability. Int. J. Sports Therapy. 8, 121–128 (2013).
Hertel, J. & Corbett, R. O. An updated model of chronic ankle instability. J. Athl Train. 54, 572–588. https://doi.org/10.4085/1062-6050-344-18 (2019).
Rodrigues, K. A., Soares, R. J. & Tomazini, J. E. The influence of fatigue in Evertor muscles during lateral ankle sprain. Foot (Edinb) 40, 98–104. https://doi.org/10.1016/j.foot.2019.05.008 (2019).
Sakai, S., Urabe, Y., Sasadai, J., Morikawa, M. & Maeda, N. Quantity and quality of the peroneal nerve muscles in leg with chronic ankle instability assessed by ultrasonography. Gait Posture 65, 413–414. https://doi.org/10.1016/j.gaitpost.2018.07.031 (2018).
Pirri, C. et al. Ultrasound imaging of crural fascia and epimysial fascia thicknesses in basketball players with previous ankle sprains versus healthy subjects. Diagnostics 11, 177 (2021).
Sakai, S. et al. Quantity and quality of the peroneus longus assessed using ultrasonography in leg with chronic ankle instability. J. Phys. Therapy Sci. 30, 1396–1400 (2018).
Sideris Vasileios, N. & Andreas, B. Nuno Enhancing neuromuscular function post ACL reconstruction. ASPETAR Sports Med. J., 318–323 (2022).
Lepley, L. K., Davi, S. M., Burland, J. P. & Lepley, A. S. Muscle atrophy after ACL injury: implications for clinical practice. Sports Health. 12, 579–586. https://doi.org/10.1177/1941738120944256 (2020).
Eken, G. et al. Effect of muscle atrophy and fatty infiltration on mid-term clinical, and functional outcomes after Achilles tendon repair. Foot Ankle Surg. 27, 730–735. https://doi.org/10.1016/j.fas.2020.09.007 (2021).
Liem, D., Lichtenberg, S., Magosch, P. & Habermeyer, P. Magnetic resonance imaging of arthroscopic supraspinatus tendon repair. J. Bone Joint Surg. Am. 89, 1770–1776. https://doi.org/10.2106/jbjs.F.00749 (2007).
Valencia, A. P. et al. Fatty infiltration is a prognostic marker of muscle function after rotator cuff tear. Am. J. Sports Med. 46, 2161–2169. https://doi.org/10.1177/0363546518769267 (2018).
Rahemi, H., Nigam, N. & Wakeling, J. M. The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. J. R. Soc. Interface 12, 20150365. https://doi.org/10.1098/rsif.2015.0365 (2015).
Gladstone, J. N., Bishop, J. Y., Lo, I. K. Y. & Flatow, E. L. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am. J. Sports Med. 35, 719–728. https://doi.org/10.1177/0363546506297539 (2007).
Yoshida, Y., Marcus, R. & Lastayo, P. Intramuscular adipose tissue and central activation in older adults. Muscle Nerve. 46, 813–816. https://doi.org/10.1002/mus.23506 (2012).
Lanza, M. B., Ryan, A. S., Gray, V., Perez, W. J. & Addison, O. Intramuscular fat influences neuromuscular activation of the gluteus medius in older adults. Front. Physiol. 11, 614415. https://doi.org/10.3389/fphys.2020.614415 (2020).
Kiyoshige, Y. & Watanabe, E. Fatty degeneration of gluteus minimus muscle as a predictor of falls. Arch. Gerontol. Geriatr. 60, 59–61. https://doi.org/10.1016/j.archger.2014.07.013 (2015).
Gribble, P. A. et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the international ankle consortium. J. Athl Train. 49, 121–127. https://doi.org/10.4085/1062-6050-49.1.14 (2014).
Hopkins, J. T., Hunter, I. & McLoda, T. Effects of ankle joint cooling on peroneal short latency response. J. Sports Sci. Med. 5, 333–339 (2006).
Kawai, T., Takamoto, K. & Bito, I. Previous hamstring muscle strain injury alters passive tissue stiffness and vibration sense. J. Bodyw. Mov. Ther. 27, 573–578. https://doi.org/10.1016/j.jbmt.2021.05.002 (2021).
