Abstract
Hepatokines play an important role in age-related metabolic disorders for example diabetes. The study҆s aim is to find how resistance training and supplementation of UA affect the levels of Hepatokines in the liver tissue of male Wistar rats that were treated with STZ and were of advanced age. Twenty-five 21-month-old male rats were randomly assigned to five equal groups: healthy control (HC), diabetic with uric acid supplementation (DU), supplementation plus resistance exercise (DRU), resistance exercise only (DR) and diabetic control (DC).The resistance training protocol was performed for 8 weeks, with 60% of the maximum voluntary contraction capacity (MVCC), climbing the ladder 14–20 times, 5 days a week. Rats were given a combination of high-fat food and 500 mg/kg of UA. Consistent with the results of one-way ANOVA, the decrease in ANGPTL6 in the DU and DR groups compared to the DCgroup is significant (p ≤ 0.05), while the decrease in the DRU group compared to theDU group was not significant (p ≥ 0.05). Fetuin Adecreased significantly (p ≤ 0.05) in the DU group compared to the DC group, but did not change significantly (p ≥ 0.05) in the DR and DRU groups. According to the results of one-way ANOVA, the decrease in FETUB, Hepassocin, LECT2, and Selenoprotein is significant in all groups (p ≤ 0.05). Based on the beneficial effects of resistance training and UA supplementation on glucose metabolism and Hepatokines, it appears that the blend of exercise training and UA supplementation has a more effective therapeutic effect on hepatic Hepatokines in elderly individuals with diabetes.
Similar content being viewed by others
Introduction
Diabetes is one of the most common chronic diseases, and its prevalence increases significantly with age. Obesity, especially when linked with increased abdominal fat distribution, is a significant factor for diabetes because it causes insulin resistance. The likelihood of developing diabetes is influenced by advancing age1. The accumulation of fat within the abdominal cavity, especially in the liver, can lead to inflammation as a consequence. The degree of insulin resistance is more strongly associated with diabetic factors than body fat mass2. Researchers have recently become interested in the liver’s involvement in the development of diabetes.Additionally, the liver is involved in common diseases such as diabetes and cardiovascular disease (CVD). The liver plays a notable role in producing glucose and regulating lipoprotein metabolism3. It is obvious today that several proteins, which are exclusively or predominantly secreted by the liver, have a significant impact on energy metabolism and the development of diabete1. Presently, the proteins that originate from the liver are known as hepatokines.Hepatokines, as signaling molecules secreted from the liver, play a pivotal role in regulating glucose, lipid, and energy metabolism. Alterations in hepatokine levels are associated with insulin resistance, chronic inflammation, and the progression of type 2 diabetes1,4.Hepatokines, including FETUB, hepasosin, ANGPTL6, Fetuin A, and selenoprotein, are of particular significance due to their pivotal roles in regulating glucose and lipid metabolism, inflammation, and oxidative stress5.
Fetuin A, a hepatokine, is known to have a significant impact on the development of metabolic diseases6, while FETUB has been found to decrease glucose absorption7.Fetuin A and FETUB have been found to inhibit insulin secretion from beta cells, leading to reduced insulin sensitivity, increased insulin resistance, and inflammation. These could have a significant impact on the pathogenesis of diabetes6,7.Recent studies have indicated that there is a positive correlation between leukocyte-derived hepatokine chemotaxin 2 (LECT2) levels and the severity of obesity and insulin resistance in humans8.
A recently discovered hepatokine, known as hepasosin (SEPP1), has been found to have specific mitogenic properties for hepatocytes. SEPP1 is a liver-produced protein involved in transporting selenium in the body. It may play a function in the enlargement of diabetes, as individuals with the condition have higher levels of SEPP1 in their blood. Higher levels of SEPP1 in the liver are also associated with insulin resistance and impaired glucose metabolism, both of which are risk factors for diabetes. Additionally, it has been found to promote hepatic lipogenesis9. Hepasosin participates in the stimulation of insulin resistance in both skeletal muscle and liver.
There is another type of hepatokine referred to as ANGPTL6, which regulates energy and glucose levels in the body. The liver is the main organ that expresses ANGPTL6. ANGPTL6 is concerned with glucose, lipid, as well as energy metabolism. ANGPTL6 improves insulin sensitivity, improves energy expenditure, and combats obesity. Researchers have shown that ANGPTL6 plays a significant function in regulating body weight and glucose metabolism. Mice lacking ANGPTL6 have been discovered to develop obesity, hyperglycemia, and insulin resistance, over expression of ANGPTL6 in mice has been demonstrated to promote weight loss and increased blood sugar and insulin sensitivity2.
Physical activity as a non-pharmacological intervention improves insulin sensitivity in diabetic and non-diabetic persons10.The combination of reduced physical activity and increased central obesity in older adults may lead to a redaction in insulin sensitivity1. Regular physical activity has a positive impact on managing symptoms of diabetes. It can lead to lower levels of fasting blood sugar (FBS), low-density lipoprotein (LDL), triglycerides, and cholesterol, which are all important markers for managing diabetes11. Resistance training has been shown to have positive effects on glucose transporter proteins (GLUT) by increasing their number, as well as increasing total muscle mass and the number of insulin receptors in muscle fibers. The American College of Sports Medicine suggested being involved in at least 3 resistance training sessions per week to help decrease the risk of developing diabetes12.also, Resistance training, as an effective intervention for improving glucose metabolism and insulin sensitivity, can influence hepatokine expression and secretion through various mechanisms, including the activation of AMPK and mTOR signaling pathways13.However, the effects of exercise training on hepatokines in diabetic subjects are still being debated3,12A study conducted by Blumenthal and colleagues investigated the effects of a 6-month aerobic exercise and weight reduction program on the levels of fetuin A in the serum of overweight senior males. The study found that although insulin resistance decreased, there was an increase in fetuin A levels3.Kihanian et al. (2019) conducted a study to compare the effect of an 8-week aerobic training program and resistance training on fetuin A, FETUB, and FGF-21 serum levels in men with diabetes.According to their research findings, men with diabetes experienced a decrease in serum fetuin Aand FETUB levels and an increase in FGF-21 levels after performing aerobic and resistance exercises12.Previous sports activity interventions on new hepatokines mostly focused on changes in fetuin A and FGF-21 levels in the blood.Hepatokines, including hepasosin, LECT2, and ANGPTL6, have not received much attention from researchers, despite their expression in liver tissue14.
