Abstract
This study compares recurrence-free survival (RFS) and overall survival (OS) in patients with non-clear cell (nccRCC) and clear cell renal cell carcinoma (ccRCC) undergoing surgical nephrectomy with thrombectomy (SNTx) for RCC with venous thrombus. Data from patients who underwent SNTx at two tertiary centers (June 1990–December 2022) were retrospectively reviewed. Patients were grouped as ccRCC or nccRCC and stratified by metastasis status at surgery. Primary endpoints were RFS and OS for metastasis-naive RCC and OS for the entire cohort, including both metastasis-naive and metastatic RCC. Kaplan–Meier analysis with log-rank tests and adjusted multivariable Cox proportional hazards models were performed, with TN adjustments for the metastasis-naive group and TNM adjustments for the entire population. Among 604 patients, 504 (83.5%) were ccRCC. In nccRCC, 44 (44.0%) were papillary, 17 (17.0%) were chromophobe, and 39 (39.0%) were rare subtypes, most commonly TFE3 rearranged RCC, followed by the RCC not otherwise specified subtype (according to the 2022 World Health Organization Classification of RCC). Median OS was 85.8 months for ccRCC, 37.7 for papillary, 90.2 for chromophobe, and 16.9 for rare subtypes. Rare RCC histology was significantly associated with worse RFS (HR 1.63, p = 0.038) and OS (HR 1.82, p = 0.039) in metastasis-naive RCC. For the entire cohort including metastatic diseases, rare subtypes had worse OS (HR 2.20, p < 0.001), while other nccRCC subtypes did not differ significantly from ccRCC in OS. In patients with RCC with venous thrombosis, rare nccRCC subtypes exhibited poorer survival outcomes, even after adjustment for TN(M) stage.
Similar content being viewed by others
Introduction
While it is well known that clear cell renal cell carcinoma (ccRCC) patients often have worse prognosis compared to non-clear cell renal cell carcinoma (nccRCC) patients in the localized disease, an interesting reversal occurs in the metastatic situation, where nccRCC patients tend to have lower survival rates1,2,3. This disparity is hypothesized to result from several factors. In metastatic disease, therapeutic development has predominantly targeted ccRCC, potentially limiting the efficacy of treatments for nccRCC3,4. Furthermore, nccRCC represents a heterogeneous group of histological subtypes, each with varying prognostic potentials5,6, and the proportion of the nccRCC component might differ by stage. In summary, tumor stage information may be the most important factor in prognosis, and certain worse prognostic subtypes should be intensively followed up to improve outcomes for nccRCC patients.
Up to 10% of RCC cases, tumor thrombus involvement of the renal vein and/or inferior vena cava is observed7,8. Standard treatment for RCC with thrombus is radical nephrectomy and/or followed by systemic adjuvant therapy9. The post-surgical survival of patients with RCC with thrombus varies, with 5-year survival rates ranging from 23 to 70%8. Various poor prognostic factors have been identified, such as lymph node invasion, tumor necrosis, invasion of the IVC wall, and concurrent metastasis10,11. Some nccRCC subtypes exhibit more aggressive behavior than ccRCC in RCC with thrombosis7,12. However, the impact of histology on survival in RCC patients with thrombosis has remained unclear.
This study aims to clarify the differences in recurrence-free survival (RFS) and overall survival (OS) between patients with ccRCC and those with nccRCC who had venous thrombosis and underwent both surgical nephrectomy with thrombectomy (SNTx), using data from two high-volume tertiary referral centers. We included not only the more common subtypes of nccRCC but also other non-clear cell RCC variants, or rare subtypes, which are often more aggressive. We conducted a comprehensive analysis covering both metastasis-naive and metastatic patient populations.
Materials and methods
Ethic statement
This study was approved by the Institutional Review Board of Asan Medical Center and the requirement for patient informed consent was waived due to the retrospective nature of the study. The study processes were performed following relevant guidelines and regulations.