Stefaniak, W., Marusiak, J. & Baczkowicz, D. Heightened tone and stiffness with concurrent Lowered elasticity of peroneus longus and tibialis anterior muscles in athletes with chronic ankle instability as measured by myotonometry. J. Biomech. 144, 111339. https://doi.org/10.1016/j.jbiomech.2022.111339 (2022).
Ekstrand, J. & Gillquist, J. The avoidability of soccer injuries. Int. J. Sports Med. 4, 124–128. https://doi.org/10.1055/s-2008-1026025 (1983).
Gajdosik, R. L. & Williams, A. K. Relation of maximal ankle dorsiflexion angle and passive resistive torque to passive-elastic stiffness of ankle dorsiflexion stretch. Percept. Mot Skills. 95, 323–325. https://doi.org/10.2466/pms.2002.95.1.323 (2002).
Kong, X., Lian, Z., Yan, Y., Yao, J. & Fan, Y. The responses of continuous knee passive stiffness following fatigue. J. Med. Biol. Eng. 43, 596–602. https://doi.org/10.1007/s40846-023-00809-9 (2023).
Walsh, G. S., Low, D. C. & Arkesteijn, M. The relationship between postural control and muscle quality in older adults. J. Mot Behav. 54, 363–371. https://doi.org/10.1080/00222895.2021.1977602 (2022).
Taş, S., Ünlüer, N. & Çetin, A. Thickness, cross-sectional area, and stiffness of intrinsic foot muscles affect performance in single-leg stance balance tests in healthy sedentary young females. J. Biomech. 99, 109530. https://doi.org/10.1016/j.jbiomech.2019.109530 (2020).
ORR.R. Contribution of muscle weakness to postural instability in the elderly. Eur. J. Phys. Rehabil Med. 46, 183–220 (2010).
Hale, S. A., Hertel, J. & Olmsted-Kramer, L. C. The effect of a 4-week comprehensive rehabilitation program on postural control and lower extremity function in individuals with chronic ankle instability. J. Orthop. Sports Phys. Ther. 37, 303–311. https://doi.org/10.2519/jospt.2007.2322 (2007).
Hall, E. A., Chomistek, A. K., Kingma, J. J. & Docherty, C. L. Balance- and Strength-Training protocols to improve chronic ankle instability deficits, part I: assessing clinical outcome measures. J. Athl Train. 53, 568–577. https://doi.org/10.4085/1062-6050-385-16 (2018).
Luan, L., Adams, R., Witchalls, J., Ganderton, C. & Han, J. Does strength training for chronic ankle instability improve balance and Patient-Reported outcomes and by clinically detectable amounts?? A systematic review and Meta-Analysis. Phys. Ther. 101 https://doi.org/10.1093/ptj/pzab046 (2021).
Mollà-Casanova, S., Inglés, M. & Serra-Añó, P. Effects of balance training on functionality, ankle instability, and dynamic balance outcomes in people with chronic ankle instability: systematic review and meta-analysis. Clin. Rehabil. 35, 1694–1709. https://doi.org/10.1177/02692155211022009 (2021).
Guo, Y. et al. A systematic review and meta-analysis of balance training in patients with chronic ankle instability. Syst. Rev. 13, 64. https://doi.org/10.1186/s13643-024-02455-x (2024).
Koshino, Y. & Kobayashi, T. Effects of Conservative interventions on static and dynamic balance in individuals with chronic ankle instability: A systematic review and Meta-analysis. Arch. Phys. Med. Rehabil. 104, 673–685. https://doi.org/10.1016/j.apmr.2022.10.014 (2023).
de Vries, J. S., Krips, R., Sierevelt, I. N. & Blankevoort, L. Dijk, C. N. Interventions for treating chronic ankle instability. Cochrane Database Syst. Rev. Cd004124. https://doi.org/10.1002/14651858.CD004124.pub3 (2011).
Mahmoud, W. S. et al. Effect of blood flow restriction as a stand-alone treatment on muscle strength, dynamic balance, and physical function in female patients with chronic ankle instability. Med. (Baltim). 102, e35765. https://doi.org/10.1097/md.0000000000035765 (2023).
Ahn, S. H., Hwang, U. J., Gwak, G. T., Yoo, H. I. & Kwon, O. Y. Comparison of the strength and electromyography of the Evertor muscles with and without toe flexion in patients with chronic ankle instability. Foot Ankle Int. 41, 479–485. https://doi.org/10.1177/1071100719898464 (2020).