In addition to exercise interventions, nutritional interventions are also very important in controlling blood sugar levels in individuals with diabetes.Ursolic Acid (UA) has a diverse range of biological functions, such as acting as an antioxidant, antimicrobial agent, anti-inflammatory agent, and anti-cancer agent15. A recent study has demonstrated that the protective effect of UA is dose-dependent. It achieves this by decreasing the activity of Alanine aminotransferase (ALT) and aspartate aminotransferase(AST) while simultaneously increasing the levels of circulating antioxidants16.Also, UA protects the liver by increasing the expression and effect of anti-aging biomarkers17. Insulin binding plays a significant role in the insulin signaling pathway. It brings about a change in the conformation of the insulin receptor, which in turn activates tyrosine kinases. These kinases are responsible for the Insulin resistance (IR) phosphorylation of downstream molecules. UA phosphorylates IR and IRS through tyrosine sites, thus activating them.Upon Protein tyrosine phosphatase 1B (PTP1B) inactivation, the protein kinase B (AKT) and Glycogen synthase kinase 3β (GSK3β) pathway is activated by IRS and PI3 K, leading to regulation of glycogen and lipid synthesis, as well as stimulation of glucose absorption18.
This study pioneers a novel approach by examining the concurrent effects of ursolic acid (UA) and resistance training on key hepatokines in an aged diabetic rat model. While prior investigations have predominantly focused on singular interventions (exercise or supplementation) or explored hepatokines in non-diabetic models, this research evaluates the synergistic effects of two distinct mechanistic interventions within an age-related diabetic context.Furthermore, this study extends beyond conventional hepatokine research by examining the impact of these interventions on specific hepatokines less established in the pathogenesis of diabetes and resistance training responses. Notably, the investigation into the effects of UA and resistance training on ANGPTL6, Fetuin A, FETUB, Hepassocin, LECT2, and Selenoprotein introduces novel insights into this research area.
To date, there have been no studies examining how this dietary supplement impacts liver metabolism concerning new hepatokines. Based on the beneficial effects of resistance training on liver metabolism and UA supplementation on glucose metabolism, it is worth investigating the potential therapeutic effect of combining these two interventions on hepatic hepatokines in elderly diabetics. Therefore, this research aim is to determine the effect of a period of resistance training along with UA supplementation on the levels of hepatokines in the liver tissue of aged male Wistar rats treated with Streptozotocin (STZ).
Materials and methods
Animal
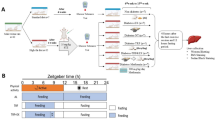
25 male Wistar rats aged 21 months were bought from the Pasteur Institute in Tehran, Iran. This study was conducted in accordance with all relevant ethical standards and national and international guidelines for the care and use of laboratory animals, including the ARRIVE guidelines. The study protocol was reviewed and approved by the Animal Ethics Committee of Shahrekord University (ethical code: IR.SKU.REC.1399.001, website: ethics.research.ac.ir) prior to commencement.Any signs of abnormality or distress in the animals were promptly assessed by trained personnel. In the event of any serious issues, a veterinary specialist in laboratory animal medicine was notified, and appropriate action was taken19.Then the rats became familiarized with the environment. Subsequently, the subjects were randomly allocated into five groups (n = 5) using simple randomization: Control (HC), A high-fat diet (HFD)/STZ-induced diabetes (DC), HFD/STZ-induced diabetes + UA (DU), resistance + HFD/STZ-induced diabetes (DR), and resistance-trained STZ-diabetic plus UA (DRU).
Acclimatization
Upon arrival at the laboratory, the rats were housed for one week under standard laboratory conditions (temperature 22 ± 2 °C, humidity 50 ± 10%, 12/12-hour light/dark cycle) to acclimate to the new environment. During this period, the rats were regularly monitored by trained personnel to ensure their health and normal behavior. Standard laboratory chow and water were provided ad libitum. Sufficient and appropriate space for movement and activity was provided within the cages. Gentle and low-stress methods were employed for rat handling. Trained personnel carefully removed the rats from their cages using soft gloves. Any rough or aggressive handling of the animals was avoided. Specialized animal handling tunnels were used for frequent transfers, when necessary. All study procedures were conducted under the close supervision of laboratory animal medicine personnel.
HFD/STZ-induced diabetes
The animals in the control group were fed with a standard rodentchow diet. The rats in the diabetes groups were fed a high-fat diet (5.21 kcal/g), which included 60% fats, 20% carbohydrates, and protein.After that, a dietary plan was considered for 8 weeks. During the fourth week, the group that had developed diabetes due to a high-fat diet and streptozotocin treatment received a small amount of streptozotocin from Sigma-Aldrich (CAS Number: 1888366-4). For the following stage, the rats were injected intraperitoneally with STZ at a dose of 30 mg/kg dissolved in 0.1 M sodium citrate buffer with a pH of 4.4. Following the initial seven days, the level of blood glucose was measured, and the rats that had blood glucose levels of 16.7 mmol/were taken into diabetic20. Afterward, the C group, consisting of sedentary non-diabetic rats, received a standard diet containing 10% fats, 75% carbohydrates, and 15% protein for the duration of the study, and male Wistar rats with Diabetes were given a high-fat diet consisting of 55% fats, 31% carbohydrates, and 14% protein. The control group, consisting of sedentary non-diabetic rats, received a standard diet containing 10% fats, 75% carbohydrates, and 15% protein for the duration of the study21. For the confirmation of the diabetes model, blood glucose levels of all animals in each group were observed during weeks 1, 2, 4, 6, and 8.For blood collection, minimally invasive techniques and fine-gauge needles were employed. The volume of blood collected at each time point was determined in accordance with ethical guidelines and with due consideration for the animals’ well-being.
