Abstract
Coronary heart disease (CHD) is a severe diabetic vascular complication and the main cause of mortality among diabetes patients. Early diagnosis of CHD could prevent its development. Both omentin-1 (Oment-1) and the long noncoding RNA MALAT1 (lncRNA MALAT1) can be detected in peripheral blood and exhibit protective or detrimental effects on CHD. However, whether these two factors could be predictive of CHD in T2DM patients remains unclear. Therefore, this study aimed to investigate the associations of circulating Oment-1 levels and the expression of MALAT1 with CHD in T2DM patients and to assess their predictive efficacy. A total of 137 T2DM patients were enrolled, including 68 patients without CHD (T2DM group) and 69 patients with CHD (T2DM + CHD group). Clinical parameters were collected, and plasma Oment-1 was measured by enzyme-linked immunosorbent assay (ELISA). RNA was isolated from peripheral monocytes, and the expression of MALAT1 was determined by quantitative PCR. Cardiac function was measured by echocardiography. Compared with that in T2DM patients, the plasma Oment-1 level was significantly lower, while the expression of MALAT1 was significantly greater in T2DM + CHD patients (all P values < 0.01). Bivariate correlation analysis indicated that Oment-1 was positively correlated with the left ventricular ejection fraction (LVEF) (P < 0.01). MALAT1 expression was negatively correlated with LVEF but positively correlated with age and DM duration (P < 0.05). Binary logistic regression suggested that Oment-1 and MALAT1 were significantly associated with the presence of CHD. Receiver operating characteristic (ROC) curve analysis demonstrated that both Oment-1 (AUC = 0.663, sensitivity = 75%, specificity = 49%) and MALAT1 (AUC = 0.749, sensitivity = 73%, specificity = 66%) had significant diagnostic value for CHD among T2DM patients. Notably, the combination of Oment-1 and MALAT1 exhibited better diagnostic efficiency (AUC = 0.771, sensitivity = 66.7%, specificity = 75.3%). In conclusion, decreased circulating Oment-1 levels and increased MALAT1 expression are closely associated with CHD in T2DM patients, and their combination offers superior diagnostic efficiency, suggesting Oment-1 and MALAT1 may serve as a non-invasive tool for the early CHD detection and risk stratification in high-risk T2DM patients. Further studies are warranted to explore the pathophysiological mechanisms of Omentin-1 and MALAT1 in the pathogenesis of CHD in T2DM and to validate their clinical utility as potential biomarkers in large cohort studies.
Similar content being viewed by others
Introduction
The global prevalence of diabetes mellitus (DM) has been increasing; the number of DM patients in China is 140.9 million, and this number is estimated to reach 174.4 million by 20451. Coronary heart disease (CHD) is a macrovascular disease of T2DM and the major cause of cardiovascular mortality2. However, the parameters used in the clinic to evaluate cardiovascular diseases are more specific to severe cardiovascular events such as myocardial infarction, which cannot provide enough information for the risk of CHD in T2DM patients. Therefore, finding predictive biomarkers would be valuable for the early diagnosis and prevention of CHD in T2DM patients.
Omentin-1 (Oment-1) is a novel adipocytokine that has long been found to be protective against cardiovascular diseases3 and T2DM-associated comorbidities4,5 because of its anti-inflammatory and antioxidative effects6,7. Reduced circulating Oment-1 levels were found in CHD patients8 and is considered a cardiovascular risk biomarker in axial spondyloarthritis patients9 and in postmenopausal women10. However, few studies have evaluated the level and value of circulating Oment-1 in T2DM patients with CHD.
The role of lncRNAs in cardiovascular diseases has been recognized in recent years, and some lncRNAs could be used as biomarkers for the diagnosis of CHD11. The lncRNA metastasis-associated lung cancer transcript 1 (MALAT1) was originally found in lung cancer but is also one of the most studied lncRNAs in cardiovascular disease12. Elevated MALAT1 expression in peripheral blood was found in patients with coronary slow flow13, CHD patients14 and CHD patients with unstable angina15, indicating that MALAT1 has diagnostic value for CHD and related events. However, few studies have examined the role of MALAT1 in CHD among T2DM patients.