Hui, J. Y. et al. Cross-cultural adaptation, reliability and validity of the Cantonese-Chinese Cumberland ankle instability tool (CAIT-HK). Foot (Edinb). 56, 102015. https://doi.org/10.1016/j.foot.2023.102015 (2023).
Faul, F., Erdfelder, E., Lang, A. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 39, 175–191. https://doi.org/10.3758/bf03193146 (2007).
Hiller, C. E., Refshauge, K. M., Bundy, A. C., Herbert, R. D. & Kilbreath, S. L. The Cumberland ankle instability tool: A report of validity and reliability testing. Arch. Phys. Med. Rehabil. 87, 1235–1241. https://doi.org/10.1016/j.apmr.2006.05.022 (2006).
Plisky, P. J., Rauh, M. J., Kaminski, T. W. & Underwood, F. B. Star excursion balance test as a predictor of lower extremity injury in high school basketball players. J. Orthop. Sports Phys. Ther. 36, 911–919. https://doi.org/10.2519/jospt.2006.2244 (2006).
Coughlan, G. F., Fullam, K., Delahunt, E., Gissane, C. & Caulfield, B. M. A comparison between performance on selected directions of the star excursion balance test and the Y balance test. J. Athl Train. 47, 366–371. https://doi.org/10.4085/1062-6050-47.4.03 (2012).
Hertel, J., Braham, R., Hale, S. & Olmsted-Kramer, L. Simplifying the star excursion balance test: analyses of subjects with and without chronic ankle instability. J. Orthop. Sports Phys. Ther. 36, 131–137. https://doi.org/10.2519/jospt.2006.36.3.131 (2006).
Gonzalez-Fernandez, F. T., Martinez-Aranda, L. M., Falces-Prieto, M., Nobari, H. & Clemente, F. M. Exploring the Y-Balance-Test scores and inter-limb asymmetry in soccer players: differences between competitive level and field positions. BMC Sports Sci. Med. Rehabil. 14, 45. https://doi.org/10.1186/s13102-022-00438-w (2022).
Sikora, D. & Linek, P. The relationship between the functional movement screen and the Y balance test in youth footballers. PeerJ 10, e13906. https://doi.org/10.7717/peerj.13906 (2022).
Bulow, A., Anderson, J. E., Leiter, J. R., MacDonald, P. B. & Peeler, J. The modified star excursion balance and Y-Balance test results differ when assessing physically active healthy adolescent females. Int. J. Sports Phys. Therapy. 14, 192–203. https://doi.org/10.26603/ijspt20190192 (2019).
Robbins, S. M., Caplan, R. M., Aponte, D. I. & St-Onge, N. Test-retest reliability of a balance testing protocol with external perturbations in young healthy adults. Gait Posture. 58, 433–439. https://doi.org/10.1016/j.gaitpost.2017.09.007 (2017).
Brenton-Rule, A. et al. Reliability of the TekScan MatScan(R) system for the measurement of postural stability in older people with rheumatoid arthritis. J. Foot Ankle Res. 5, 21. https://doi.org/10.1186/1757-1146-5-21 (2012).
Simpson, J. D. et al. Bilateral Spatiotemporal postural control impairments are present in participants with chronic ankle instability. Phys. Ther. Sport. 39, 1–7. https://doi.org/10.1016/j.ptsp.2019.06.002 (2019).
Verhagen, E. et al. The effect of a balance training programme on centre of pressure excursion in one-leg stance. Clin. Biomech. (Bristol Avon). 20, 1094–1100. https://doi.org/10.1016/j.clinbiomech.2005.07.001 (2005).
Goetschius, J., Feger, M., Hertel, J. & Hart, J. Validating Center-of-Pressure Balance Measurements Using the MatScan® Pressure Mat. J. Sport Rehabil. 27. https://doi.org/10.1123/jsr.2017-0152 (2018).
Kelln, B., McKeon, P., Gontkof, L. & Hertel, J. Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active,young adults. J. Sport Rehabil. 17, 160–170. https://doi.org/10.1123/jsr.17.2.160 (2008).