Study population
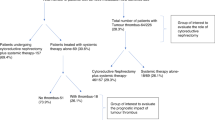
Medical records of patients who underwent surgical nephrectomy with thrombectomy at two tertiary centers from January 1990 to December 2022 were retrospectively reviewed. All procedures were performed using an open surgical approach. Patients with pathologic M1 disease were also included, and some of them underwent cytoreductive radical nephrectomy, rather than radical nephrectomy with curative intent. Patients diagnosed with single kidney, non-renal cell carcinoma, such as urothelial carcinoma or sarcoma, and RCC with bilateral involvement were excluded from the analysis. The demographic data collected included age and sex, along with pathological information such as histological subtypes (clear cell, papillary, chromophobe, and rare) and cancer stage. The pathologic subtypes were classified using 2022 World Health Organization (WHO) Classification of RCC13. The TNM stage was determined according to the 2017 AJCC TNM classification system14. RFS was defined as the time period following the surgical removal of renal cell carcinoma with thrombus, during which there were no signs of cancer recurrence. OS was defined as the duration from the surgical removal of RCC with thrombus until the patient’s death.
Statistical analysis
For the comparison, patients were divided into two groups: ccRCC and nccRCC. The nccRCC group included papillary (pRCC), chromophobe (chRCC), and rare (rRCC) subtypes. The Student’s t-test and chi-square analysis were used to compare these groups. To evaluate differences in RFS and OS between the ccRCC and nccRCC groups, the Kaplan–Meier method with log-rank tests was applied. This analysis was conducted separately for patients with metastasis-naive disease and for the entire cohort, which included both metastasis-naive and metastatic patients. For this study, the term ‘metastasis-naive’ was defined to encompass both M0 and Mx, with Mx indicating cases where clinical metastatic status was not assessed preoperatively.
Additionally, to assess the prognostic effect of pathological subtypes on oncologic outcomes, we used the TN(M) stage-adjusted multivariable Cox proportional hazards model. The results of the Cox regression were expressed in terms of hazard ratios (HR) and 95% confidence intervals. Statistical significance was determined with a p-value of less than 0.05. Notably, RFS was evaluated solely in the metastasis-naive RCC group, as it cannot be accurately assessed in the entire cohort due to the inclusion of metastatic patients. In contrast, OS was analyzed in both the metastasis-naive group and the entire cohort. All statistical analyses were conducted using the R project (version 4.1.1).
Results
Patient demographics
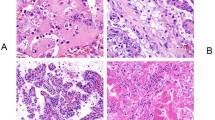
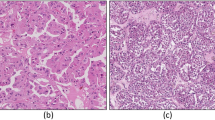
Of the 604 patients who underwent surgical nephrectomy with venous thrombectomy at two tertiary referral centers, 253 patients (42%) were enrolled from one center, while 351 patients (58%) were enrolled from the other. Among the nccRCC patients (n = 100), the majority were of the pRCC (n = 44, 44.0%) and chRCC (n = 17, 17.0%). The remaining 39 patients (39.0%) had rRCC, with TFE3-rearranged RCC (n = 15) and RCC not otherwise specified (RCC NOS, n = 16) being the two most common subtypes. (Fig. 1) A total of 503 (83.3%) patients were classified as metastasis-naive (including M0 and Mx), with 101 patients (16.7%) having pathologic M1 and 56 patients (9.3%) having Mx. Pathologic N1 was present in 92 patients (15.2%) and Nx in 349 patients (57.8%). The thrombus level was predominantly level I in 417 patients (69.0%) and level II in 126 patients (20.9%), according to the Mayo classification15.
Univariate analysis revealed that nccRCC showed statistically significant higher T stage (p < 0.001), thrombus level (p = 0.004), pathologic N stage (p = 0.006), and M stage (p < 0.001) than ccRCC, but not in age or sex (Table 1).
Non-parametric survival analysis: RFS and OS of the specific RCC subtypes
Figure 2, panel A represents the RFS of metastasis-naive RCC patients (n = 503) by RCC histology. Of the 435 patients with ccRCC, the median RFS was 32.3 months. In comparison, median RFS of the pRCC (n = 27), chRCC (n = 15) and rRCC (n = 26) groups were 9.3, 36.3 and 3.3 months respectively. In the Kaplan–Meier analysis for RFS, pRCC and rRCC showed worse outcomes compared to ccRCC (p < 0.001).