Atherosclerosis and inflammation are the primary pathological alterations in CHD. Intriguingly, Oment-1 is an anti-inflammatory and anti-atherogenic adipokine16, and circulating Omentin-1 levels are negatively correlated with atherosclerosis17. In contrast, MALAT1 has been shown to promote inflammation18, and its circulating expression is positively correlated with the progression of atherosclerosis14. However, the correlation between omentin-1 and MALAT1 has not yet been investigated.
Therefore, this study aimed to evaluate the roles of Oment-1 and MALAT1 in CHD among T2DM patients and to explore the potential roles of Oment-1, MALAT1 and their combination as specific noninvasive markers for CHD in T2DM patients.
Materials and methods
Patient recruitment
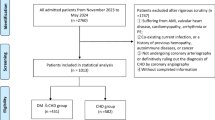
This study was conducted in the Department of Endocrinology, the Third Hospital of Hebei Medical University, from October 2021 to October 2023. A total of 137 T2DM patients aged 18–75 years were ultimately enrolled according to the inclusion and exclusion criteria. The inclusion criterion was as follows: T2DM diagnosed according to the WHO criteria (1999)19. The exclusion criteria were as follows: T2DM patients who were pregnant; presented with diabetes-related acute complications, such as diabetic ketoacidosis, hyperglycemic hyperosmolar state, and lactic acidosis; or had comorbidities, such as infectious diseases, autoimmune diseases, malignancies, heart failure, and renal and hepatic functional impairment.
CHD was diagnosed by meeting one of the following criteria20: at least one coronary artery stenosis > 50%, confirmed by previous coronary angiography or coronary CTA; a history of myocardial infarction; stable angina pectoris; and asymptomatic myocardial ischemia.
Patients were divided into two groups according to CHD history: the T2DM without CHD group (T2DM, n = 68) and the T2DM with CHD group (T2DM + CHD, n = 69). All patients with CHD received standard medical therapy, including aspirin and statins, and remained free from new episodes of myocardial infarction, angina pectoris, and heart failure.
This study was approved by the Ethics Committee of the Third Hospital of Hebei Medical University (Approval Code. W2021-088-1) and performed in accordance with relevant guidelines, and written informed consent was obtained from each participant. The study was performed in accordance with the Declaration of Helsinki.
Patient data and biochemical parameter collection
The baseline data of the patients, including age, sex, height, weight, duration of diabetes, family history, smoking status and alcohol consumption, were recorded. The abdominal circumference, height and weight were measured, and BMI was calculated as body weight (kg)/height (m)2 (kg/m2).
Blood samples were collected from the patients after 8 h of overnight fasting. The levels of triglycerides (TGs), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), very low-density lipoprotein cholesterol (VLDL-C), uric acid (UA), creatinine (Cr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), C-reactive protein (CRP), and homocysteine (Hcy) were measured via photoelectric colorimetry. Hemoglobin A1c (HbA1c) was measured via high-performance liquid chromatography. Fasting C-peptide (FC-P) and 25-hydroxy vitamin D (25(OH)D) were measured via MAGLUMI@ Chemiluminescence Immunoassy System(Snibe Co.,Ltd.Shenzhen) and available kits(cat.no.130205001M, cat.no.130261004M, Snibe Co.,Ltd.Shenzhen). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation21.
Echocardiography
Transthoracic echocardiography was carried out using a color ultrasound diagnostic instrument (PHILIPS EPIQ7 C). The thickness of the ventricular wall and left ventricular end-diastolic diameter (LVEDD) were measured by M-mode ultrasound. The method of LVESD measurement was the same as that of LVEDD, and the left ventricular ejection fraction (LVEF) was calculated via the following formula: LVEF = (LVEDD- LVESD)/LVEDD.
Oment-1 measurement
Fasting blood (5 ml) was collected from each patient, and plasma was collected after centrifugation. The plasma samples were stored at − 80 °C before every measurement. The plasma level of Oment-1 was measured by enzyme-linked immunosorbent assay (ELISA) by commercial kits (CUSABIO, Wuhan; Catalog No. CSB-E09745 h) according to the manufacturer’s protocol.