Fong, D. T., Wang, D., Chu, V. W. & Chan, K. M. Myoelectric stimulation on peroneal muscles with electrodes of the muscle belly size attached to the upper Shank gives the best effect in resisting simulated ankle sprain motion. J. Biomech. 46, 1088–1091. https://doi.org/10.1016/j.jbiomech.2013.01.019 (2013).
Stegeman, D. F. & Hermen, H. J. Standards for surface electromyography: the European project surface EMG for non-invasive assessment of muscles (SENIAM) Enschede: Roessingh Research and Development (2007).
Arima, S. et al. Morphological and functional characteristics of the peroneus muscles in patients with lateral ankle sprain: an Ultrasound-Based study. Medicina (Kaunas) 58. https://doi.org/10.3390/medicina58010070 (2022).
Saeki, J., Ikezoe, T., Nakamura, M., Nishishita, S. & Ichihashi, N. The reliability of shear elastic modulus measurement of the ankle plantar flexion muscles is higher at dorsiflexed position of the ankle. J. Foot Ankle Res. 10, 18. https://doi.org/10.1186/s13047-017-0199-0 (2017).
Hug, F., Tucker, K., Gennisson, J. L., Tanter, M. & Nordez, A. Elastography for muscle biomechanics: toward the Estimation of individual muscle force. Exerc. Sport Sci. Rev. 43, 125–133. https://doi.org/10.1249/JES.0000000000000049 (2015).
Pillen, S. et al. Skeletal muscle ultrasonography: visual versus quantitative evaluation. Ultrasound Med. Biol. 32, 1315–1321. https://doi.org/10.1016/j.ultrasmedbio.2006.05.028 (2006).
Heckmatt, J. Z. & Dubowitz, V. Ultrasound imaging and directed needle biopsy in the diagnosis of selective involvement in muscle disease. J. Child. Neurol. 2, 205–213. https://doi.org/10.1177/088307388700200307 (1987).
Moreta, M. C. et al. Reliability and validity of the modified heckmatt scale in evaluating muscle changes with ultrasound in spasticity. Arch. Rehabil Res. Clin. Transl. 2, 100071. https://doi.org/10.1016/j.arrct.2020.100071 (2020).
Lambert, Z. V. & Durand, R. M. Some precautions in using canonical analysis. J. Mark. Res. 12, 468–475. https://doi.org/10.2307/3151100 (1975).
McVey, E. D., Palmieri, R. M., Docherty, C. L., Zinder, S. M. & Ingersoll, C. D. Arthrogenic muscle Inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int. 26, 1055–1061. https://doi.org/10.1177/107110070502601210 (2005).
Palmieri-Smith, R., Ty Hopkins, J. & Brown, T. Peroneal activation deficits in persons with functional ankle instability. Am. J. Sports Med. 37, 982–988. https://doi.org/10.1177/0363546508330147 (2009).
Uezumi, A., Fukada, S., Yamamoto, N., Takeda, S. & Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell. Biol. 12, 143–152. https://doi.org/10.1038/ncb2014 (2010).
Farup, J., Madaro, L., Puri, P. L. & Mikkelsen, U. R. Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease. Cell. Death Dis. 6, e1830. https://doi.org/10.1038/cddis.2015.198 (2015).
Zhu, Y. et al. Fatty infiltration in the musculoskeletal system: pathological mechanisms and clinical implications. Front. Endocrinol. (Lausanne). 15, 1406046. https://doi.org/10.3389/fendo.2024.1406046 (2024).
Vandervoort, A. A. Aging of the human neuromuscular system. Muscle Nerve. 25, 17–25. https://doi.org/10.1002/mus.1215 (2002).
Piasecki, M. et al. Failure to expand the motor unit size to compensate for declining motor unit numbers distinguishes sarcopenic from non-sarcopenic older men. J. Physiol. 596, 1627–1637. https://doi.org/10.1113/jp275520 (2018).
Kiyoshige, Y. & Watanabe, E. Fatty degeneration of gluteus minimus muscle as a predictor of falls. Arch. Gerontol. Geriatr. 60, 59–61. https://doi.org/10.1016/j.archger.2014.07.013 (2015).
Addison, O. et al. Role of hip abductor muscle composition and torque in protective stepping for lateral balance recovery in older adults. Arch. Phys. Med. Rehabil. 98, 1223–1228. https://doi.org/10.1016/j.apmr.2016.10.009 (2017).
