Abstract
Geopolymers have long been used to stabilise unique soils, and the stabilising conditions and freeze–thaw cycles (FTs) have a substantial impact on the engineering qualities of stabilised soils. The mechanical characteristics and microstructure of lime (Ca(OH)2) fly ash (FA) stabilised saline soils were investigated in this study using the Unconfined Compressive Strength (UCS), Splitting Strength, and Residual Strength (IR) tests in conjunction with X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), and Thermogravimetry (TG). The results indicated that the mechanical properties were optimal at 3% Ca(OH)2 with constant 13% FA content and stabilised soils with UCS and splitting strength of 8.78 MPa and 1.43 MPa, respectively. The stabilised soils strength showed a trend of rapid decrease and then stabilisation with increasing FTs, and frost resistance was optimal at 3% Ca(OH)2. At FTs = 20, the UCS and splitting strength IR were 40.94% and 32.51%, respectively, which were higher than those of other proportions of stabilised soils. This was attributed to the fact that calcium assisted FA stabilisation was primarily attributed to the formation of dense network structure of hydrated calcium silicate and hydrated calcium aluminate gels, as well as the generation of ettringite with sulphate.
Similar content being viewed by others
Introduction
Permafrost arises when temperatures fall below 0 °C, and it is a widespread occurrence around the planet1. Frozen soils are made up of four phases: soil particles, water, ice, and gas, which are non-homogeneous and anisotropic2,3. Frozen soils can be classed into two types: persistently frozen soils and seasonally frozen soils. In late winter and early spring, seasonally frozen zones are subjected to freeze–thaw cycles (FTs) effect4, due to large temperature variations, which can lead to frost heave5,6 and freeze-thaw7. In addition, saline frozen soils are formed in soils with high saline content in the frozen zone soils, sulphates in the seasonally frozen zone can cause saline bloom8, and chloride slimes can cause corrosion of reinforcing steel and other engineering problems9. Therefore, it is of great significance to explore the improvement technology of saline soils in seasonally frozen zones in order to avoid and minimize the adverse effects of the above-mentioned engineering diseases on structures10,11.
Methods of stabilised soil are being investigated to improve the stability of saline soils in the seasonally frozen zones12. Geopolymer is an inorganic polymer material synthesized from alkali activated aluminosilicates, and as a gel material, it is regarded as an environmentally acceptable bonding agent13. Geopolymer has several advantages, including rapid hardening, high strength, strong material interface bonding ability, good corrosion resistance, and durability14. In the last decade, various industrial wastes and by-products such as fly ash (FA)15,16, slag17,18, silica fume (SF)19, and rice husk ash (RHA)20 have been the key sources of aluminosilicates for geopolymers, with FA being the most often utilized primary feedstock. Geopolymer activation has received substantial attention over the last several decades21. H. Taghvayi et al.22 observed significant differences in the structural, mechanical, and physical properties of geopolymers derived from various sources of solid waste. FA alkali activation, which includes the dissolution of calcium and aluminum in the formation of calcium (aluminium) silicate hydrate (C–(A)–S–H) gels, enhances mechanical properties and durability in geopolymer system14,23. The composition of gels in alkali activated composite systems is greatly influenced by raw material composition, reactivity, mixing ratios, and activator selection24,25.
B. Walkley et al. found that the strength of alkali activated FA geopolymers develops through the formation of three-dimensional sodium aluminosilicate hydrate (N–A–S–H) type gels26, that the major reaction product formed in the geopolymer system is N–A–S–H, and these gel materials can be used to stabilised soils27. Á. Palomo and coworkers et al.28,29 discovered that large concentrations of alkali exciters inhibit ion mobility, delaying gels formation. The addition of calcium to an adequate quantity of alkali solution causes the development of calcium silicate hydrates (C–A–H) gels, which fill the spaces between soil particles, enhancing compressive strength30. Alkali solution concentration impacts and activates hydrolysis and polymerization reactions to produce appropriate aluminosilicates, with Na+ and K+ increasing strength by promoting aluminosilicate production and OH– decreasing gels strength31.
Hamid et al.32 investigated the impact of alkali activated slag cement on the mechanical strength of saline sandy soils. The unconfined compressive strength (UCS) of geopolymer treated soils rose by 340–952% compared to untreated soils, and the geopolymer gels, C–A–S–H/N–A–S–H gels, joined the soil particles. Lv et al. 33 studied the curing effect of lime, lime-FA, and lime, FA, and sodium silicate on sulphate saline soils. The best results were obtained when the sulphate content was 1% cured, and the two types of stabilised soils, lime-FA and sodium silicate, had a higher UCS than the other two types of stabilised soils when the saline content was kept constant. Ca2+ plays a vital role in FA activation hardening and mechanical properties34,35, and dissolved Ca2+ in FA acts as a charge-balancing cation and also forms C–S–H gels. Van Deventer 36 showed that Ca2+ plays a significant part in the rapid hardening of gel materials by providing additional nucleation sites. Temuujin et al.37 found that adding active calcium compounds such as CaO and Ca(OH)2 to FA increases mechanical characteristics while speeding up the cementation procedure. Zhao et al.38 discovered that adding Ca(OH)2 to FA considerably boosted strength, but that adding too much Ca(OH)2 increases the danger of strength decrease. Duan et al.39 investigated the addition of SF to FA, which expedited the consolidation reaction via Ca(OH)2 consumption and dramatically increased compressive strength at 28 days40. Ca2+ plays an important role in the hardening and mechanical characteristics of geopolymers. There has also been little investigation into using NaOH as an alkali activator and Ca2+ as a supplement material to increase FA geopolymer resistance to saline soils stabilisation.
The mechanical properties and frost resistance of FA stabilised saline soils were investigated in this research employing Ca(OH)2 as supplementary material for Ca2+ and NaOH as an excitatory agent, as well as the UCS, splitting strength, and freeze–thaw cycle tests. Meanwhile, X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR), and Thermogravimetric analysis (TG–DSC) were used to investigate the microscopic properties of the stabilised soils as well as the stabilisation mechanism of the Ca2+ enhanced FA stabilised soils.