RNA isolation and RT‒qPCR
Fasting blood samples were collected, and peripheral monocytes were isolated by commercially available kits (Beijing Solabor Technology Co., Ltd.). RNA was extracted via the TRIzol method (cat. no. 15596026; Invitrogen; Thermo Fisher Scientific, Inc.), and RNA quality and concentration were assessed by UV spectroscopy at 260 and 280 nm. A HiFiScript gDNA Removal cDNA Synthesis Kit (cat. no. CW2582S; CoWin Biosciences) was used for reverse transcription. MonAmp ChemoHS qPCR mix (cat. no. rn04005M; Monad Biotech Co., Ltd.) was used to detect MALAT1 expression, and β-actin was used as a housekeeping gene. The thermocycling conditions were as follows: 95 °C for 15 min; 40 cycles at 95 °C for 10 s, 56 °C for 30 s and 72 °C for 30 s. Relative gene expression was calculated via the 2−△△CT method.
The PCR primers were designed and synthesized by Sangon Biotech Co., Ltd. (Shanghai). The sequences of primers used were as follows:
β-Actin: forward primer, 5’-AAGGCCAACCGCGAGAA-3’; reverse primer, 5’-ATGGGGGAGGGCATACC-3’. LncRNA MALAT1: forward primer 5’-TACCTAACCAGGCATAACA-3’; reverse primer 5’-GTAGACCAACTAAGCGAAT-3’.
Statistical analysis
All the statistical analyses were performed using SPSS software (version 26). The normality of the continuous data was examined via the Shapiro–Wilk test. Normally distributed data are expressed as the means ± standard deviations (means ± SDs), whereas nonnormally distributed data are expressed as medians and interquartile ranges (IQRs). Independent sample t tests or Mann‒Whitney U tests were performed to assess the differences between two groups. Categorical variables are expressed as numbers and were compared using the chi-square test. Spearman’s rank correlation analysis and Pearson’s correlation analysis were performed to evaluate the correlations between the study factors and the clinical and biochemical parameters. Binary logistic regression was performed to determine the contributions of the study parameters to the prediction of CHD onset. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic value of parameters for CHD in T2DM patients. A two-sided P value < 0.05 was considered statistically significant.
Results
Demographic, clinical, and biochemical characteristics of the study subjects
In the present study, there were no significant differences in patients’ baseline characteristics between T2DM patients and T2DM + CHD patients, including sex, BMI, family history, smoking status, or alcohol consumption, (all P > 0.05). Compared with T2DM patients without CHD, the duration of diabetes was significantly longer in T2DM patients with CHD (P = 0.019).
Compared with those in T2DM patients, SBP, HbA1c and FBG were significantly greater in T2DM + CHD patients (all P < 0.05), whereas LVEF, TC, LDL-C and eGFR were significantly lower in T2DM + CHD patients (all P < 0.05). There were no differences in DBP or other biochemical parameters, including TG, HDL-C, VLDL-C, ALT, AST, Cr, UA, CRP, FC-P, Hcy and 25(OH)d, between the two groups (all P > 0.05) (Table 1).
Oment-1 levels and MALAT1 expression in circulation
In the present study, compared to T2DM patients, plasma Oment-1 levels were significantly decreased in T2DM + CHD patients (15.55 ± 4.55 pg/ml vs. 13.09 ± 5.35 pg/ml, P = 0.0043; Fig. 1A), whereas MALAT1 expression in peripheral blood cells was significantly increased in T2DM + CHD patients (1.25 ± 0.42 vs. 1.69 ± 0.54, P < 0.0001; Fig. 1B). These findings indicated that reduced plasma Oment-1 levels and increased MALAT1 expression might be involved in the pathogenesis of CHD in T2DM patients.
Oment-1 levels and MALAT1 expression in the circulation of T2DM and T2DM + CHD patients. Compared with that in T2DM patients, the level of Oment-1 was significantly decreased (A), whereas the expression of MALAT1 (B) was significantly increased in the circulation of T2DM + CHD patients. * p < 0.05 for T2DM + CHD vs. T2DM.
Correlation analysis of Oment-1 and MALAT1
Bivariate correlation analysis was performed for Oment-1 levels and MALAT1 expression to determine the relevant factors. Oment-1 was positively correlated with LVEF (r = 0.223, p = 0.001) and had no correlation with other parameters, including age, BMI, HbA1c, FBG, CRP, TC, TG, HDL-C, LDL-c, or eGFR. The expression of MALAT1 was negatively correlated with LVEF (r =−0.253, p = 0.007) but positively correlated with age (r = 0.451, p = 0.002) and DM duration (r = 0.201, p = 0.019) (Table 2).