The supplement intake
Animals in the supplement groups received a daily dose of 500 mg UA per kilogram of high-fat diet, equating to approximately 250 mg UA per kilogram of body weight per day22.The selection of this dosage was based on scientific rationale derived from prior studies and safety considerations. Evidence suggests that this dosage level, in short-term applications (8 weeks), has not demonstrated significant toxicity in animal models and has also exhibited positive metabolic effects23,24,25.
The resistance training protocol
The maximal velocity concentric contraction (MVCC) test was employed to determine training intensity and facilitate progressive overload adjustments. This test involves attaching 75% of a rat’s body weight to its tail and climbing a ladder while carrying a load. The training load was progressively increased by 30 g with each successful repetition, with a 2-minute rest interval between climbs. MVCC is defined as the point at which the rat fails to climb the ladder productively for three consecutive attempts. MVCC was measured at three distinct time points: beginning of week 1, week 4, and end of week 826.
Following a familiarization period with vertical climbing without added weight, the rats performed resistance training using 80% of their determined MVCC on a specific training ladder (with a 2 cm grid, 110 cm height, and 85° incline). Training was conducted five times a week for eight weeks, with 9–10 lifts per session27.
Progressive overload adjustments were implemented by re-evaluating MVCC at weeks 4 and 8. The training load was then adjusted to 80% of the newly determined MVCC, ensuring a consistent challenge throughout the training period. This approach to progressive overload allows for continuous adaptation and optimization of the training stimulus.
Collection of tissue
Animals were initially anesthetized using a combination of xylazine (10 mg/kg) and ketamine (90 mg/kg) administered intraperitoneally, ensuring a humane and painless induction of anesthesia.The depth of anesthesia was continuously monitored by trained personnel to ensure the absence of pain and distress in the animals during the procedure. Following anesthesia, blood samples were collected via cardiac puncture to measure blood glucose levels. Subsequently, animals were euthanized by cervical dislocation, a method chosen to minimize suffering and ensure a rapid and painless death. All animal procedures were performed in strict accordance with the guidelines of the Shahrekord University of Medical Sciences Animal Ethics Committee and relevant national and international regulations. We believe that this approach to anesthesia, blood collection, and euthanasia is both humane and effective, aligning with the highest standards of animal welfare.
Measurement of variables
Western blot analysis was conducted to quantify the protein levels of ANGPTL6 (ab155189, 1:1000), Fetuin-A (sc-166531, 1:200), Fetuin-B (sc-73978, 1:200), Hepassocin (sc-514057, 1:300), LECT2 (sc-398072, 1:200), Selenoprotein (sc-376858, 1:300) and anti-β-actin (sc-47778, 1:500) obtained from Abcam and Santa Cruz Biotechnology company. Tissue samples were mechanically homogenized using a Tissuelyzer II (Qiagen) at 30 Hz for 2 min in 1 mL of Lysis Buffer containing Tris-HCl (50 mM, pH 8.0), EDTA (1 mM), NaCl (150 mM), Sodium Deoxycholate (0.1%), SDS (0.1%), Protease Inhibitor Cocktail (1 tablet, Sigma, Catalog #P8340), and NP-40 (1%). Lysates were immediately placed on ice and then centrifuged at 12,000 rpm for 10 min at 4 °C in an Eppendorf 5415R centrifuge. The supernatant was collected, aliquoted into microcentrifuge tubes, and stored at −80 °C for future use. Next, SDS-PAGE was performed using a 10% separating gel and a 5% stacking gel. Electrophoresis was carried out at 120 V for approximately 45 min in a Mini-PROTEAN Tetra Cell (Bio-Rad) using running buffer containing 25 mM Tris, 192 mM glycine, and 0.1% SDS at room temperature. Protein transfer was then performed using a semi-dry transfer apparatus (Bio-Rad Trans-Blot Turbo Transfer System) at 2.5 A for 7 min at room temperature. The membrane was blocked with 5% non-fat dry milk in TBS-T for 1 h at room temperature with gentle orbital shaking. The membrane was subsequently incubated with the primary antibodies specific to the aforementioned proteins in 5% BSA in TBS-T for 16–18 h at 4 °C with gentle rocking. After washing three times with TBS-T, the membrane was incubated with the secondary antibody (anti-β-actin, sc-47778, Santa Cruz Biotechnology) diluted 1:500 in 5% BSA in TBS-T for 1 h at room temperature with gentle shaking. Finally, protein bands were visualized using enhanced chemiluminescence (ECL) with the Pierce ECL Plus Western Blotting Substrate. The blots were then stripped by striping buffer (25 mM glycin, 0.1% SDS, 1% Tween-20 at pH 2.0), re-blocked and reprobed with above mentioned primary and secondary antibodies. Images were captured using a radiography x-ray film (CP-GU, Fuji Film). Band intensities were quantified by densitometry using Image Lab software (Bio-Rad) with background subtraction. To normalize the data, the densitometric value of each protein band was divided by that of β-actin within the same sample. This normalized ratio was used for statistical analysis and group comparisons. β-actin served as a loading control.
Statistical analysis
The study was conducted in two stages. In the first stage, parameters were compared between the C and HFD groups. In the second stage, the HFD group rats were treated with UA diet and resistance training, and different parameters were compared between the C, D, U, R, and RU groups.The mean ± standard error of the mean was. The changes in maximal running speed and MVCC (in 3 levels), blood glucose (in 5 levels), and body weight (in 8 levels) were analyzed using repeated-measures analysis of variance (ANOVA). Repeated-measures analysis of variance was also used to determine the impact of group and time status. One-way ANOVA and Tukey’s test were used to measure the remaining parameters. The significance of differences was considered at a level of p < 0.05.
Result
-
The confirmation of the diabetes model.
The blood glucose levels of all animals in each group were observed during weeks 1, 2, 4, 6, and 8, and the data is presented in Fig. 1.
-
The weight and blood glucose levels change.
Following the end of the protocol, there was no significant interaction effect among supplement, time, and training on body weight (p = 0.9, ηp2 = 0.024) and glucose (p = 0.08, ηp2 = 0.031) on repeated-measures ANOVA. However, there was a significant interaction effect between time and training for glucose.
-
The levels of liver hepatokines in the diabetic model.