Material and methods
Materials
The saline soils were collected in Urumqi, Xinjiang, China, a typical seasonally frozen zone with a minimum winter temperature of –20°C and a maximum yearly temperature variation of more than 60°C. The region is heavily salinized, and the results show that the total soluble saline content of the soil is highest at a depth of 100–120 cm, up to 1%. Table 1 shows the basic physical properties of the saline soils, and Fig. 1 shows the particle size distribution of the saline soils. Therefore, for this research, saline soils at this depth were selected for the preparation of FA stabilised soils with different calcium contents.
FA was provided by 102 Mission in the Xinjiang region of China, and its chemical composition was evaluated using X-ray Fluorescence Spectrometry (XRF), with the results shown in Table 2. Lime was provided by Xinjiang Hongyanchi No. 2 Power Generation Co. Ltd., and the XRD of FA and lime was shown in Fig. 2. Na2SO4 was supplied by General-Reagent Co. (powdered, purity ≥ 99.0%). NaOH was provided by Zhongtai Chemical Co. Ltd (flake, purity ≥ 98%). The saline soils, FA, and Ca(OH)2 were processed as follows: the saline soils were soaked in water, allowed to settle and precipitate several times, subsequently dried at 105°C–110°C, crushed, and passed through 2.00 mm sieve, while the FA and Ca(OH)2 were sieved through a 0.075 mm sieve.
Experimental
Specimen preparation
Firstly, all the materials were weighed proportionally, soils, FA, and Ca(OH)2 were mixed thoroughly and prepared, then NaOH and Na2SO4 were dissolved in water. The stabilised soils were then created by spraying the mixed solution in a mixture of soils, Ca(OH)2, and FA before being placed in a sealed bag for 12 h to ensure consistent distribution of water and saline. The stabilised soils were weighed and poured into the mould in three layers. A circular specimen with a diameter of 100 × 100 mm was created by static pressure, wrapped in cling film, and placed in the curing room for 28 days. The stabilised soils contained 13% FA, 12.5% moisture content, and a maximum dry density of 1.894 g/cm3. The Ca(OH)2 mass percentages of 1%, 2%, 3%, and 4% were designated as L1, L2, L3, and L4, respectively.
Mechanical property experiment
The UCS is the maximum axial stress when the specimen is destroyed under axial pressure without any lateral restraint, as shown in Fig. 3a,b. Splitting strength is the important indicator of tensile strength, which responds to the ability of the material to resist cracking damage, as shown in Fig. 3c,d. UCS and splitting strength were important indicators of the effectiveness of consolidation. UCS and splitting tests were researched on stabilised soils using a cement flexural and compressive integrated machine (YAW-SERIES) with different content of stabilisers curing for 28 days and different number of FTs. The displacement rate was set at 1.0 mm/min during the experiment and the peak load was recorded at the time of specimen damage.
Freeze–thaw cycle test
Since saline soils exhibit smaller changes in water content during freezing and thawing, a freeze–thaw pattern with constant water content was utilized. After 28 days of stabilised soils curing, it was frozen and thawed for 12 h each at –20°C and + 20°C, resulting in a single freeze–thaw cycles (FTs = N, N = 1, 3, 5, 10, and 20). The measure of IR after freezing and thawing expression was used to assess the frost resistance of stabilised soils; the higher the IR, the better the stabilised soils’ frost resistance.
where \({qu}_{0}\) is the strength before freeze–thaw cycles; \({qu}_{n}\) is the strength after FTs = N.
Instrumentation
Following the splitting tests, internal soil samples from the specimens were collected and dried at 60°C for 24 h for XRD, SEM, FTIR, and TG analysis. XRD (D8 Advance, Bruker, U.S.) test material is crushed and fed through a 200 mesh screen, with a test ranges from 10° to 80°, with a step of 0.02° and measurement speed of 10°/min. The SEM test was performed using scanning electron microscope (Hitachi S4800, Hitachi, Tokyo, Japan) that had been gold sprayed twice prior to the test to see the stabilised soils and gel materials at magnifications of 1000 and 2000 times. FTIR (Great 10, CK Ruijie Technology Co., Tianjin, China) was carried out using potassium bromide press with a material to KBr ratio of 1:200 and 32 scans ranging from 400 cm–1 to 4000 cm–1 to explore the functional groups of the products. TG tests were performed using thermogravimetric synchronous thermal analyser (STA7300, Hitachi, Tokyo, Japan) for TG analysis of hydration products at a ramp rate of 10 ℃/min from room temperature to 900℃.
Results
Mechanical performance analysis
Figure 4 shows the relationship between UCS, splitting strength and Ca(OH)2 content of L1, L2, L3, and L4 stabilised soils after 28 days of curing.
The UCS of stabilised soils after 28 days were 7.68 MPa, 8.37 MPa, 8.78 MPa, and 8.53 MPa, respectively. The UCS tended to grow and then drop as Ca(OH)2 content increased, with a peak value appearing at L3. Figure 4b demonstrates that the splitting strength of stabilised soils maintained for 28 days is 1.11 MPa, 1.27 MPa, 1.43 MPa, and 1.36 MPa, respectively, and that the splitting strength increases with the amount of Ca(OH)2 applied, with a similar trend and maximum value as UCS. It indicates that the link between mechanical characteristics and FA/Ca(OH)2 ratio in improved saline soils is not individually, but rather that there is an optimal mixing amount.
When FA based geopolymer was mixed in saline soils, FA reacted with Ca(OH)2 to produce more C–S–H and C–A–H, and the higher the Ca(OH)2 mixing, the more hydration products were produced, resulting in better cementation between soil particles to form a whole and thus increased tensile capacity of stabilised soils. In addition to the need for a cementing material to interconnect the soil particles, the increase in compressive strength necessitates a sufficient volume of substance to fill the pores, improving the microstructure’s density. FA and Ca(OH)2 particles as microfillers dispersed in the geopolymer occupy the internal space of the microstructure of the stabilised soils, resulting in a filler effect.
Frost resistance
The frost resistance of L1, L2, L3, and L4 stabilised soils is shown in Fig. 5.
