Abstract
Muscle wasting occurs early during critical illness. It is associated with poor PICU outcomes. Ultrasonography can detect muscle thickness in critically ill children. Study quadriceps muscle thickness to assess the nutritional status on admission and follow the muscle thickness change throughout the first week of admission in PICU using point-of-care ultrasonography. This cohort study was conducted on 55 critically ill mechanically ventilated children aged between 2 months and 14 years. Serial measurements of quadriceps muscle thickness were done by ultrasound, during the first week of admission. Quadriceps muscle thickness decreased by 16.4% over the 1st week of admission. The muscle wasting was significantly correlated with STRONGkids score, the inability to achieve target caloric requirements during the 1st week of admission, duration of ventilation, PRISM score, and mortality. Point of care ultrasonography is an easy tool for assessing PICU muscle wasting. Loss of muscle mass during the first week of PICU admission is correlated to mortality, thus it can be utilized in predicting PICU outcomes.
Similar content being viewed by others
Introduction
Critical illness is associated with metabolism, immunity, and endocrine system alterations, such as muscle catabolism and insulin resistance during the early phase of critical illness1,2. In critically ill adults, muscle wasting occurs due to low nutritional intake, immobilization, and catabolic hormones released during hospital stay3. Also, adult ICU patients requiring bed rest on admission had muscle wasting more obvious in the lower limb than in the upper limb4. In critically ill surgical adults, accurate muscle assessment by traditional examination is challenging in the ICU due to edema and hemodynamic instability5.
Quadriceps femoris muscle thickness changes occur on days 3 and 7 of admission compared to baseline thickness on the day of admission in the pediatric intensive care unit (PICU)6. The nutritional intake in critically ill children affects quadriceps femoris muscle thickness.7 This wasting is associated with longer PICU stays and poor outcomes8,9,10.
MRI is not suitable for serial muscle assessment in the PICU11. Fortunately, muscle ultrasonography can pick up muscle changes during PICU admission12. Regardless of the clinical case severity, ultrasonography could be done to avoid the hazards of ionizing radiation13. The quadriceps femoris muscle was chosen for muscle wasting assessment in preterm infants due to its large size and easy accessibility14. There is a lack of validated methods for muscle assessment in PICU15. And, there are a few studies on measuring muscle thickness using POCUS in critically ill children15,16,17,18.
This study was conducted in a developing country aiming to measure the quadriceps femoris muscle thickness in critically ill children on mechanical ventilation, using Point-of-Care Ultrasonography (POCUS) and explore the utility of this measurement to evaluate the nutritional status on admission and correlate it to nutritional intake and other factors during the first week of PICU stay. And investigate the correlation between muscle wasting and outcomes.
Patients and methods
This cohort study was conducted in the PICU of Cairo University Children’s Hospital from January 2021 to August 2022. This 26-bed PICU manages general and post-surgical pediatric cases not including cardiac surgery (Renal replacement therapy is available) admitting children within the age range of 2 months to 14 years. Mechanically ventilated children were enrolled in this study on the day of admission. Sedatives were used but no muscle relaxants were utilized. Exclusion criteria included patients with muscular disease, absolute contraindication to enteral feeding as intestinal obstruction, on steroids in the past year on muscle relaxants and those admitted less than 3 days.
The Research Ethical Committee of the Faculty of Medicine, Cairo University approved the research protocol (code MD-34-2021) following the Helsinki Declaration 1964, as revised in 2000.
Before enrolment, informed written consent was obtained from the patient’s guardian.
A complete history was documented including age, sex, history of chronic diseases, operations, developmental pattern, type of feeding (breast or formula feeding), and use of any supplements before admission as a part of the assessment of nutritional status. Physical examinations including vital signs on admission (blood pressure, heart rate, and respiratory rate for age, saturation, and temperature) were accomplished.
Anthropometric measurements were plotted according to the guidelines of WHO growth charts. Head circumference was measured in children < 36 months. For children < 2 years, growth chats of weight for age and weight for length were used. For children > 2 years, weight for height and BMI were utilized. Mid-upper arm circumference (MUAC) was assessed in children up to 5 years old8,19.