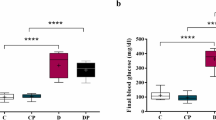
The analysis of variance results (Table 1) indicates that HFD-STZ has a significant impact on the levels of liver hepatokines in diabetic model rats.According to the comparison of average data, ANGPTL6, Fetuin A, Hepassocin, LECT2, and Selenoprotein showed a significant increasing trend in HFD group mice with 1.59 times, 1.68 times, 2.32 times, 1.58 times, and 1.40 times respectively, compared to HC group samples. Only the FETUB factor showed a significant decrease in HFD group mice by 1.12 times compared to HC group samples.
The impact of supplement consumption, resistance training, and their combined effect on the levels of various factors in diabetic rats exposed to the HFD-STZ agent is shown in Table 2. The results show that both supplement consumption and resistance training had a significant impact on changing the levels of all tested factors, except for Fetuin A. The interaction between supplement consumption and resistance training also had a significant impact on the changes in liver hepatokine levels, except for ANGPTL6 and Fetuin Afactors where the changes were not significant.
The average changes in liver hepatokine levels were compared among the HC, DC, DU, DR, and DRU groups to assess the impact of supplementation and resistance training on mitigating the effects of diabetes on these factors. In the DC group of rats, there was a significant increase in the levels of ANGPTL6, Fetuin A, Hepassocin, LECT2, and Selenoprotein factors. However, in the DU, DR, and DRU groups, these factors showed a significant decrease. The most notable reduction in ANGPTL6 was observed in the DRU and DU groups, with reductions of 2.18 and 1.56 times, respectively, compared to the DC group. Similarly, the DRU group exhibited the highest reduction in Fetuin A, Hepassocin, LECT2, and Selenoprotein factors, with reductions of 1.21, 1.62, 1.45, and 2.15 times, respectively, compared to the DC group. Regarding the changes in FETUB levels, supplementation and resistance training had a decreasing effect, without moderating the impact of the diabetes factor. However, both supplementation and resistance training, similar to the diabetes factor, decreased the levels of FETUB in the tested mice.
-
MVCC changes.
The interaction between training and time (p = 0.0001, ηp2 = 0.434) was significant for the MVCC. The significant level of interactive effect among supplement, time, and training was shown (p = 0.024, ηp2 = 0.212). (Table 3).
-
Fetuin A.
Diabetes has led to a significant increase in Fetuin Acompared to the healthy control group (p ≤ 0.05). Resistance training combined with supplementation UA has led to a significant reduction in Fetuin A (p ≤ 0.05). Although the supplement alone caused a decrease in Fetuin A, This reduction was not significant (p > 0.05). On the other hand, resistance training alone did not have a significant effect on Fetuin A in diabetic rats compared to the control group (p > 0.05))Fig. 2, a
-
FETUB Changes.
Compared to the control group, FETUB showed a significant reduction of 1.12 times in the diabetic group (p ≤ 0.05). The UA supplement and resistance training (with or without together) had no significant difference with the DC group (p > 0.05))Fig. 2, b
-
LECT2changes.
Diabetes has led to a significant increase in LECT2 compared to the healthy control group. Compared to the diabetes control group LECT2 levels in all groups (resistance exercise, supplement consumption, and resistance exercise along with supplement consumption) showed a significant decrease (p ≤ 0.05).The reduction in the resistance training group was greater than in the other groups. In this research, the difference in SELECT2 levels between diabetic rats that underwent resistance training and took supplements, and rats in the healthy group, was not significant. In other words, taking the supplement at the same time as performing resistance training reduced LECT2 levels in diabetic rats to the same level as in healthy rats. No significant difference was observed in LECT2 levels between the resistance group and the supplement group. Furthermore, there was no significant difference in the level of LECT2 between the resistance training group with supplement consumption and the resistance training group alone. However, resistance training combined with supplement consumption led to a significant decrease in LECT2 compared to taking the supplement alone (Fig. 2, c).
-
ANGPTL6changes.
Diabetes led to a significant increase in ANGPTL6 levels compared to the healthy control group.NGPTL6 had a significant decrease in all intervention groups compared to the diabetes control group.UA supplementation could significantly reduce ANGPTL6 levels in diabetic rats to the level of the healthy control group. ANGPTL6 levels were significantly decreased in diabetic rats that performed resistance training compared to the diabetic control group. However, it still had a significant increase compared to the healthy control group. UA supplementation caused a significant decrease in ANGPTL6 compared to the resistance group in diabetic rats. Furthermore, receiving UA supplements along with resistance training has led to a significant decrease in ANGPTL6 compared to the healthy control group (Fig. 2, d).
-
Hepassocinchanges.
Diabetes has led to a significant increase in Hepassocin levels compared to the healthy control group (HC). Hepassocin levels in all intervention groups have decreased significantly compared to the healthy control group(HC). Furthermore, there was no significant difference between the changes in Hepassocin levels between the UA supplement group (DU) and the resistance training group (DR). However, resistance training with UA supplementation in (DRU) has led to a significant decrease in Hepassocin levels compared to the UA supplementation (DU) and resistance training groups (DR). Therefore, it can be said that performing resistance training with supplementation consumption was more effective in reducing Hepassocin levels than performing resistance training or supplementation consumption alone (Fig. 2, e).
-
Selenoprotein changes.
Selenoprotein levels showed a significant increase in the diabetic group (DC) compared to the control group (HC). Taking the supplement and performing resistance training (DRU), either together or separately, resulted in a significant decrease in selenoprotein levels. Additionally, no significant difference was shown in selenoprotein levels between the resistance training group (DR) and the supplement group (DU). Resistance training combined with supplementation (DRU) resulted in a greater reduction in selenoprotein levels compared to when each factor was used alone (Fig. 2, f).
The effect of a period of resistance training along with UA supplementation on the levels of Fetuin A(a), FETUB(b), LECT2 (c), ANGPTL6 (d), Hepassocin (e), and Selenoprotein (f) levels in the liver tissue of aged male Wistar rats treated with STZ. A Bar graph with varying letters indicates a notable distinction (as determined by ANOVA and subsequent Tukey’s HSD, with a significance level of p < 0.05).Control (C), A high-fat diet (HFD)/STZ-induced diabetes (D), HFD/STZ-induced diabetes + UA (U), resistance + HFD/STZ-induced diabetes (R), and resistance-trained STZ-diabetic plus UA (RU).Bar with different letters indicates a significant difference (ANOVA and subsequent Tukey’s HSD, p < 0.05).