The stabilised soils L1, L2, L3, and L4 exhibit similar frost resistance, where their UCS and splitting strength decrease with the number of FTs. The degradation curve of frost resistance in stabilised soils may be separated into two stages: the first stage was a rapid deterioration stage, primarily during the 5 FTs, and the second stage was a progressive stabilisation stage. Before the FTs, as the Ca(OH)2 concentration grew, the UCS and splitting strength exhibited a progressive increase followed by slow decline; after a specific number of FTs, this pattern persisted but changed. When the stabilised soils were subjected to 20 FTs, their UCS and splitting strength IR were L3, L4, L2, and L1, respectively. The IR of the stabilised soils decreased as the Ca(OH)2 concentration increased, while the frost resistance of the stabilised soils improved with increasing Ca(OH)2 admixture. Because the higher the Ca(OH)2 content, the lower the content of unconsolidated Na2SO4 in stabilised soils, the smaller the damage effect of freezing, saline expansion, and melting corrosion, so it can maintain higher agglomerate cementation, which is macroscopically manifested in the slowing of the decline of stabilised soils IR. It can be seen that the Ca(OH)2 and FA inclusion lessens the damage to the soil structure caused by freezing and thawing, owing primarily to its limiting of soil frost heave, which makes movement between soil particles difficult.
Microstructural investigation
XRD analyses
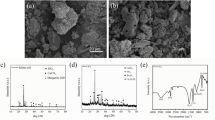
Figure 6 shows XRD pictures of calcium assisted excitation FA at 1, 3, 7, and 28 days.
It can be found that the spectra in the hydration products of alkali activated composite systems show similarities at different calcium concentrations. Ca(OH)2 reacts with sodium sulphate in L1, L2, L3, and L4 and is partially converted to CaSO4, as well as CaCO3 generated by carbonation. The diffraction peaks gradually strengthen with the increase of Ca(OH)2, and a large number of diffraction peaks of unreacted Ca(OH)2 can also be seen. The diffraction broad peaks at 25°–35° shows that the main reaction products in the excited system are all C–S–H, C–A–H, and the peak intensities gradually increase with the prolongation of the reaction time, indicating that the reaction process continues and the amount of products generated is gradually increasing. In Fig. 6 (a), the L1 at 33.54° produces a substantial amount of CaSO4, and the diffraction peak lowers over time. The diffraction peak amplification is mostly caused by the interaction of Ca(OH)2 with Na2SO4 to produce CaSO4 and NaOH. The loss of the diffraction peaks can be attributed to two reasons: the created NaOH continues to stimulate the FA, resulting in the formation of a gel material to cover the surface, and the reaction of CaSO4 with C–A–H, which produces AFt. The diffraction peaks of CaCO3 and Ca(OH)2 gradually grow and decrease with time for L1. The main reason is that Ca(OH)2 produces C–A–H and C–S–H with FA, and a portion of Ca(OH)2 absorbs CO2 and progressively converts to CaCO3, as was shown for L2, L3, and L4.
FA hydration process was complex due to its chemical makeup, in which Al2O3 reacted with SO42− and Ca(OH)2 to form AFt (Eq. (2))33. Ca(OH)2 combines with SiO2 and Al2O3 to generate C–S–H and C–A–H, respectively (Eqs. (3) and (4))41, and C–A–H reacts with CaSO4 to form AFt (Eq. (5)). AFt was the primary hydration product of C–A–H and CaSO4, and the whole reaction sequence can be incorporated into Eq. (5).
SEM analyses of calcium assisted FA excitation
Figure 7 illustrates the image of calcium assisted excitation FA within 1, 3, 7, and 28 days by SEM. Figure 7a–d indicates that a substantial amount of C–S–H and C–A–H gel materials is clearly visible on the FA surface within the orange circles, Ca(OH)2 is shown by the red arrows, and needle-like AFt is indicated by the blue arrows. Increasing Ca(OH)2 causes an increase in flaky Ca(OH)215, needle-like AFt10, and flocculated gel materials. The primary reason is that NaOH reacts with FA to quickly dissolve and generate C–S–H and C–A–H rates42, and the slow generation of NaOH from the reaction of Ca(OH)2 with Na2SO4 with lower solubility was insufficient to support the rapid dissolution of FA, resulting in slower generation, and the production of C–A–H reacts more and more rapidly with sulphate to be converted into more AFt. Ca(OH)2 enters a solvation equation state with ionized OH– and Ca2+, which are still in high concentrations, and then reacts with FA to form C–S–H and C–A–H43,44, which cover the AFt and Ca(OH)2 surfaces. The higher the Ca(OH)2 content, the more C–S–H and C–A–H are produced by its conversion, the greater the C–A–H reaction with sulphate, and the faster the rate of AFt formation.
Figure 7 (e, f, g, and h) and (i, j, k, and l) show SEM images for excitation at 3 and 7 days, respectively. Figure 7 (e, f, g, and h) indicates that the amount of flaky Ca(OH)2 decreased while the amount of flocculated gel materials increased. Figure 7 (i, j, k, and l) illustrates a decrease in the amount of flocculated gel materials as the excitation time increases. This was because the produced C–S–H and C–A–H cover the surfaces of FA, Ca(OH)2, and AFt, slowing Ca(OH)2 dissolution and FA-OH– interaction. OH– penetrates the cracks and pores of the hydration products to react with FA, and the excitedly formed [SiO4]4− and [AlO4]5− propagate to the outer layer through the cracks and pores, resulting in slower C–S–H and C–A–H development due to the restricted diffusion rate. The SEM (Fig. 7 (m, n, o, and p)) at 28 days of excitation shows an increase in the flocculated gel materials C–A–H, C–S–H, and AFt, as well as a decrease in the layered Ca(OH)2, which corresponds to a drop in the hydration layer and a shift in the rate control step.
SEM analysis of specimens after undergoing freeze–thaw cycles
The horizontal direction of Fig. 8 depicts the morphological changes in the surface microstructure of the stabilised soils following various FTs using the same FA and varying amounts of Ca(OH)2. As the frequency of FTs rises, the stabilised soils microstructure develops fissures. Figure 8 (a, b, c, and d) shows that the presence of C–S–H and C–A–H gels in the red circles resulted in a denser microstructure around the soil particles in the yellow circles of the stabilised soils. Ca(OH)2 and FA interacted to produce additional C–S–H and C–A–H gels, increasing the strength of stabilised soils. Combining the results in Figs. 6, 7, and 8 shows that the increase in UCS and splitting strength of stabilised soils, one in which Ca(OH)2 and FA act as the microaggregates, fills the internal voids of saline soils and increases the density of the microstructure, resulting in a packing effect. Second, the interaction of Ca(OH)2 with Na2SO4 produces CaSO4 bonded soil particles. Third, FA reacts with OH– to produce reaction products such as C–S–H and C–A–H gels, while directly or indirectly curing sulphate hence improving the mechanical qualities of stabilised soils.