We also investigated the correlation between Oment-1 and MALAT1 and found that the level of plasma Oment-1 was negatively correlated with MALAT1 expression (r = −0.19, p = 0.026) (Fig. 2).
Association of Oment-1 and MALAT1 with the presence of CHD
Binary logistic regression analysis was performed with the presence of CHD in T2DM patients as the dependent variable and the study variables (Oment-1, MALAT1) as independent predictors. The regression models and data are shown in Table 3. In the first regression model, only the study variables were taken as predictors, and both Oment-1 and MALAT1 were significantly associated with the presence of CHD. In the second model, after adjusting for age, sex, duration of diabetes and HbA1c, Oment-1 and MALAT1 were still significantly associated with the presence of CHD. These associations were still significant even after adjusting for age, sex, duration of diabetes, HbA1c, BMI, LDL-C and eGFR, as shown in the third model (Table 3).
ROC curve analysis
ROC curve analysis was performed to determine the diagnostic value of Oment-1 and MALAT1 for the presence of CHD in T2DM patients. On the basis of analyses of the ROC curves (Fig. 1), plasma Oment-1 levels with cutoff values ≤ 12.45 pg/ml were used to discriminate CHD patients from non-CHD patients with T2DM, with an AUC of 0.633 (95% CI: 0.540–0.725; p < 0.001), a sensitivity of 75%, a specificity of 49%, a PPV of 59.9%, and an NPV of 65.9%. The cutoff value of MALAT1 expression was ≥ 1.367, with an AUC was 0.749 (95% CI: 0.668–0.830; p < 0.001), a sensitivity of 73%, a specificity of 66%, a PPV of 68.6%, and an NPV of 70.7%. Additionally, the AUC of Oment-1 combined with MALAT1 was 0.771 (95% CI: 0.694–0.848; p < 0.001), the sensitivity was 66.7%, the specificity was 75.3%, the PPV was 73.3%, and the NPV was 69.1%, indicating that the combination of Oment-1 and MALAT1 had better diagnostic value for CHD in T2DM patients. (Fig. 3)
Discussion
T2DM patients are at a greater risk of developing cardiovascular disease, which is the leading cause of mortality among this population22. In the present study, we investigated the correlations between circulating Oment-1 levels and MALAT1 expression in T2DM patients with CHD. Data have indicated that, compared with those in T2DM patients without CHD, circulating Oment-1 levels are significantly lower, whereas MALAT1 expression is increased in CHD patients. Additionally, the plasma Oment-1 level was negatively correlated with LVEF, whereas MALAT1 expression was positively correlated with age, DM duration and LVEF. Notably, Oment-1 and MALAT1 were inversely correlated with each other. Both factors, either independently or in combination, exhibited predictive value for CHD in T2DM patients.
In the present study, we found that the level of circulating Oment-1 was lower in T2DM patients with CHD and that it has predictive value for CHD. Similar results have also been reported previously in CHD patients with diabetes23 or without diabetes24,25. The favorable effect of Oment-1 on CHD might be due to its endothelial cell protective and anti-atherosclerotic effects. Oment-1 has been found to partly ameliorate FFA-induced endothelial cell injury26 and improve endothelial function by activating the Akt/eNOS/NO27 and AMPK/PPARδ pathways28, contributing to increased NO production and the inhibition of ER stress and oxidative stress. Oment-1 can modulate macrophage function and inhibit atherosclerosis formation and development29,30,31.
Intriguingly, this was the first study to find that circulating Oment-1 levels were positively correlated with LVEF. Similar results have been reported in patients with dilated cardiomyopathy32 and in mouse models of heart failure33, indicating that Oment-1 levels might also be related to ventricular diastolic function. In vivo studies have shown that Oment-1 can prevent pathological cardiac remodeling following ischemia34 and ameliorate ischemia-induced myocardial injury by activating mitophagy and maintaining dynamic mitochondrial homeostasis35. An in vitro study indicated that Oment-1 could protect H9 C2 cells from docetaxel-induced damage by reducing endoplasmic reticulum stress36. Therefore, Oment-1 is a protective factors against cardiomyocytes, and its effect on diabetic cardiomyopathy is a prospective area that needs to be further investigated.