Discussion
The production of cytokines, adipokines, and hepatokines is directly associated with metabolic disorders like diabetes. This study showed that FETUB can be decreased following resistance training, and UA supplementation, with or without together (p ≤ 0.05). Resistance training + UA group showed a significantly greater reduction than the other two groups. Furthermore, FetuinA was significantly decreased in the ursolic acid + resistance training group compared to the diabetic control group, but there was no significant change in the resistance training group and the ursolic acid group alone (p ≥ 0.05).
Research on the effect of exercise activity on the fetuin family, especially Fetuin A and FETUB, in diabetes patients is very limited. The results of the present study were consistent with those of Sakr et al. (2014), and Malin et al. (2014)28,29. Proposed mechanisms include the regulation of insulin signaling pathways, increased glucose uptake and utilization, and reduced inflammation. Both aerobic and resistance exercise have been shown to be beneficial, with regular physical activity leading to decreased blood glucose levels, reduced body weight and fat percentage, and improved insulin sensitivity. Further research is needed to fully understand the underlying mechanisms and optimize exercise interventions for individuals with metabolic disorders30,31.
In this research, LECT2 decreased by 25.56% after 8 weeks of resistance training, by 31.85% after resistance training with supplementation consumption, and by 20.74% after supplement consumptionThe decrease in LECT2 observed in this study can be explained by its sensitivity to metabolic changes. LECT2, a hepatokine, is upregulated in response to positive energy balance and hepatic fat accumulation. Exercise, by improving insulin sensitivity and activating protein AMP-activated protein kinase (AMPK), can reduce LECT2 secretion. Additionally, the correlation between LECT2 and metabolic markers like BMI, waist circumference, and HOMA-IR suggests that exercise-induced improvements in these parameters can indirectly contribute to reduced LECT2 levels32. Our findings contradict those of Sargent et al. (2018), who observed no change in LECT2 serum concentrations following one hour of moderate-intensity aerobic exercise. Discrepancies between the studies may be attributed to differences in supplementation, participant characteristics (obese versus healthy men), exercise intensity, and modality (aerobic versus resistance)32.
Among the findings of the present study, it is worth mentioning that ANGPTL6 was reduced by 23.58% after eight weeks of resistance training, by 36.15% in the supplement group, and by 54.15% in the resistance training with the supplement group. This suggests that combining exercise with supplement use is more effective in reducing ANGPTL6 than either factor alone. Previous studies have shown that exercise can reduce the levels of the hepatokine ANGPTL6, which is elevated in metabolic disorders like diabetes33. Improvements in insulin sensitivity and glucose metabolism, along with exercise-induced changes in adipokine profiles, particularly leptin, may contribute to the reduction of ANGPTL6 levels. Leptin, a hormone produced by adipose tissue, is associated with metabolic stress and can influence hepatic gene expression, including that of ANGPTL6. By modulating energy balance and reducing inflammation, exercise can lead to decreased leptin levels and subsequent downregulation of ANGPTL633.
In the present study, it was observed that there was a significant decrease in Selenoprotein levels in all three groups: resistance training group (29.62%), supplement group (37.49%), and resistance training with the supplement group (53.65%). The most significant decrease was observed in the training with the supplement group. Additionally, Hepassocin levels decreased significantly in all three groups, with the most significant decrease occurring in the training with the supplement group. Selenoprotein is a protein secreted by the liver, and previous research has indicated a significant relationship between Selenoprotein mRNA expression and insulin resistance34. Miso et al. have suggested that Selenoprotein may play a role in causing insulin resistance and hyperglycemia in liver cells and skeletal muscles by disrupting the insulin messenger pathway through inactivation of AMPK35. Very limited research has been done on the effects of exercise training on selenoprotein levels. In this regard, Sargent et al. (2018) examined the effect of acute exercise training on hepatokines in overweight and obese people. Their results indicated that exercise had no significant effect on selenoprotein32. However, research has shown that in mice with selenoprotein P deficiency, after the intervention of sports training, the endurance capacity has increased through the regulation of reactive oxygen species and AMPK32. Selenium plays a crucial role in maintaining homeostasis and overall health. Individuals with low levels of selenium may benefit from selenium supplementation. However, excessive intake of selenium may have adverse effects, especially for those who already have sufficient to high levels of selenium. It is recommended that individuals with a serum or plasma selenium concentration of 122 g per liter or higher should avoid consuming selenium36. During exercise, the level of selenoprotein P decreases gradually in both trained and untrained individuals, as reported by a study Selenoprotein P is regulated by hepatic AMPK, which is activated by exercise in an intensity-dependent manner37. In the present study, resistance exercise likely triggered this cellular mechanism resulting in a decrease in Selenoprotein. Studies on rats have demonstrated that Selenoproteins regulate the concentration of apolipoproteins, plasma cholesterol, and the expression of genes involved in cholesterol biosynthesis, metabolism, and transport. This suggests that Selenoproteins have a main role in the regulation of lipoprotein biosynthesis. Additionally, there is a correlation between Selenoprotein and fat indicators, specifically triglycerides38. It is possible that resistance training in this study led to a significant decrease in Selenoprotein levels by affecting blood and liver fat indices. However, more research is needed to draw a definitive conclusion about the impact of sports activity on Selenoprotein. According to Huang et al. (2020), Hepassocin increases hepatic lipogenesis and is involved in inducing insulin resistance in skeletal muscle and liver9. It is possible that the decrease in Hepassocin observed in this study was a result of improved insulin resistance and a reduction in lipid profile.