Figure 8 (e, f, g, and h) illustrates the impact of various Ca(OH)2 contents on the mechanical properties of stabilised soils following 3 FTs. The results show that the structure of stabilised soils under the action of FTs was destroyed by cleavage in the area indicated by the red arrows, and integrity deteriorated to varying degrees, as did macroscopic UCS and splitting strength IR45. The stabilised soils with 3% Ca(OH)2 content were less damaged than L1, L2, and L4, indicating that they had the best mechanical qualities.
Figure 8 (b, f, and j) shows SEM images of L2 after varying numbers of FTs. Figure 8 shows that increasing the number of FTs causes partial separation of the gels. Furthermore, in Fig. 8 (f, j), microcracks were detected between the particles in the area indicated by the arrows, exposing the soil particles enclosed by the gels and resulting in an uneven particle surface5. Figure 8 (i, k, and l) A similar phenomenon was observed in L1, L3, and L4 stabilised soils after 20 FTs, with the emergence of gaps and voids resulting in a steady decline in the strength of the stabilised soils.
After adding Ca(OH)2 and FA to the saline soils, Ca(OH)2 and FA acted as microaggregates, filling the internal voids and increasing the bulk density of the saline soils’ microstructure. Moreover, Ca(OH)2 reacts with Na2SO4 to form slightly soluble CaSO4, which enhances the bonding between soil particles. In addition, FA reacted with OH– to form alkali activation products such as C–S–H, C–A–H, and N–A–S–H10, which directly or indirectly healed the sulphate and improved the mechanical qualities of saline soils.
FTIR analyses
The variation in the characteristic spectra of the FITR spectrum provides a more accurate indicator of the excited system’s excitation characteristics. Figure 9 shows the FTIR of calcium assisted FA excited gel materials within 1, 3, 7, and 28 days. The vibrational bands observed in all FTIR were identical and compatible with the C–S–H and C–A–H characteristic signals in Table 3. In Fig. 9a, absorption peaks of 1 day near 3445 cm–1 and 1650 cm–1 were the extensional motions of –OH of the bound water and the bending motions of the unbound water of the gel materials46,47, which were primarily from the C–S–H, C–A–H hydration products48,49 and the hydroxyl stretching vibrational bands in AFt50. The intensity of the molecular water characteristic peak grows dramatically as the Ca(OH)2 content increases. Figure 9a Absorption in the 1412 cm–1~1513 cm–1 region, exhibiting asymmetric stretching of O–C–O51, is associated with the interaction of soluble bases, such as Ca(OH)2, with CO2 to create carbonates10. Figure 9a At 1367 cm–1, there were C–S–H, C–A–H gels with asymmetric stretching vibrations of Si–O–Si(Al)52. The absorption peak at 1085 cm–1 indicates SO42–, which increased and then weakened with time, corresponding to the conversion of Na2SO4 to CaSO4 and finally to AFt53. Absorption peaks at 850 cm–1~1050 cm–1 indicate Si–O and Al–O bond vibrations in C–S–H, C–A–H gels54. The Si–O–T bond also represents the polymerization process; the lower the Si–O–T absorption, the greater the degree of polymerization and silica rich crosslinking55. Figure 9c shows the high-degree polymerization of C–S–H and C–A–H gels. Figure 9d The peak at 670 cm–1 is created by T-O asymmetric stretching vibrations, which migrate to the lower band over time as the number of aluminium tetrahedra in the gel structure increases56. The peak about 450 cm–1 corresponds to the Si–O in-plane bending vibrations in [SiO4]57.
TG–DSC analyses
Figure 10 shows the TG–DSC of calcium assisted FA geopolymer excitation for 28 days period. We can find that the decomposition process is divided into four decomposition temperature ranges: 20 °C–80 °C, 80 °C–280 °C, 350 °C–550 °C, and 550 °C–650 °C. The mass loss in the I–temperature region is related to pore water and was mainly due to C–S–H, C–A–H gels49, C–(A)–S–H65 and N–A–S–H gels41 dehydration. In the II–temperature region, the dehydroxylation reaction occurs, which was correlated with mass loss due to the removal of bound water from the hydrated gel materials66, and in agreement with the FTIR and XRD results. Ca(OH)2 boosted weight loss in the II–temperature zone, indicating that it encouraged the production of C–S–H and C–A–H gels. Combined with SEM, FTIR, and XRD analyses, the weight loss at 20℃–280℃ was attributed to gel decomposition such as C–S–H and C–A–H produced by the excitation of FA by NaOH and Ca(OH)2. In the III–temperature region, continuous warming causes exothermic carbon material in FA; however, the FA loss on burnout was 4.32%, and the calculated loss on burnout as a percentage of total mass for L1-L4 should be 4.01%, 3.74%, 3.51%, and 3.30%, respectively, according to the proportion of Ca(OH)2 added; thus, the excess mass loss was caused by the decomposition related to of Ca(OH)267, which accounts for 2.04%, 1.62%, 1.72%, and 1.09%, respectively. The IV–temperature zone is associated with carbonate breakdown, and the presence of carbonates is consistent with the XRD and FTIR results, and their size increases with increasing Ca(OH)2 content.
Discussion
The XRD, SEM, and FTIR data demonstrated that the Si–O and Al–O bonds were broken in an alkaline environment, and the SiO2 diffraction peaks in FA were weakened, resulting in C–A–H and C–S–H gel layers68. While Ca(OH)2 decreasing with increasing time, C–S–H and C–A–H gels increased13, and subsequent generation of gels covered AFt, FA (Fig. 7 (i, j, k, and l)). The hydration layer limits the migration of OH–, Ca2+, [AlO4]5−, and [SiO4]4−, and the conversion of the gels’ outer layer to AFt with sulphate was accelerated, allowing OH– to penetrate through the hydration layer and react with FA.