In this study, we detected the expression of the lncRNA MALAT1 in peripheral blood mononuclear cells from T2DM patients and found that the expression of MALAT1 in the circulation was increased in T2DM patients with CHD. Similar results have also been reported by Sohrabifar et al.37 in Iranian patients. Elevated expression of MALAT1 in the circulation has been reported in CHD patients38,39 and is associated with increased CHD severity38 and unstable angina40, indicating that MALAT1 could be a biomarker for CHD screening and surveillance.
In the present study, we found that MALAT1 expression in the circulation of T2DM patients was positively correlated with age and diabetes duration. No similar results have been reported previously in T2DM patients. A previous study performed in patients with periodontitis and healthy subjects revealed that blood MALAT1 expression was not correlated with age41. The data from the present study suggest that MALAT1 might be more susceptible to the influences of aging and duration in T2DM patients. MALAT1 is known to promote inflammation and oxidative stress. Thus, the elevated MALAT1 levels in older patients with longer durations of diabetes might reflect cumulative inflammatory damage, potentially exacerbating cardiovascular complications.
Additionally, this was the first study to find that MALAT1 expression was negatively correlated with LVEF in patients with T2DM. A previous study performed by Qi et al.42 reported that, in hemodialysis patients with heart failure, lncRNA ENST00000561762 expression in peripheral blood mononuclear cells was negatively correlated with LVEF. Therefore, MALAT1 expression in peripheral blood might also play a crucial role in the diagnosis of cardiac function in T2DM patients. Some evidence has indicated that MALAT1 contributes to high glucose-induced cardiomyocyte damage by causing mitochondrial damage and oxidative stress43. It remains unclear whether MALAT1 expression in peripheral blood mononuclear cells reflects MALAT1 expression in heart tissue.
To the best of our knowledge, this is the first study to investigate the correlation between circulating Oment-1 levels and MALAT1 expression in patients with T2DM. Our results revealed a significant negative correlation between Oment-1 and MALAT1. Given that Oment-1 has anti-inflammatory and antioxidative effects, whereas MALAT1 is associated with proinflammatory activity, our findings suggest a potential regulatory network involving adipokines (e.g., Oment-1) and lncRNAs (e.g., MALAT1) in the pathogenesis of diabetic vascular complications. However, further studies are needed to elucidate the underlying mechanisms and clinical implications.
This study has several limitations. First, it is a single-center study with a relatively small sample size, which may cause patient selection bias. Second, the use of hypoglycemic or lipid-lowering drugs could influence circulating Oment-1 levels and MALAT1 expression. Additionally, since T2DM patients with CHD are older than those without CHD are, age-related factors might also affect Oment-1 and MALAT1 levels. Third, serum 25(OH)D levels could be influenced by vitamin D supplementation; therefore, its role requires further investigation. Future studies with larger sample sizes are needed to better understand the clinical implications of these findings.
Conclusions
Decreased circulating Oment-1 levels and elevated MALAT1 expression are significantly associated with CHD in patients with T2DM, suggesting their potential as biomarkers for the noninvasive early detection of CHD in T2DM patients. However, further validation through large-scale, multicenter studies is needed to confirm their diagnostic efficacy and determine clinically relevant cutoff values.
Data availability
Data are available on request from the corresponding author.
References
Sun, H. et al. Diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119 (2022).
Strain, W. D. & Paldánius, P. M. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc. Diabetol. 17 (1), 57 (2018).
Bai, P. et al. Association between coronary artery disease and plasma Omentin-1 levels. Cureus 13 (8), e17347 (2021).
Biscetti, F. et al. Association between plasma omentin-1 levels in type 2 diabetic patients and peripheral artery disease. Cardiovasc. Diabetol. 18 (1), 74 (2019).
Eimal Latif, A. H. et al. Association of plasma Omentin-1 levels with diabetes and its complications. Cureus, 13(9): p. e18203. (2021).
Binti Kamaruddin, N. A. et al. Cytoprotective role of omentin against oxidative Stress-Induced vascular endothelial cells injury. Molecules, 25(11), 2534 (2020).
Zhao, A. et al. Omentin-1: a newly discovered warrior against metabolic related diseases. Expert Opin. Ther. Targets. 26 (3), 275–289 (2022).
Du, Y. et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc. Diabetol. 15, 90 (2016).
Genre, F. et al. Omentin: a biomarker of cardiovascular risk in individuals with axial spondyloarthritis. Sci. Rep. 10 (1), 9636 (2020).