(A) Kaplan–Meier recurrence-free survival curves for metastasis-naive RCC patients by pathologic subtypes. (B) Kaplan–Meier overall survival curves for metastasis-naive RCC patients by pathologic subtypes. (C) Kaplan–Meier overall survival curves for the entire cohort including metastasis-naive and metastatic patients by pathologic subtypes.
Figure 2 panel B represents the OS of metastasis-naive RCC patients. In ccRCC, the median OS is 85.8 months. In comparison, median OS of the pRCC, chRCC, and rRCC groups were 37.7, 90.2 and 16.9 months respectively. Consistent with RFS trends, the OS of the pRCC and rRCC were worse than that of ccRCC (p < 0.001).
Figure 2, Panel C shows the OS of the entire cohort (n = 604). For ccRCC (n = 504), the median OS was 76.2 months. The median OS for pRCC patients (n = 44) was 67.8 months, and for chRCC (n = 17), it was 66.3 months. Among all subtypes, patients with rRCC (n = 39) exhibited a significantly lower median OS of 13.0 months (p < 0.001).
Adjusted multivariate Cox-proportional hazard regression for RFS and OS
In Table 2, a TN-adjusted Cox proportional hazards model for metastasis-naive disease showed that rRCC subtypes were significantly associated with worse RFS (HR 1.63, 95% CI 1.03–2.60, p = 0.038) and OS (HR 1.82, 95% CI 1.03–3.20, p = 0.039) compared to ccRCC. The pRCC and chRCC subtypes did not show a significant association with oncological outcomes after adjusting for covariates by TN stage.
Table 3 represents a TNM-adjusted Cox proportional hazards model for the entire cohort. rRCC subtypes had a significantly higher risk of overall death compared to ccRCC (HR 2.20, 95% CI 1.42–3.40, p < 0.001). However, pRCC and chRCC subtypes did not show a worse OS than ccRCC.
Discussion
The prognostic value of RCC subtypes in the context of venous thrombosis remains poorly investigated. In this venous thrombosis RCC cohort, nccRCC cases were more likely to present with advanced TNM stage at diagnosis (Table 1), consistent in prior findings7,8. However, after adjustment for TN(M) stage, the majority of nccRCC subtypes, pRCC and chRCC, did not show worse RFS and OS compared to ccRCC. While pRCC has been reported with worse survival outcomes in cases with venous thrombosis7, our study observed this disparity only in unadjusted analyses, which resolved after TNM stage adjustment. This suggests that the poorer prognosis observed in nccRCC is largely attributable to higher TNM stage at presentation rather than intrinsic subtype-specific characteristics. Also, these findings suggest that pRCC and chRCC may align with those for ccRCC, as no survival differences were observed after TNM stage adjustment16,17. In contrast, the poor survival outcomes of rRCC, even after adjusting for TNM staging, highlight the need for therapeutic strategies specifically tailored to its unique biology.
rRCC, which comprises only about 2–6% of all RCCs, encompasses a mix of high-grade histologic features and variable clinical behaviors, making it a particularly formidable challenge in RCC management18. Although the total proportion of rRCC represents only a small fraction of all RCC subtypes, in our dataset, almost half of the nccRCC was rRCC, as reported in a previous study7. This suggests that in the context of advanced RCC, rRCC may be more prevalent than our current conception.
In this study, rRCC were associated with a significantly worse OS, both in metastasis-naive and metastatic cases. The aggressive nature of rRCC underscores the need for treatment strategies beyond ccRCC paradigms18,19. Despite advancements in targeted and immunotherapies, outcomes for rRCC remain poor, highlighting the limitations of treatments extrapolated from ccRCC managements4,20,21. Even pembrolizumab, an anti–programmed death 1 (PD-1) antibody renowned for effective adjuvant treatment for RCC since 2021, has been studied for locally advanced ccRCC primarily22,23. rRCC remain underrepresented in clinical trials due to their low prevalence and molecular complexity, contributing to significant knowledge gaps and suboptimal care1,4,22.