The macroscopic mechanical properties of stabilised soil are connected to the hydration products of Ca(OH)2 assisted FA, with higher Ca(OH)2 levels resulting in more hydration products. The FA hydration procedure enhances the UCS, splitting strength, and frost resistance of stabilised soil by reacting with sulphates and filling saline soil voids. Ca(OH)2 interacts with Na2SO4, NaOH, and CaSO4, resulting in increased FA solubility, [SiO4]4−, [AlO4]3− monomer release, and gels formation22. When Ca(OH)2 quantities reach 3%, the strength begins to decline, as strength is connected not only to hydration products but also to the degree of polymerization of the gel material. The increase in alkaline materials leads to an increase in pH, which hinders further polymerization69, as evidenced by the faster rate of mass loss of L3 and L4 at 100 °C–280 °C.
Saline soils allow free water to travel and accumulate by capillary action. Following a temperature drop, the internal free water of the stabilised soils specimens had a phase shift and increased in volume by approximately 9%70, resulting in expansion strains. This expansion stress can readily cause stress concentrations in interaction with the soils, resulting in huge pressure and fissures. As the temperature rises, the ice crystals gradually melt and the stress dissipates, shattering the stabilised soils structure71. Under the constant action of freeze–thaw cycles, the early interior structure ruptures and the strength rapidly decreases.
Calcium assisted FA excited coagulation material increases the strength of stabilised soils by attaching the particles, and the volume expansion of free water reduces the damaging impact of high pressure and enhances frost resistance. Free water content is also important for stabilised soils’ frost tolerance. TG discovered that the higher the Ca(OH)2 concentration, the bigger the first and second mass loss peaks of the gelling material, implying that there was more bound water in the gel materials. The gel components restrict movement of free water in stabilised soils, hence boosting frost resistance. The unreacted Ca(OH)2 and CaSO4 during freeze–thaw cycle acted as microsoluble bonding substances and rebonded some of the particles after FTs, resulting in a slower decrease in strength. When Ca(OH)2 is converted to CaCO3, it also improves the strength of stabilised soils, so that stabilised soils with high Ca(OH)2 content have the most and richest gel material, making the IR of stabilised soils decrease slower. After several freeze–thaw cycles, the pores in the stabilised soil will enlarge enough to support the volume expansion of ice crystals. As a result, the stress concentration between the stabilised soils will be greatly reduced, and the stabilised soils will retain their high strength following the fast phase.
Conclusions
In order to investigate the effect of calcium content on the properties of FA stabilised soils, alkaline solutions and various Ca(OH)2 contents were used to prepare FA calcium containing geopolymers. The following conclusions were drawn from the study of frost resistance and microstructural changes in stabilised soils after different number of FTs:
-
(1)
Ca(OH)2 combines with Na2SO4 to form CaSO4 with NaOH, and NaOH activates the FA reaction, resulting in a geopolymer containing a large number of C–S–H, C–A–H, and N–A–S–H gels that reacts with cured sulphate to generate AFt. The XRD, SEM, FTIR, and TG of FA and Ca(OH)2 stabilised sulphate geopolymers show no significant changes, yet there are differences that confirm the experimental results.
-
(2)
Different Ca(OH)2 contents have a stronger impact on the UCS and splitting strength of FA stabilised soils. With a constant FA concentration, the optimal ratio was found to be 3% Ca(OH)2. SEM revealed that gels bonding and physical filling improved the integrity of the stabilised soils, increasing the UCS and splitting strength, and that the microstructure of the stabilised soils with a 3% Ca(OH)2 content was the most dense and homogeneous.
-
(3)
The FTs weakens stabilised saline soils and damages the bonding substance between soil particles. The SEM results reveal that as the number of FTs increases, the agglomeration structure of stabilised soils gradually develops micro-cracks while maintaining high integrity, and Ca(OH)2 assisted excitation can effectively improve stabilised soils’ frost resistance.
-
(4)
The mechanical characteristics of geopolymers can be improved by adding moderate amounts of Ca(OH)2. Geopolymers such as C–A–H and C–S–H were generated in alkali activated matrices, although high calcium content can result in poor mechanical and durability qualities.
The results of this research will add to our understanding of alkali activated systems and widen their applications in FA geopolymer system. More importantly, the results of this study show that using the geopolymer system improves the freeze–thaw resistance of stabilised soils. Research indicates that the calcium assisted to FA mixing ratio and FTs have a significant impact on the microstructure of saline soils, with an appropriate ratio result in higher mechanical characteristics. However, in the field of chemical curing of saline soils in the seasonally frozen zone, curing time, water content, saline type, and salinity are among the factors influencing the curing effect that were not investigated in this report and will hopefully be investigated in future work.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Zhang, Y., Tian, R.-Z. & Wang, T.-L. Study on salt expansion mechanism of subgrade-culvert transition section in saline soils and cold regions. Cold Regions Sci. Technol. 205, 103701. https://doi.org/10.1016/j.coldregions.2022.103701 (2023).
Zhang, S., Zhang, J., Gui, Y., Chen, W. & Dai, Z. Deformation properties of coarse-grained sulfate saline soil under the freeze-thaw-precipitation cycle. Cold Regions Sci. Technol. 177, 103121. https://doi.org/10.1016/j.coldregions.2020.103121 (2020).
Li, H., Li, S., Kang, X., Wu, L. & Ding, Y. Understanding unsaturated sulfate saline soil in cold regions: A comprehensive hydraulic–thermal–air–salt–mechanical model with experimental research. Cold Regions Sci. Technol. 215, 103970. https://doi.org/10.1016/j.coldregions.2023.103970 (2023).
Hewage, S. A., Tang, C.-S., Mehta, Y. & Zhu, C. Investigating cracking behavior of saline clayey soil under cyclic freezing-thawing effects. Eng. Geol. 326, 107319. https://doi.org/10.1016/j.enggeo.2023.107319 (2023).
Wan, X. et al. Experimental study on pore water phase transition in saline soils. Cold Regions Sci. Technol. 203, 103661. https://doi.org/10.1016/j.coldregions.2022.103661 (2022).