Christodoulatos, G. S. et al. Circulating Omentin-1 as a biomarker at the intersection of postmenopausal breast Cancer occurrence and cardiometabolic risk: an observational Cross-Sectional study. Biomolecules, 11(11), 1609 (2021).
Zhang, Y. et al. MicroRNAs or long noncoding RNAs in diagnosis and prognosis of coronary artery disease. Aging Dis. 10 (2), 353–366 (2019).
Yan, Y., Song, D., Song, X. & Song, C. The role of LncRNA MALAT1 in cardiovascular disease. IUBMB Life. 72 (3), 334–342 (2020).
Zhao, C. et al. The LncRNA MALAT1 participates in regulating coronary slow flow endothelial dysfunction through the miR-181b-5p-MEF2A-ET-1 axis. Vascul Pharmacol. 138, 106841 (2021).
Qiu, S. & Sun, J. lncRNA-MALAT1 expression in patients with coronary atherosclerosis and its predictive value for in-stent restenosis. Exp. Ther. Med. 20 (6), 129 (2020).
Barbalata, T., Niculescu, L. S., Stancu, C. S., Pinet, F. & Sima, A. V. Elevated levels of Circulating LncRNAs LIPCAR and MALAT1 predict an unfavorable outcome in acute coronary syndrome patients. Int. J. Mol. Sci. 24(15), 12076 (2023).
Sena, C. M. Omentin: A key player in glucose homeostasis, atheroprotection, and Anti-Inflammatory potential for cardiovascular health in obesity and diabetes. Biomedicines 12(2), 284 (2024).
Kadoglou, N. P. E., Kassimis, G., Patsourakos, N., Kanonidis, I. & Valsami, G. Omentin-1 and Vaspin serum levels in patients with pre-clinical carotid atherosclerosis and the effect of Statin therapy on them. Cytokine 138, 155364 (2021).
Shi, Z., Zheng, Z., Lin, X. & Ma, H. Long noncoding RNA MALAT1 regulates the progression of atherosclerosis by miR-330-5p/NF-κB signal pathway. J. Cardiovasc. Pharmacol. 78 (2), 235–246 (2021).
Grimaldi, A. & Heurtier, A. [Diagnostic criteria for type 2. diabetes] Rev. Prat. 49 (1), 16–21 (1999).
Knuuti, J. et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41 (3), 407–477 (2020).
Kidney Disease. Improving global outcomes (KDIGO) glomerular diseases work group.kdigo 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 100 (4s), S1–s276 (2021).
Einarson, T. R., Acs, A., Ludwig, C. & Panton, U. H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 17 (1), 83 (2018).
Ali, S. et al. Evaluation of serum adipokines (omentin-1 and visfatin) in coronary artery disease at a North Indian hospital. Endocr. Regul. 57 (1), 262–268 (2023).
Onur, I. et al. Serum omentin 1 level is associated with coronary artery disease and its severity in postmenopausal women. Angiology 65 (10), 896–900 (2014).
Shibata, R. et al. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis 219 (2), 811–814 (2011).
Chen, Y. et al. Omentin-1 Ameliorated Free Fatty Acid-Induced Impairment in Proliferation, Migration, and Inflammatory States of HUVECs. Cardiol Res Pract, 2020: p. 3054379. (2020).
Dong, Q. et al. Tetrahydroxystilbene glycoside improves endothelial dysfunction and hypertension in obese rats: the role of omentin-1. Biochem. Pharmacol. 186, 114489 (2021).
Liu, F. et al. Omentin-1 protects against high glucose-induced endothelial dysfunction via the AMPK/PPARδ signaling pathway. Biochem. Pharmacol. 174, 113830 (2020).
Hiramatsu-Ito, M. et al. Omentin attenuates atherosclerotic lesion formation in Apolipoprotein E-deficient mice. Cardiovasc. Res. 110 (1), 107–117 (2016).
Lin, X. et al. Omentin-1 modulates macrophage function via integrin receptors Αvβ3 and Αvβ5 and reverses plaque vulnerability in animal models of atherosclerosis. Front. Cardiovasc. Med. 8, 757926 (2021).
Tan, Y. L. et al. Tanshinone IIA promotes macrophage cholesterol efflux and attenuates atherosclerosis of apoE-/- mice by Omentin-1/ABCA1 pathway. Curr. Pharm. Biotechnol. 20 (5), 422–432 (2019).