Studies have shown that taking UA supplements can enhance the benefits of resistance training. This supplementaids in controlling glycogen and lipid production, while also enhancing the absorption of glucose39. Research has indicated that UA supplements have a positive effect on the new hepatokines. The amplified effect of resistance training on liver tissue hepatokines in the group taking UA supplements may be due to the supplement strengthening the effect of resistance training. Recent studies have also suggested that UA has the potential to improve insulin resistance, hyperinsulinemia, and inflammation commonly observed in obesity and diabetes40.Evidence indicates that certain hepatokines, including fetuin-A and LECT2, contribute to the disruption of insulin signaling in hepatocytes and extrahepatic tissues. They achieve this by inducing macrophage inflammatory responses through increased pro-inflammatory cytokine production and by promoting hepatic steatosis via the upregulation of lipogenic genes (e.g., Fetuin-A, LECT2, and Hepassocin)41,42.Conversely, UA, a plant-derived triterpenoid, exerts protective effects by modulating metabolic signaling and reducing hepatic oxidant levels. UA contributes to hepatic health by regulating key signaling pathways, including the NF-κB and apoptotic pathways. It mitigates hepatic cellular damage by attenuating inflammation and oxidative stress. Furthermore, UA facilitates systemic metabolic improvement by modulating insulin signaling in adipose tissue and metabolic signaling in skeletal muscle.Studies have demonstrated that UA can improve insulin sensitivity by reducing body weight, body mass index, waist circumference, and fasting blood glucose levels. Additionally, UA reduces fat mass, triglyceride (TG) levels, plasma leptin concentrations, and lipid accumulation. Conversely, UA increases high-density lipoprotein (HDL) cholesterol, brown adipose tissue, insulin sensitivity, fatty acid uptake, and β-oxidation in high-fat diet (HFD)-induced obese animal models, indicating enhanced energy expenditure41,42,43.
Physical activity has been shown to reduce disability in older individuals [39], while resistance training may impact Hepatokine levels by influencing blood and liver fat indices.Resistance training elicits improvements in glucose metabolism and insulin sensitivity through the modulation of hepatokine expression, a process mediated, in part, by the activation of the AMPK and mTOR signaling pathways, which are pivotal for metabolic adaptation. Specifically, hepatic-secreted hepatokines, which are critical regulators of systemic metabolic homeostasis, undergo alterations in response to resistance training. This leads to enhanced insulin sensitivity, primarily through augmented glucose uptake, facilitated by AMPK-mediated glucose transport in the skeletal muscle and a concomitant reduction in hepatic glucose output44. Furthermore, mTOR activation contributes to muscle hypertrophy and potentiates insulin sensitivity. Mechanistically, augmentation of muscle mass, enhancement of mitochondrial function, and attenuation of systemic inflammation collectively contribute to improved glucose disposal and insulin action. Consequently, resistance training is effective in improving glycemic control in individuals with type 2 diabetes. Notably, specific hepatokines, such as Fetuin-A, implicated in the pathogenesis of metabolic disorders, are modulated by this exercise modality44.also, Exercise interventions generally exert positive effects on health through significant alterations in body composition and metabolic indices. These interventions result in an increase in fat-free mass (FFM) and a decrease in fat mass, which are indicative of improved body composition. Concerning lipid metabolism, exercise leads to an elevation in HDL and a reduction in LDL, total cholesterol (TC), and TG, signifying an enhanced lipid profile and a reduced risk of metabolic diseases. Furthermore, exercise contributes to a decrease in blood glucose, insulin, and the homeostatic model assessment for insulin resistance (HOMA-IR), demonstrating improved insulin sensitivity and a diminished risk of type 2 diabetes mellitus43,45,46.
Research suggests that combining resistance training with UA supplementation can have a more effective therapeutic effect on hepatic Hepatokines in elderly individuals with diabetes, potentially leading to improved lifestyle for older people.On one hand, engaging in physical activity has the potential to decrease disability among older individuals.
The limitations of the research
Despite physiological similarities, rodents exhibit significant differences in human physiology, particularly in glucose metabolism and insulin response, which may impact the translatability of our findings. Furthermore, the aging process and therapeutic response profiles differ between these species. The STZ -induced diabetes model does not fully replicate the complex genetic and environmental factors contributing to human type 2 diabetes. Additionally, controlled laboratory conditions deviate from real-world human environments, potentially influencing observed outcomes. Discrepancies in drug metabolism, responsiveness to resistance training, and the limited genetic and environmental diversity inherent in rodent models pose challenges for extrapolating results to human populations. Nevertheless, our data suggest that UA and resistance training may exert beneficial effects on hepatokines, providing a foundation for future clinical trials. Subsequent human studies should encompass diverse populations, considering factors such as age, sex, and ethnicity, and evaluate the long-term impact of these interventions on hepatokine levels and clinical endpoints.
Conclusion
We can suggest that combining resistance training with UA supplementation may be more beneficial in treating hepatic hepatokines in elderly diabetics. This combination is seen as effective due to the positive impacts both interventions have on liver function and glucose metabolism. By integrating resistance training and UA supplementation, a more potent therapeutic effect may be achieved in improving the health of elderly diabetics, specifically targeting hepatic hepatokines.
Data availability
The data that support the findings of this study are available from the corresponding author, Mohammad Faramarzi, upon reasonable request.
Abbreviations
- CVD :
-
Cardiovascular disease
- MVCC:
-
Maximum voluntary contraction capacity
- LECT2:
-
Leukocyte-derived hepatokine chemotaxin 2
- SEPP1:
-
Hepasosin
- FBS:
-
Fasting blood sugar
- LDL:
-
Low-density lipoprotein
- GLUT :
-
Glucose transporter proteins
- ALT :
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- UA:
-
Ursolic Acid
- IR:
-
Insulin resistance
- PTP1B:
-
Protein tyrosine phosphatase 1B
- AKT:
-
Protein kinase B
- GSK3β:
-
Glycogen synthase kinase 3β
- STZ :
-
Streptozotocin
- ANOVA:
-
Repeated-measures analysis of variance
- HFD A:
-
High-fat diet
- AMPK AMP:
-
Activated protein kinase
References
Iroz, A., Couty, J. P. & Postic, C. Hepatokines: unlocking the multi-organ network in metabolic diseases. Diabetologia 58, 1699–1703. https://doi.org/10.1007/s00125-015-3634-4 (2015).
Fan, K. C. et al. Serum angiopoietin-like protein 6, risk of type 2 diabetes, and response to hyperglycemia: a prospective cohort study. JCEM 105, e1949–e1957. https://doi.org/10.1210/clinem/dgaa1033 (2020).