Yang, J. et al. Influence of anionic polyacrylamide on the freeze–thaw resistance of silty clay. Cold Regions Sci. Technol. 219, 104111. https://doi.org/10.1016/j.coldregions.2023.104111 (2024).
Liu, B. et al. Study on electrical properties of saline frozen soil and influence mechanism of unfrozen water content. Cold Regions Sci. Technol. 220, 104146. https://doi.org/10.1016/j.coldregions.2024.104146 (2024).
Lai, Y., Wu, D. & Zhang, M. Crystallization deformation of a saline soil during freezing and thawing processes. Appl. Therm. Eng. 120, 463–473. https://doi.org/10.1016/j.applthermaleng.2017.04.011 (2017).
Ding, S. et al. Changing of mechanical property and bearing capacity of strongly chlorine saline soil under freeze-thaw cycles. Sci. Rep. 14, 6203. https://doi.org/10.1038/s41598-024-56822-8 (2024).
Li, H. et al. Characterization and mechanism study of sulfate saline soil solidification in seasonal frozen regions using ternary solid waste-cement synergy. Constr. Build. Mater. 427, 136263. https://doi.org/10.1016/j.conbuildmat.2024.136263 (2024).
Shu, H., Yu, Q., Niu, C., Sun, D. & Wang, Q. The coupling effects of wet-dry and freeze–thaw cycles on the mechanical properties of saline soil synergistically solidified with sulfur-free lignin, basalt fiber and hydrophobic polymer. Catena 238, 107832. https://doi.org/10.1016/j.catena.2024.107832 (2024).
Liu, Z. et al. Study on the changing pattern of the salt-freeze swelling force of sulfate saline soil containing sodium chloride under variable temperature environment. Phys. Chem. Earth A/B/C 129, 103365. https://doi.org/10.1016/j.pce.2023.103365 (2023).
Sun, B., Ye, G. & de Schutter, G. A review: Reaction mechanism and strength of slag and fly ash-based alkali-activated materials. Constr. Build. Mater. 326, 126843. https://doi.org/10.1016/j.conbuildmat.2022.126843 (2022).
Zhao, J. et al. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J. Clean. Prod. https://doi.org/10.1016/j.jclepro.2021.127085 (2021).
Li, H. et al. Characteristics of carbide-slag-activated GGBS–fly ash materials: Strength, hydration mechanism, microstructure, and sustainability. Constr. Build. Mater. 422, 135796. https://doi.org/10.1016/j.conbuildmat.2024.135796 (2024).
Chen, K., Lin, W.-T. & Liu, W. Effect of NaOH concentration on properties and microstructure of a novel reactive ultra-fine fly ash geopolymer. Adv. Powder Technol. 32, 2929–2939. https://doi.org/10.1016/j.apt.2021.06.008 (2021).
Ren, C., Wang, J., Duan, K., Li, X. & Wang, D. Effects of steel slag on the hydration process of solid waste-based cementitious materials. Materials 17, 1999. https://doi.org/10.3390/ma17091999 (2024).
Li, N., Shi, C. & Zhang, Z. Understanding the roles of activators towards setting and hardening control of alkali-activated slag cement. Compos. B Eng. 171, 34–45. https://doi.org/10.1016/j.compositesb.2019.04.024 (2019).
Chen, X., Niu, Z., Wang, J., Zhu, G. R. & Zhou, M. Effect of sodium polyacrylate on mechanical properties and microstructure of metakaolin-based geopolymer with different SiO2/Al2O3 ratio. Ceram. Int. 44, 18173–18180. https://doi.org/10.1016/j.ceramint.2018.07.025 (2018).
Pachla, E. C., Silva, D. B., Stein, K. J., Marangon, E. & Chong, W. Sustainable application of rice husk and rice straw in cellular concrete composites. Constr. Build. Mater. 283, 122770. https://doi.org/10.1016/j.conbuildmat.2021.122770 (2021).
Nayak, D. K., Abhilash, P. P., Singh, R., Kumar, R. & Kumar, V. Fly ash for sustainable construction: A review of fly ash concrete and its beneficial use case studies. Clean. Mater. 6, 100143. https://doi.org/10.1016/j.clema.2022.100143 (2022).
Taghvayi, H., Behfarnia, K. & Khalili, M. The effect of alkali concentration and sodium silicate modulus on the properties of alkali-activated slag concrete. J. Adv. Concr. Technol. 16, 293–305. https://doi.org/10.3151/jact.16.293 (2018).
Zhou, H., Wang, X., Wu, Y. & Zhang, X. Mechanical properties and micro-mechanisms of marine soft soil stabilized by different calcium content precursors based geopolymers. Constr. Build. Mater. 305, 124722. https://doi.org/10.1016/j.conbuildmat.2021.124722 (2021).
Ismail, I. et al. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cement Concr. Compos. 45, 125–135. https://doi.org/10.1016/j.cemconcomp.2013.09.006 (2014).
Garcia-Lodeiro, I., Palomo, A., Fernández-Jiménez, A. & Macphee, D. E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cement Concrete Res. 41, 923–931. https://doi.org/10.1016/j.cemconres.2011.05.006 (2011).
Walkley, B. et al. Phase evolution of C-(N)-A-S-H/N-A-S-H gel blends investigated via alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 89, 120–135. https://doi.org/10.1016/j.cemconres.2016.08.010 (2016).
Xia, W. et al. Experimental investigation of the erodibility of soda saline-alkali soil under freeze-thaw cycle from a microscopic view. Catena 232, 107430. https://doi.org/10.1016/j.catena.2023.107430 (2023).
Palomo, Á., Alonso, S., Fernandez-Jiménez, A., Sobrados, I. & Sanz, J. Alkaline Activation of Fly Ashes: NMR Study of the Reaction Products. J. Am. Ceram. Soc. 87, 1141–1145. https://doi.org/10.1111/j.1551-2916.2004.01141.x (2008).
Wilson, W., Sorelli, L. & Tagnit-Hamou, A. Unveiling micro-chemo-mechanical properties of C–(A)–S–H and other phases in blended-cement pastes. Cem. Concr. Res. 107, 317–336. https://doi.org/10.1016/j.cemconres.2018.02.010 (2018).