Dong, S. et al. Can arthrogenic muscle Inhibition exist in peroneal muscles among people with chronic ankle instability?? A Cross-sectional study. Sports Med. Open. 10. https://doi.org/10.1186/s40798-024-00710-y (2024).
Visser, M. et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J. Gerontol. Biol. Sci. Med. Sci. 60, 324–333. https://doi.org/10.1093/gerona/60.3.324 (2005).
Engelke, K. et al. The effect of ageing on fat infiltration of thigh and paraspinal muscles in men. Aging Clin. Exp. Res. 34, 2089–2098. https://doi.org/10.1007/s40520-022-02149-1 (2022).
Strasser, E. M., Draskovits, T., Praschak, M., Quittan, M. & Graf, A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr). 35, 2377–2388. https://doi.org/10.1007/s11357-013-9517-z (2013).
Oranchuk, D. J., Bodkin, S. G., Boncella, K. L. & Harris-Love, M. O. Exploring the associations between skeletal muscle echogenicity and physical function in aging adults: A systematic review with meta-analyses. J. Sport Health Sci. https://doi.org/10.1016/j.jshs.2024.05.005 (2024).
Hill, M. W., Roberts, M., Price, M. J. & Kay, A. D. Association between knee extensor and ankle plantarflexor muscle thickness and echo intensity with postural sway, mobility and physical function in older adults. Exp. Gerontol. 150, 111385. https://doi.org/10.1016/j.exger.2021.111385 (2021).
Wood, L. K. et al. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J. Appl. Physiol. (1985). 117, 363–369. https://doi.org/10.1152/japplphysiol.00256.2014 (2014).
Di Ludovico, A. et al. Skeletal muscle as a pro- and anti-inflammatory tissue: insights from children to adults and ultrasound findings. J. Ultrasound. https://doi.org/10.1007/s40477-024-00917-5 (2024).
Mongold, S. J., Ricci, A. W., Hahn, M. E. & Callahan, D. M. Skeletal muscle compliance and echogenicity in Resistance-Trained and nontrained women. J. Strength. Cond Res. 38, 671–680. https://doi.org/10.1519/jsc.0000000000004669 (2024).
Yuan, H. & Kim, M. Meta-Analysis on the association between echo intensity, muscle strength, and physical function in older individuals. Ann. Geriatr. Med. Res. 27, 329–337. https://doi.org/10.4235/agmr.23.0101 (2023).
Fukumoto, Y. et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur. J. Appl. Physiol. 112, 1519–1525. https://doi.org/10.1007/s00421-011-2099-5 (2012).
Mota, J. A. & Stock, M. S. Rectus femoris echo intensity correlates with muscle strength, but not endurance, in younger and older men. Ultrasound Med. Biol. 43, 1651–1657. https://doi.org/10.1016/j.ultrasmedbio.2017.04.010 (2017).
Wang, H., Yu, H., Kim, Y. H. & Kan, W. Comparison of the effect of resistance and balance training on isokinetic Eversion strength, dynamic balance, hop test, and ankle score in ankle sprain. Life (Basel) 11 https://doi.org/10.3390/life11040307 (2021).
Gabriner, M. L., Houston, M. N., Kirby, J. L. & Hoch, M. C. Contributing factors to star excursion balance test performance in individuals with chronic ankle instability. Gait Posture 41, 912–916. https://doi.org/10.1016/j.gaitpost.2015.03.013 (2015).
Williams, V. et al. Prediction of dynamic postural stability during Single-Leg jump landings by ankle and knee flexibility and strength. J. Sport Rehabil. 25, 266–272. https://doi.org/10.1123/jsr.2015-0001 (2016).
Kaminski, T. & Hartsell, H. Factors contributing to chronic ankle instability: A strength perspective. J. Athl Train. 37, 394–405 (2002).
Fereydounnia, S. et al. Improvements in strength and functional performance after Kinesio taping in semi-professional male soccer players with and without functional ankle instability. Foot (Edinb). 41, 12–18. https://doi.org/10.1016/j.foot.2019.06.006 (2019).
Ko, D., Choi, Y. & Lee, K. Effects of Peroneus Brevis versus Peroneus Longus Muscle Training on Muscle Function in Chronic Ankle Instability: A Randomized Controlled Trial. Healthcare 12, 547. https://doi.org/10.3390/healthcare12050547 (2024).