Blumenthal, J. B., Gitterman, A., Ryan, A. S. & Prior, S. J. Effects of exercise training and weight loss on plasma Fetuin-A levels and insulin sensitivity in overweight older men. J. Diabetes Res. 2017, 1492581. https://doi.org/10.1155/2017/1492581 (2017).
Jensen-Cody, S. O. & Potthoff, M. J. Hepatokines and metabolism: Deciphering communication from the liver. Mol. Metabolism. 44, 101138. https://doi.org/10.1016/j.molmet.2020.101138 (2021).
Stefan, N., Schick, F., Birkenfeld, A. L., Häring, H. U. & White, M. F. The role of hepatokines in NAFLD. Cell Metabol. 35 (2), 236–252. https://doi.org/10.1016/j.cmet.2023.01.006 (2023).
Ward, K., Mulder, E., Frings-Meuthen, P., O’Gorman, D. J. & Cooper, D. Fetuin-A as a potential biomarker of metabolic variability following 60 days of bed rest. Front. Physiol. 11, 573581. https://doi.org/10.3389/fphys.2020.573581 (2020).
Meex, R. C. et al. Fetuin B is a secreted hepatocyte factor linking steatosis to impaired glucose metabolism. Cell. Metab. 22, 1078–1089. https://doi.org/10.1016/j.cmet.2015.09.023 (2015).
Yoo, H. J. et al. Association of leukocyte cell-derived chemotaxin 2 (LECT2) with NAFLD, metabolic syndrome, and atherosclerosis. PloS One. 12, e0174717. https://doi.org/10.1371/journal.pone.0174717 (2017).
Huang, R. L. et al. Discovery of a role of the novel hepatokine, Hepassocin, in obesity. Biofactors 46, 100–105. https://doi.org/10.1002/biof.1574 (2020).
Colberg, S. R. et al. Physical activity/exercise and diabetes: a position statement of the American diabetes association. Diabetes Care. 39, 2065–2079. https://doi.org/10.2337/dc16-1728 (2016).
Li, R. Y. & Guo, L. Exercise in diabetic nephropathy: protective effects and molecular mechanism. Int. J. Mol. 25, 3605. https://doi.org/10.3390/ijms25073605 (2024).
Keihanian, A., Arazi, H. & Kargarfard, M. Effects of aerobic versus resistance training on serum fetuin-A, fetuin-B, and fibroblast growth factor-21 levels in male diabetic patients. Physiol. Int. 106, 70–80. https://doi.org/10.1556/2060.106.2019.01 (2019).
Tayebi, S. M., Hasannezhad, P., Saeidi, A. & Fadaei, M. R. Intense circuit resistance training along with Zataria multiflora supplementation reduced plasma retinol binding protein-4 and tumor necrosis factor-α in postmenopausal females. Jundishapur J. Nat. Pharm. Prod. 13 (2), e38578. https://doi.org/10.5812/jjnpp.38578 (2018).
Weigert, C., Hoene, M. & Plomgaard, P. Hepatokines—a novel group of exercise factors. Pflugers Arch. - Eur. J. Physiol. https://doi.org/10.1007/s00424-018-2216-y (2019). 471:383 – 96.
Ogasawara, R., Sato, K., Higashida, K., Nakazato, K. & Fujita, S. Ursolic acid stimulates mTORC1 signaling after resistance exercise in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 305, E760–E765. https://doi.org/10.1152/ajpendo.00302.2013 (2013).
Wang, Z. H., Hsu, C. C., Huang, C. N. & Yin, M. C. Anti-glycative effects of oleanolic acid and ursolic acid in kidney of diabetic mice. Eur. J. Pharmacol. 628 https://doi.org/10.1016/j.ejphar.2009.11.019 (2010). :255 – 60.
Gharibi, S., Bakhtiari, N. & Bakhtiari, F. Ursolic acid mediates hepatic protection through enhancing of anti-aging biomarkers. Curr. Aging Sci. 11, 16–23. https://doi.org/10.2174/1874609810666170531103140 (2018).
Liu, Q., Chen, L., Hu, L., Guo, Y. & Shen, X. Small molecules from natural sources, targeting signaling pathways in diabetes. BBA Gene Regul. Mech. 1799, 854–865. https://doi.org/10.1016/j.bbagrm.2010.06.004 (2010).
Karp, N. A. et al. Applying the ARRIVE guidelines to an in vivo database. PLoS Biol. 13 (5), e1002151. https://doi.org/10.1371/journal.pbio.1002151 (2015).
de Bem, G. F. et al. Antidiabetic effect of Euterpe oleracea Mart.(açaí) extract and exercise training on high-fat diet and streptozotocin-induced diabetic rats: A positive interaction. PLoS One. 13, e0199207. https://doi.org/10.1371/journal.pone.0199207 (2018).
Li SongTao, L. S. et al. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS One. 9, e86724. https://doi.org/10.1371/journal.pone.0086724 (2014).
Kazemi Pordanjani, M., Banitalebi, E., Roghani, M. & Hemmati, R. Ursolic acid enhances the effect of exercise training on vascular aging by reducing oxidative stress in aged type 2 diabetic rats. Food Sci. Nutr. 11, 696–708. https://doi.org/10.1002/fsn3.3105 (2023).
Villegas Vílchez, L. F., Ascencios, J. H. & Dooley, T. P. GlucoMedix®, an extract of Stevia rebaudiana and Uncaria tomentosa, reduces hyperglycemia, hyperlipidemia, and hypertension in rat models without toxicity: a treatment for metabolic syndrome. BMC Complement. Med. Ther. 22 (1), 62. https://doi.org/10.1186/s12906-022-03538-9 (2022).
Jackson, T. M., Rawling, J. M., Roebuck, B. D. & Kirkland, J. B. Large supplements of nicotinic acid and nicotinamide increase tissue NAD + and Poly (ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J. Nutr. 125 (6), 1455–1461. https://doi.org/10.1093/jn/125.6.1455 (1995).
Shatynska, O. et al. Dietary supplementation with magnesium citrate May improve pancreatic metabolic indices in an alloxan-induced diabetes rat model. Slovak J. Food Sciences/Potravinarstvo. 14 (1), 836–846. https://doi.org/10.5219/1375 (2020).