Lei, B. et al. The hydration mechanisms of co-stabilization saline soils by using multiple solid wastes. Processes 11, 2679. https://doi.org/10.3390/pr11092679 (2023).
Wang, T. et al. Retardation effect of the pozzolanic reaction of low-calcium supplementary cementitious materials on clinker hydration at later age: Effects of pore solution, foreign ions, and pH. Cement Concrete Res. 177, 107416. https://doi.org/10.1016/j.cemconres.2023.107416 (2024).
Hamid, W. & Alnuaim, A. Evaluation of the durability and strength of stabilized sabkha soil with geopolymer. Case Stud. Constr. Mater. 18, e02051. https://doi.org/10.1016/j.cscm.2023.e02051 (2023).
Lv, Q., Jiang, L., Ma, B., Zhao, B. & Huo, Z. A study on the effect of the salt content on the solidification of sulfate saline soil solidified with an alkali-activated geopolymer. Constr. Build. Mater. 176, 68–74. https://doi.org/10.1016/j.conbuildmat.2018.05.013 (2018).
Oh, J. E., Moon, J., Oh, S.-G., Clark, S. M. & Monteiro, P. J. M. Microstructural and compositional change of NaOH-activated high calcium fly ash by incorporating Na-aluminate and co-existence of geopolymeric gel and C-S–H(I). Cem. Concr. Res. 42, 673–685. https://doi.org/10.1016/j.cemconres.2012.02.002 (2012).
Palomo, A., Fernández-Jiménez, A., Kovalchuk, G., Ordoñez, L. M. & Naranjo, M. C. Opc-fly ash cementitious systems: Study of gel binders produced during alkaline hydration. J. Mater. Sci. 42, 2958–2966. https://doi.org/10.1007/s10853-006-0585-7 (2007).
van Deventer, J. S. J., Provis, J. L., Duxson, P. & Lukey, G. C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 139, 506–513. https://doi.org/10.1016/j.jhazmat.2006.02.044 (2007).
Temuujin, J., van Riessen, A. & Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 167, 82–88. https://doi.org/10.1016/j.jhazmat.2008.12.121 (2009).
Zhao, X. et al. Investigation into the effect of calcium on the existence form of geopolymerized gel product of fly ash based geopolymers. Cement Concr. Compos. 103, 279–292. https://doi.org/10.1016/j.cemconcomp.2018.11.019 (2019).
Duan, P., Yan, C. & Zhou, W. Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cement Concr. Compos. 78, 108–119. https://doi.org/10.1016/j.cemconcomp.2017.01.009 (2017).
Maddalena, R., Roberts, J. J. & Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 186, 933–942. https://doi.org/10.1016/j.jclepro.2018.02.138 (2018).
Yang, J. et al. Sustainable clinker-free solid waste binder produced from wet-ground granulated blast-furnace slag, phosphogypsum and carbide slag. Constr. Build. Mater. 330, 127218. https://doi.org/10.1016/j.conbuildmat.2022.127218 (2022).
Xue, Z. et al. Analysis of compressive strength, durability properties, and micromechanisms of solidified loess using industrial solid waste: Slag–white mud–calcium carbide residue. J. Build. Eng. 84, 108511. https://doi.org/10.1016/j.jobe.2024.108511 (2024).
Wang, R. et al. Study on the design method of multi-component industrial solid waste low carbon cementitious material with cement as the activator. Case Stud. Constr. Mater. 21, e03478. https://doi.org/10.1016/j.cscm.2024.e03478 (2024).
Su, C., Zhang, J. & Ding, Y. Research on reactivity evaluation and micro-mechanism of various solid waste powders for alkali-activated cementitious materials. Constr. Build. Mater. 411, 134374. https://doi.org/10.1016/j.conbuildmat.2023.134374 (2024).
Han, Y. et al. Effect of freeze-thaw cycles on shear strength of saline soil. Cold Reg. Sci. Technol. 154, 42–53. https://doi.org/10.1016/j.coldregions.2018.06.002 (2018).
Tchadjié, L. N. et al. Potential of using granite waste as raw material for geopolymer synthesis. Ceram. Int. 42, 3046–3055. https://doi.org/10.1016/j.ceramint.2015.10.091 (2016).
Yang, G. et al. Effect of carbide slag and steel slag as alkali activators on the key properties of carbide slag-steel slag-slag-phosphogypsum composite cementitious materials. Front. Mater. https://doi.org/10.3389/fmats.2024.1353004 (2024).
Rahmati, M. & Toufigh, V. Evaluation of geopolymer concrete at high temperatures: An experimental study using machine learning. J. Clean. Prod. 372, 133608. https://doi.org/10.1016/j.jclepro.2022.133608 (2022).
Chen, S., Lu, P., Bie, Y., Wang, L. & Guo, L. Mechanical properties and micro mechanism of alkali-activated tannery sludge/fly ash composite cement-based recycled concrete. Constr. Build. Mater. 391, 131813. https://doi.org/10.1016/j.conbuildmat.2023.131813 (2023).
Djouani, F., Connan, C., Delamar, M., Chehimi, M. M. & Benzarti, K. Cement paste–epoxy adhesive interactions. Constr. Build. Mater. 25, 411–423. https://doi.org/10.1016/j.conbuildmat.2010.02.035 (2011).
Kapeluszna, E., Kotwica, Ł, Różycka, A. & Gołek, Ł. Incorporation of Al in C-A-S-H gels with various Ca/Si and Al/Si ratio: Microstructural and structural characteristics with DTA/TG, XRD, FTIR and TEM analysis. Constr. Build. Mater. 155, 643–653. https://doi.org/10.1016/j.conbuildmat.2017.08.091 (2017).
Yan, S. et al. Effects of graphene oxide on the geopolymerization mechanism determined by quenching the reaction at intermediate states. RSC Adv. 7, 13498–13508. https://doi.org/10.1039/c6ra26340b (2017).
Han, Y., Xia, J., Chang, H. & Xu, J. The influence mechanism of ettringite crystals and microstructure characteristics on the strength of calcium-based stabilized soil. Materials 14, 1359. https://doi.org/10.3390/ma14061359 (2021).