Ko, D., Choi, Y. & Lee, K. Effects of peroneus brevis versus peroneus longus muscle training on muscle function in chronic ankle instability: A randomized controlled trial. Healthcare (Basel) 12. https://doi.org/10.3390/healthcare12050547 (2024).
Neamatallah, Z. et al. Correlation between level of functional ability and sports performance among football players with chronic ankle instability (CAI). J. Pharm. Res. Int., 415–424. https://doi.org/10.9734/jpri/2021/v33i64B35780 (2021).
Ko, K. R., Lee, H., Lee, W. Y. & Sung, K. S. Ankle strength is not strongly associated with postural stability in patients awaiting surgery for chronic lateral ankle instability. Knee Surg. Sports Traumatol. Arthrosc. 28, 326–333. https://doi.org/10.1007/s00167-018-4960-0 (2020).
Wang, L., Yu, G., Zhang, X., Wang, Y. Z. & Chen, Y. P. Relationship between ankle pain, range of motion, strength and balance in individuals with functional ankle instability: a cross-sectional study. BMC Musculoskelet. Disord. 24, 955. https://doi.org/10.1186/s12891-023-07079-1 (2023).
Payne, K. A., Berg, K. & Latin, R. W. Ankle injuries and ankle strength, flexibility, and proprioception in college basketball players. J. Athl Train. 32, 221–225 (1997).
Bell, D. R., Padua, D. A. & Clark, M. A. Muscle strength and flexibility characteristics of people displaying excessive medial knee displacement. Arch. Phys. Med. Rehabil. 89, 1323–1328. https://doi.org/10.1016/j.apmr.2007.11.048 (2008).
Taniguchi, M. et al. Enhanced echo intensity in Vastus medialis is associated with worsening of functional disabilities and symptoms in patients with knee osteoarthritis: a 3 years longitudinal study. Rheumatol. Int. 43, 953–960. https://doi.org/10.1007/s00296-022-05246-6 (2023).
Chmielewski, T. L. et al. Investigation of clinician agreement in evaluating movement quality during unilateral lower extremity functional tasks: a comparison of 2 rating methods. J. Orthop. Sports Phys. Ther. 37, 122–129. https://doi.org/10.2519/jospt.2007.2457 (2007).
Grindstaff, T. L., Dolan, N. & Morton, S. K. Ankle dorsiflexion range of motion influences lateral step down test scores in individuals with chronic ankle instability. Phys. Ther. Sport. 23, 75–81. https://doi.org/10.1016/j.ptsp.2016.07.008 (2017).
Lee, J. & Yoon, T. Effective treatment for chronic ankle instability during lateral Step-Down-Kinesiology tape, resistance exercise, or both accompanied with heel Raise-Lower exercise?? J. Sport Rehabil. 28, 809–816. https://doi.org/10.1123/jsr.2018-0073 (2019).
Rabin, A. & Kozol, Z. Measures of range of motion and strength among healthy women with differing quality of lower extremity movement during the lateral step-down test. J. Orthop. Sports Phys. Ther. 40, 792–800. https://doi.org/10.2519/jospt.2010.3424 (2010).
Serra-Añó, P., Inglés, M., Espí-López, G. V., Sempere-Rubio, N. & Aguilar-Rodríguez, M. Biomechanical and viscoelastic properties of the ankle muscles in men with previous history of ankle sprain. J. Biomech. 115, 110191. https://doi.org/10.1016/j.jbiomech.2020.110191 (2021).
Koldenhoven, R. M., Hart, J., Abel, M. F., Saliba, S. & Hertel, J. Running gait biomechanics in females with chronic ankle instability and ankle sprain copers. Sports Biomech. 21, 447–459 (2022).
Lapointe, S. J. et al. Changes in the flexibility characteristics of the ankle complex due to damage to the lateral collateral ligaments: an in vitro and in vivo study. J. Orthop. Res. 15, 331–341. https://doi.org/10.1002/jor.1100150304 (1997).
Siegler, S., Chen, J. & Schneck, C. D. The effect of damage to the lateral collateral ligaments on the mechanical characteristics of the ankle joint–an in-vitro study. J. Biomech. Eng. 112, 129–137. https://doi.org/10.1115/1.2891163 (1990).