Advances in molecular profiling, acknowledged in the updated WHO RCC classification, emphasize tailored treatments to specific cancer entities13,24. In our cohort, identifying TFEB and TFE3 translocations helped distinguish rRCC into subtypes with different prognosis. TFEB translocations were associated with better survival outcomes due to their generally indolent behavior, whereas TFE3 translocations, found in 38% of our rRCC cases (n = 15), exhibited a relatively more aggressive clinical course25,26. This high proportion of TFE3 translocations may have impacted the poor survival outcomes observed in the rRCC group. Likewise, the molecular reclassification may further uncover subgroups with distinct prognostic and therapeutic profiles.
This study’s retrospective nature and the extensive temporal span of data collection, from 1990 to 2022, present several limitations. Over these three decades, advancements in surgical techniques and pharmacological treatments, including the introduction of modern agents like pembrolizumab, have not been uniformly reflected across the data set22,23. This variability complicates the interpretation of survival outcomes, particularly overall survival (OS), which is heavily influenced by the era-specific medical interventions. Similarly, the variability in patient treatment regimens and post surgical follow-up care, and the lack of detailed data on the timing and type of treatments following recurrence, impede precise evaluation of RFS and OS outcomes.
Furthermore, due to data collection limitations, our analysis did not account for potential prognostic factors such as patient age, performance status, tumor size, sarcomatoid changes, presence of necrosis, and surgical resection margin status. While we deliberately focused on examining associations between pathological subtypes and oncological outcomes, inclusion of these variables as baseline characteristics and their incorporation into both univariate and multivariate analyses would have strengthened our statistical approach and controlled for potential confounding effects.
Moreover, the classification of RCC subtypes in this study was based on the diagnostic standards at the time of surgery. This raises the possibility that some cases categorized as ‘rare’ might be classified using contemporary classification systems; however, our study could not confirm the pathology with the latest standards set by molecular pathologists. Reassessing past cases with modern classifications could refine the subtype classification and associated survival outcomes.
Lastly, the analysis is limited by its focus on data from only two tertiary centers, which may not represent the broader demographic and clinical variations seen in a wider healthcare setting. Although collected over a vast timespan, the relatively small sample sizes for nccRCC subgroups could have restricted the statistical power of the findings to detect subtle differences in treatment effects.
Conclusion
In patients with RCC and venous thrombosis, rare nccRCC subtypes exhibit markedly worse RFS and OS, even after adjustment for tumor stage. While other nccRCC subtypes, such as papillary and chromophobe RCC, did not significantly differ in survival outcomes compared to ccRCC, rare nccRCC demonstrated significantly poorer outcomes. These findings highlight the need for further investigation and targeted treatment strategies for these aggressive subtypes.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to patient confidentiality and institutional guidelines but can be made available from the corresponding author on reasonable request, with appropriate permissions from the participating institutions’ review boards.
Change history
05 December 2025
The original online version of this Article was revised: The Acknowledgements section was incomplete. The correct information now accompanies the original Article.
References
De Velasco, G. et al. Comprehensive analysis of survival outcomes in non-clear cell renal cell carcinoma patients treated in clinical trials. Clin. Genitourin. Cancer 15, 652-660.e1 (2017).
Rosiello, G. et al. Comparison of survival outcomes in patients with metastatic papillary vs. clear-cell renal cell carcinoma: A propensity-score analysis. World J. Urol. 39, 461–472 (2021).
Vera-Badillo, F. E. et al. Systemic therapy for non-clear cell renal cell carcinomas: A systematic review and meta-analysis. Eur. Urol. 67, 740–749 (2015).
Sepe, P. et al. Characteristics and treatment challenges of non-clear cell renal cell carcinoma. Cancers 13, 3807 (2021).
Monda, S. M. et al. The metastatic risk of renal cell carcinoma by primary tumor size and subtype. Eur. Urol. Open Sci. 52, 137–144 (2023).
Adashek, J. J., Genovese, G., Tannir, N. M. & Msaouel, P. Recent advancements in the treatment of metastatic clear cell renal cell carcinoma: A review of the evidence using second-generation p-values. Cancer Treat. Res. Commun. 23, 100166 (2020).
Rabinowitz, M. J. et al. Characterizing tumor thrombus arising from non-clear cell renal cell carcinoma. Eur. Urol. Open Sci. 43, 28–34 (2022).