Robayo-Salazar, R. A., Mejía de Gutiérrez, R. & Puertas, F. Effect of metakaolin on natural volcanic pozzolan-based geopolymer cement. Appl. Clay Sci. 132–133, 491–497. https://doi.org/10.1016/j.clay.2016.07.020 (2016).
Wang, J. et al. Effect of Ca/Si and Al/Si on micromechanical properties of C(-A)-S-H. Cement Concrete Res. 157, 106811. https://doi.org/10.1016/j.cemconres.2022.106811 (2022).
Zhang, H., Chai, W. & Cao, Y. Flotation separation of quartz from gypsum using benzyl quaternary ammonium salt as collector. Applied Surface Science 576, 15183. https://doi.org/10.1016/j.apsusc.2021.151834 (2022).
Aboulayt, A. et al. Alkali-activated grouts based on slag-fly ash mixtures: From early-age characterization to long-term phase composition. Constr. Build. Mater. 260, 120510. https://doi.org/10.1016/j.conbuildmat.2020.120510 (2020).
Aboulayt, A. et al. Stability of a new geopolymer grout: Rheological and mechanical performances of metakaolin-fly ash binary mixtures. Constr. Build. Mater. 181, 420–436. https://doi.org/10.1016/j.conbuildmat.2018.06.025 (2018).
Ismail, I., Bernal, S. A., Provis, J. L., Hamdan, S. & van Deventer, J. S. J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 46, 361–373. https://doi.org/10.1617/s11527-012-9906-2 (2012).
Yu, P., Kirkpatrick, R. J., Poe, B., McMillan, P. F. & Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 82, 742–748. https://doi.org/10.1111/j.1151-2916.1999.tb01826.x (2004).
Mollah, M., Kesmez, M. & Cocke, D. An X-ray diffraction (XRD) and Fourier transform infrared spectroscopic (FT-IR) investigation of the long-term effect on the solidification/stabilization (S/S) of arsenic(V) in Portland cement type-V. Sci. Total Environ. 325, 255–262. https://doi.org/10.1016/j.scitotenv.2003.09.012 (2004).
Aboulayt, A. et al. Properties of metakaolin based geopolymer incorporating calcium carbonate. Adv. Powder Technol. 28, 2393–2401. https://doi.org/10.1016/j.apt.2017.06.022 (2017).
García Lodeiro, I., Macphee, D. E., Palomo, A. & Fernández-Jiménez, A. Effect of alkalis on fresh C-S–H gels. FTIR analysis. Cement Concrete Res. 39, 147–153. https://doi.org/10.1016/j.cemconres.2009.01.003 (2009).
Criado, M., Fernández-Jiménez, A. & Palomo, A. Alkali activation of fly ash: Effect of the SiO2/Na2O ratio. Microporous Mesoporous Mater. 106, 180–191. https://doi.org/10.1016/j.micromeso.2007.02.055 (2007).
He, S., Li, Y., Yu, P. & Zhou, Y. Effect of lime mud under wet grinding on the compressive strength and hydration of cement mortar. Cement Concrete Compos. 140, 105067. https://doi.org/10.1016/j.cemconcomp.2023.105067 (2023).
Zhang, Y., Zhang, J., Jiang, J., Hou, D. & Zhang, J. The effect of water molecules on the structure, dynamics, and mechanical properties of sodium aluminosilicate hydrate (NASH) gel: A molecular dynamics study. Constr. Build. Mater. 193, 491–500. https://doi.org/10.1016/j.conbuildmat.2018.10.221 (2018).
Zhang, J. et al. Effects of fly ash on MgO-based shrinkage-compensating cement: Microstructure and properties. Constr. Build. Mater. 339, 127648. https://doi.org/10.1016/j.conbuildmat.2022.127648 (2022).
Zimar, Z. et al. Application of coal fly ash in pavement subgrade stabilisation: A review. J. Environ. Manag. 312, 114926. https://doi.org/10.1016/j.jenvman.2022.114926 (2022).
Ma, H. et al. Effect of shrinkage reducing admixture on drying shrinkage and durability of alkali-activated coal gangue-slag material. Constr. Build. Mater. 270, 121372. https://doi.org/10.1016/j.conbuildmat.2020.121372 (2021).
Chen, Q., Ghimire, B., Su, L. & Liu, Y. Micro-scale investigations on the mechanical properties of expansive soil subjected to freeze-thaw cycles. Cold Regions Sci. Technol. 219, 104128. https://doi.org/10.1016/j.coldregions.2024.104128 (2024).
Li, Q., Dang, B., Li, D. & Hu, X. Strength deterioration of earthen sites loess solidified by calcined ginger nuts under dry-wet and freeze-thaw cycles. Atmosphere 14, 868. https://doi.org/10.3390/atmos14050868 (2023).
Funding
This research was funded by the Major Science and Technology Project of the Xinjiang Production and Construction Corps Science and Technology Bureau [No. 2024AA007], the Scientific and Technological Research Programs in key Areas of Xinjiang Production and Construction Corps Science and technology Bureau [No. 2023AB013-01], the Science and Technology Development Plan Project of the Innovation-driven Development Experimental Zone of the Silk Road Economic Belt and the National Independent Innovation Demonstration Zone of Urumqi-Changji-Shihezi [No.2023LQ03002], the Major Science and Technology Special Projects in Xinjiang Uygur Autonomous Region [No.2023A03004-04], the Desert Sand Composite Industrial Solid Waste Green Low Carbon Engineered Materials Innovation Team [No.20243125985] and the Xinjiang Uygur Autonomous Region Science and Technology Department [No.2023B03011-3].
Author information
Authors and Affiliations
Contributions
Sining Li: Writing – review & editing, Writing – original draft. Yong Huang: Resources, Funding acquisition, Writing – review.. Qiushuang Cui: Visualization, Data curation. Ruyun Bai: Formal analysis, Data curation. Huan Li: Investigation, Formal analysis, Data curation. Liran Jiao: Formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, S., Huang, Y., Cui, Q. et al. Effect of calcium content on geopolymer consolidation of saline soils in the seasonally frozen zone. Sci Rep 15, 16168 (2025). https://doi.org/10.1038/s41598-025-00307-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-00307-9