Singulani, M. P. et al. Effects of strength training on osteogenic differentiation and bone strength in aging female Wistar rats. Sci. Rep. 7, 42878. https://doi.org/10.1038/srep42878 (2017).
Farsani, Z. H., Banitalebi, E., Faramarzi, M. & Bigham-Sadegh, A. Effects of different intensities of strength and endurance training on some osteometabolic MiRNAs, Runx2 and PPARγ in bone marrow of old male Wistar rats. Mol. Biol. Rep. 46, 2513–2521. https://doi.org/10.1007/s11033-019-04695-w (2019).
Sakr, H. F. et al. Preventive roles of swimming exercise and Pioglitazone treatment on hepatic dysfunction in a rat model of metabolic syndrome. Cjpp 92, 162–170. https://doi.org/10.1139/Cjpp-2013-0043 (2014).
Malin, S. K., Del Rincon, J. P., Huang, H. & Kirwan, J. P. Exercise-induced Lowering of fetuin-A May increase hepatic insulin sensitivity. Med. Sci. Sports Exerc. 46, 2085. https://doi.org/10.1249/MSS.0000000000000338 (2014).
Cullberg, K. B. et al. Effect of weight loss and exercise on angiogenic factors in the circulation and in adipose tissue in obese subjects. Obesity 21, 454–460. https://doi.org/10.1002/oby.20060 (2013).
Shabkhiz, F., Khalafi, M., Rosenkranz, S., Karimi, P. & Moghadami, K. Resistance training attenuates Circulating FGF-21 and myostatin and improves insulin resistance in elderly men with and without type 2 diabetes mellitus: A randomised controlled clinical trial. EJSS 21, 636–645. https://doi.org/10.1080/17461391.2020.1762755 (2021).
Sargeant, J. A. et al. The influence of adiposity and acute exercise on Circulating hepatokines in normal-weight and overweight/obese men. Appl. Physiol. Nutr. Metab. 43, 482–490. https://doi.org/10.1139/apnm-2017-0639 (2018).
Kim, M. J. et al. Leptin regulates the expression of angiopoietin-like 6. BBRC 502, 397–402. https://doi.org/10.1016/j.bbrc.2018.05.180 (2018).
Burk, R. F. & Hill, K. E. Selenoprotein P—expression, functions, and roles in mammals. BBA Gen. Subj. 1790, 1441–1447. https://doi.org/10.1016/j.bbagen.2009.03.026 (2009).
Misu, H. et al. Deficiency of the hepatokine Selenoprotein P increases responsiveness to exercise in mice through upregulation of reactive oxygen species and AMP-activated protein kinase in muscle. Nat. Med. 23, 508–516. https://doi.org/10.1038/nm.4295 (2017).
Burk, R. F. & Hill, K. E. Selenoprotein P an extracellular protein with unique physical characteristics and a role in selenium homeostasis. Annu. Rev. Nutr. 25, 215–235. https://doi.org/10.1146/annurev.nutr.24.012003.132120 (2005).
Ennequin, G., Sirvent, P. & Whitham, M. Role of exercise-induced hepatokines in metabolic disorders. Am. J. Physiol. Endocrinol. Metab. 317, E11–24. https://doi.org/10.1152/ajpendo.00433.2018 (2019).
Stranges, S. et al. Higher selenium status is associated with adverse blood lipid profile in British adults. J. Nutr. 140, 81–87. https://doi.org/10.3945/jn.109.111252 (2010).
Cione, J. G. et al. No additional effects of ursolic acid supplementation associated with combined exercise program on metabolic syndrome of postmenopausal women: A double-blind, randomized, placebo-controlled trial. Clin. Nutr. ESPEN. 44, 143–149. https://doi.org/10.1016/j.clnesp.2021.05.031 (2021).
Ramirez-Rodriguez, A. M., González-Ortiz, M., Martínez-Abundis, E. & Acuna Ortega, N. Effect of ursolic acid on metabolic syndrome, insulin sensitivity, and inflammation. J. Med. Food. 20, 882–886. https://doi.org/10.1089/jmf.2017.0003 (2017).
Kim, T. H., Hong, D. G. & Yang, Y. M. Hepatokines and Non-Alcoholic Fatty Liver Disease: Linking Liver Pathophysiology to Metabolism. Biomedicines9(12):1903. (2021). https://doi.org/10.3390/biomedicines9121903
Seo, D. Y. et al. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 22 (3), 235–248. https://doi.org/10.4196/kjpp.2018.22.3.235 (2018).
Saeidi, A. et al. Differential effects of exercise programs on neuregulin 4, body composition and cardiometabolic risk factors in men with obesity. Front. Physiol. https://doi.org/10.3389/fphys.2021.797574 (2021).
Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98 (4), 2133–2223. https://doi.org/10.1152/physrev.00063.2017 (2018).
Atashak, S. et al. High-intensity interval training improves Lipocalin-2 and Omentin-1 levels in men with obesity. Int. J. Sports Med. 43 (4), 328–335. https://doi.org/10.1055/a-1560-5401 (2022).
Ataeinosrat, A. et al. Intensity dependent effects of interval resistance training on myokines and cardiovascular risk factors in males with obesity. Front. Endocrinol. (Lausanne). https://doi.org/10.3389/fendo.2022.895512 (2022).
Acknowledgement
The authors would like to acknowledge that the Iran National Science Foundation (INSF) has supported this study with approval code 4001286.
Author information
Authors and Affiliations
Contributions
Z GK: Conceptualization, Methodology, Supervision, Writing, review & editing. M F: Conceptualization, Data curation, Funding acquisition. Z HF: Investigation, Writing, review & editing. H A and MH: Formal analysis, Software.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The Acknowledgement section in the original version of this Article was omitted. It now reads: “The authors would like to acknowledge that the Iran National Science Foundation (INSF) has supported this study with approval code 4001286”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Farsani, Z.H., Faramarzi, M., Karaji, Z.G. et al. Resistance training and ursolic acid consumption affect hepatokines in aged diabetic rat liver. Sci Rep 15, 24326 (2025). https://doi.org/10.1038/s41598-025-01156-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01156-2