Dondero, K., Friedman, B., Rekant, J., Landers-Ramos, R. & Addison, O. The effects of myosteatosis on skeletal muscle function in older adults. Physiol. Rep. 12, e16042. https://doi.org/10.14814/phy2.16042 (2024).
Blackburn, J. T., Padua, D. A., Riemann, B. L. & Guskiewicz, K. M. The relationships between active extensibility, and passive and active stiffness of the knee flexors. J. Electromyogr. Kinesiol. 14, 683–691. https://doi.org/10.1016/j.jelekin.2004.04.001 (2004).
Secomb, J. L. et al. Relationships between Lower-Body muscle structure and Lower-Body strength, power, and muscle-Tendon complex stiffness. J. Strength. Conditioning Res. 29, 2221–2228. https://doi.org/10.1519/JSC.0000000000000858 (2015).
He, X. et al. Decreased passive muscle stiffness of Vastus medialis is associated with poorer quadriceps strength and knee function after anterior cruciate ligament reconstruction. Clin. Biomech. (Bristol Avon) 82 https://doi.org/10.1016/j.clinbiomech.2021.105289 (2021).
Willems, T. M. et al. Intrinsic risk factors for inversion ankle sprains in male subjects: A prospective study. Am. J. Sports Med. 33, 415–423. https://doi.org/10.1177/0363546504268137 (2005).
Mendez-Rebolledo, G. et al. Individuals with chronic ankle instability show altered regional activation of the peroneus longus muscle during ankle Eversion. Scand. J. Med. Sci. Sports. 34, e14535. https://doi.org/10.1111/sms.14535 (2024).
Turvey, M. T. & Fonseca, S. T. The medium of haptic perception: A tensegrity hypothesis. J. Mot. Behav. 46, 143–187. https://doi.org/10.1080/00222895.2013.798252 (2014).
Isabelle, M., Sylvie, Q. B. & Chantal, P. Electromechanical assessment of ankle stability. Eur. J. Appl. Physiol. 88, 558–564 (2003).
Theret, M., Rossi, F. M. V. & Contreras, O. Evolving roles of Muscle-Resident Fibro-Adipogenic progenitors in health, regeneration, neuromuscular disorders, and aging. Front. Physiol. 12, 673404. https://doi.org/10.3389/fphys.2021.673404 (2021).
Hill, M. W., Wdowski, M. M., Rosicka, K., Kay, A. D. & Muehlbauer, T. Exploring the relationship of static and dynamic balance with muscle mechanical properties of the lower limbs in healthy young adults. Front. Physiol. 14, 1168314. https://doi.org/10.3389/fphys.2023.1168314 (2023).
119 Needle, A. R. et al. The relationship between joint stiffness and muscle activity in unstable ankles and copers. J. Sport Rehabil. 26, 15–25. https://doi.org/10.1123/jsr.2015-0061 (2017).
Lieber, R. L. & Binder-Markey, B. I. Biochemical and structural basis of the passive mechanical properties of whole skeletal muscle. J. Physiol. 599, 3809–3823. https://doi.org/10.1113/jp280867 (2021).
Tanen, L., Docherty, C. L., Van Der Pol, B., Simon, J. & Schrader, J. Prevalence of chronic ankle instability in high school and division I athletes. Foot Ankle Spec. 7, 37–44. https://doi.org/10.1177/1938640013509670 (2014).
Acknowledgements
The authors wish to acknowledge Miss Yang Yang Cheng who is a registered sonographer recognised for her ultrasound experience and teaching. Most importantly, we are grateful to all the precious patients who participated in the study.
Author information
Authors and Affiliations
Contributions
SMCC participated in the data collection/analysis and writing of the manuscript. SCF is a biostatistician and provides advice and guidance on biostatistics. WM and ZY participated in the revision of the manuscript. KMCV participated in the inter-rater methodology and provided ultrasound advice and support. KMCV, KKSL and YSHP supported the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study has been approved by the CUHK-NTEC clinical research committee with reference number 2022.263-T. The data have not been communicated to a third party.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chia, C.S.M., Fu, SC., Ko, V.MC. et al. A cross-sectional study on peroneal muscle echogenicity changes and their effects on balance functions in individuals with chronic ankle instability. Sci Rep 15, 15090 (2025). https://doi.org/10.1038/s41598-025-00175-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00175-3