Hatakeyama, S. et al. Prognostic benefit of surgical management in renal cell carcinoma patients with thrombus extending to the renal vein and inferior vena cava: 17-year experience at a single center. BMC Urol 13, 47 (2013).
Bukavina, L. et al. Epidemiology of renal cell carcinoma: 2022 Update. Eur. Urol. 82, 529–542 (2022).
Cao, C. et al. Long-term survival and prognostic factors for locally advanced renal cell carcinoma with renal vein tumor thrombus. BMC Cancer 19, 144 (2019).
Chen, Z. et al. Outcomes of renal cell carcinoma with associated venous tumor thrombus: Experience from a large cohort and short time span in a single center. BMC Cancer 21, 766 (2021).
Kaushik, D. et al. The impact of histology on clinicopathologic outcomes for patients with renal cell carcinoma and venous tumor thrombus: A matched cohort analysis. Urology 82, 136–141 (2013).
Moch, H. et al. The 2022 World Health Organization classification of tumours of the urinary system and male genital organs—Part A: Renal, penile, and testicular tumours. Eur. Urol. 82, 458–468 (2022).
Paner, G. P. et al. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur. Urol. 73, 560–569 (2018).
Neves, R. J. & Zincke, H. Surgical treatment of renal cancer with vena cava extension. Br. J. Urol. 59, 390–395 (1987).
Albiges, L. et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): A single-arm, multicentre, phase 2 trial. Lancet Oncol. 24, 881–891 (2023).
McDermott, D. F. et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. JCO 39, 1029–1039 (2021).
Sirohi, D., Smith, S. C., Agarwal, N. & Maughan, B. L. Unclassified renal cell carcinoma: Diagnostic difficulties and treatment modalities. RRU 10, 205–217 (2018).
Zoumpourlis, P., Genovese, G., Tannir, N. M. & Msaouel, P. Systemic therapies for the management of non-clear cell renal cell carcinoma: What works, what doesn’t, and what the future holds. Clin. Genitourin. Cancer 19, 103–116 (2021).
Ishihara, H. et al. Effect of improved systemic therapy on patient survival in metastatic non-clear-cell renal cell carcinoma. Int. J. Urol. 28, 605–607 (2021).
Wilson, N. R., Acikgoz, Y. & Hasanov, E. Advances in non-clear cell renal cell carcinoma management: From heterogeneous biology to treatment options. Int. J. Cancer 154, 947–961 (2024).
Choueiri Toni, K. et al. Overall survival with adjuvant pembrolizumab in renal-cell carcinoma. New Engl. J. Med. 390, 1359–1371 (2024).
Choueiri Toni, K. et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. New Engl. J. Med. 385, 683–694 (2021).
Izarn, F. et al. Real world data of diagnosis, survival, and treatment outcomes in patients with metastatic non clear cell renal cell carcinoma. Clin. Genitourin. Cancer 21, e35–e43 (2023).
Caliò, A., Segala, D., Munari, E., Brunelli, M. & Martignoni, G. MiT family translocation renal cell carcinoma: From the early descriptions to the current knowledge. Cancers 11, 1110 (2019).
Caliò, A. et al. TFE3 and TFEB-rearranged renal cell carcinomas: An immunohistochemical panel to differentiate from common renal cell neoplasms. Virchows Arch. 481, 877–891 (2022).
Acknowledgements
This study was supported by the Department of Urology at Asan Medical Center and Seoul National University Hospital, and by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (RS-2024-00392315). We thank the patients and staff who contributed to this research.
Author information
Authors and Affiliations
Contributions
D.S. and J.S. wrote the main manuscript text and performed the statistical analysis, prepared all figures and tables. B.L., C.S., D.Y., I.G.J., J.H.H., H.A., B.H., C.W.J., and J.H.H. contributed to data collection, with J.H.H. especially contributing to data collection from Seoul National University Hospital. D.S., J.S., and J.H.H reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shin, D., Lim, B., Song, C. et al. Comparative analysis of oncologic outcomes in surgically treated patients with renal cell carcinoma and renal vein thrombosis by pathologic subtypes. Sci Rep 15, 15946 (2025). https://doi.org/10.1038/s41598-025-00452-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-00452-1