Huang, Y. et al. Circulating Omentin-1 levels are decreased in dilated cardiomyopathy patients with overt heart failure. Dis. Markers. 2016, p6762825 (2016).
Li, F. et al. YiQiFuMai powder injection ameliorates chronic heart failure through cross-talk between adipose tissue and cardiomyocytes via up-regulation of Circulating adipokine omentin. Biomed. Pharmacother. 119, 109418 (2019).
Ito, M. et al. Omentin modulates chronic cardiac remodeling after myocardial Infarction. Circ. Rep. 5 (2), 46–54 (2023).
Hu, J. et al. Omentin1 ameliorates myocardial ischemia-induced heart failure via SIRT3/FOXO3a-dependent mitochondrial dynamical homeostasis and mitophagy. J. Transl Med. 20 (1), 447 (2022).
Lage, R., Cebro-Márquez, M., Rodríguez-Mañero, M., González-Juanatey, J. R. & Moscoso, I. Omentin protects H9c2 cells against docetaxel cardiotoxicity. PLoS One. 14 (2), e0212782 (2019).
Sohrabifar, N., Ghaderian, S. M. H., Alipour Parsa, S., Ghaedi, H. & Jafari, H. Variation in the expression level of MALAT1, MIAT and XIST LncRNAs in coronary artery disease patients with and without type 2 diabetes mellitus. Arch. Physiol. Biochem. 128 (5), 1308–1315 (2022).
Lv, F., Liu, L., Feng, Q. & Yang, X. Long non-coding RNA MALAT1 and its target microRNA-125b associate with disease risk, severity, and major adverse cardiovascular event of coronary heart disease. J. Clin. Lab. Anal. 35 (4), e23593 (2021).
Zhu, Y., Yang, T., Duan, J., Mu, N. & Zhang, T. MALAT1/miR-15b-5p/MAPK1 mediates endothelial progenitor cells autophagy and affects coronary atherosclerotic heart disease via mTOR signaling pathway. Aging (Albany NY). 11 (4), 1089–1109 (2019).
Liu, S., Hou, J., Gu, X., Weng, R. & Zhong, Z. Characterization of LncRNA expression profile and identification of functional LncRNAs associated with unstable angina. J. Clin. Lab. Anal. 35 (11), e24036 (2021).
Gholami, L. et al. The LncRNA ANRIL is down-regulated in peripheral blood of patients with periodontitis. Noncoding RNA Res. 5 (2), 60–66 (2020).
Qi, X. et al. The expression profile analysis and functional prediction of LncRNAs in peripheral blood mononuclear cells in maintenance Hemodialysis patients developing heart failure. Sci. Rep. 14 (1), 29577 (2024).
Wang, T. et al. MALAT1/miR-185-5p mediated high glucose-induced oxidative stress, mitochondrial injury and cardiomyocyte apoptosis via the RhoA/ROCK pathway. J. Cell. Mol. Med. 27 (17), 2495–2506 (2023).
Acknowledgements
The authors would like to thank Xinli Jiang (Department of Ophthalmology, The Third Hospital of Hebei Medical University, Shijiazhuang, China) for technical help and discussion of the results obtained in the experiments.
Funding
This work was supported by the Government-funded Clinical Medicine Outstanding Talent Training Project (ZF2025150), the Natural Science Foundation of Hebei Province (H2020206478), the Central Government Guides Local Science and Technology Development Project (246Z7711G) and the Projects of Medical Science Research of the Health Commission of Hebei Province, China (20210725, 20210513, 20210372 and 20170642).
Author information
Authors and Affiliations
Contributions
M.M.T., Y.K.L. and Y.L. conceived and designed the study. M.M.T., J.C.C. and M.L. performed the experiments and wrote, reviewed and revised the manuscript. M.M.T. and P.P.L. were involved in the analysis and interpretation of the data and performed the statistical analysis. Y.L., H.J.M. and Y.K.L. confirmed the authenticity of all the raw data. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the ethics committee of the hospital and was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tian, M., Cao, J., Li, M. et al. Associations of circulating omentin-1 levels and long noncoding RNA MALAT1 expression with coronary heart disease in patients with type 2 diabetes mellitus. Sci Rep 15, 16376 (2025). https://doi.org/10.1038/s41598-025-01153-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01153-5