Routine PICU investigations were performed on admission including random blood sugar level, CBC, CRP, serum electrolytes (Na, K, Mg), calcium profile (Ca, PO4, ALP), liver function tests, kidney function tests, and serum albumin. coagulation profile and cultures as blood, sputum, CSF, and urine if indicated and chest x-ray. Arterial blood gas is a part of investigations to confirm the indication of mechanical ventilation. Data collection included the diagnosis on admission, use of inotropes, need for dialysis, steroids or sedatives use during PICU stay, duration of mechanical ventilation, reintubation, and PICU length of stay.
Pediatric risk of Mortality Score (PRISM) was calculated in the first 12–24 h of admission. In 2015 a new update for PRISM improved its quality to predict mortality risk in PICU20,21. STRONGkids score was used to assess nutritional risk on admission22. Nutritional intake was calculated using the actual caloric and was compared to goal requirements guided by the Schofield equation23.
According to ASPEN guidelines, the goal for nutritional intake during the first week of admission is to reach at least two-thirds of the target requirements, beginning within 24–48 h and progressing in a stepwise manner. Enteral nutrition is the preferred method in the PICU, with a minimum protein intake of 1.5 g/kg/day. For critically ill children who are unable to receive enteral nutrition, parenteral nutrition should be initiated within the first week of admission19.
Extubating readiness in PICU is determined by PH > 7.25, Po2/Fio2 ≥ 150 or oxygen saturation is 90–95% on Fio2 ≤ 30% and positive end-expiratory pressure (PEEP) < 6, respiratory rate is set < 45, mean airway pressure is 7 and tidal volume is 4–5 ml/kg. Regarding clinical parameters, the patient should be vitally stable, and conscious with a gag reflex. The cause of respiratory failure is reversed with spontaneous breathing and hemoglobin level ≥ 7 mg/dl. The spontaneous breathing trial is switching the ventilator mode to CPAP, setting the pressure support to zero with the same PEEP level for 3 min then setting the pressure support to 5–8 cm H2O2 for 7 min. If any of the following parameters are present: bradycardia < 100/min more than 5 min, saturation < 85% with a 15% increase in FiO2 or increase in work of breathing, failure of spontaneous breathing trial is then documented24. Discharge criteria include hemodynamic stability and stable respiratory status with de-escalation of the level of care. Communication is essential with subspecialists if needed. A written summary and discharge plan should be deliberately delivered25.
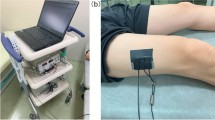
On day 1 of admission, days 3, and 7 measurements of quadriceps femoris muscle thickness were done. Three readings were measured on each day and the average was recorded. Muscle ultrasonography was done with a linear probe, using a sufficient gel quantity to prevent image distortion15. The muscle thickness was measured in one transverse plane as repeated measurements in different planes are not commonly used in the literature17. The probe was placed perpendicular to the skin. The anatomical landmark was midway between the anterior superior iliac spine and the superior patellar edge16. The maximal pressure was applied to ensure accuracy and compress lower limb edema if present5. No discrepancy would be displayed if measurements were done midway between the landmarks or at 1/3 from the distal landmark26. The muscle thickness was the distance between the muscle superior border and the Femur cortex15. To minimize inter-rater variability, the radiologist (consultant of diagnostic and interventional radiology) performed the measurement each time using a GE ultrasound machine with a liner probe (11 L HZ). Since children’s muscle thickness varies by age group, the muscle wasting was computed as a percentage of change from the initial measurement.
Statistical analysis
Data were analyzed using the IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, USA) release 22 for Microsoft Windows ). The Kolmogorov–Smirnov test was used to assess normality for continuous variables. Descriptive analyses were performed to obtain the means, and deviations for quantitative data when normally distributed, and the median and IQR for skewed data. Also, numbers and frequencies for qualitative data were obtained.
Bivariate analyses were performed for normally distributed data using the independent sample t-test, paired student’s t-test, and one-way ANOVA test. Non-normally distributed variables were compared using the Mann–Whitney test (U test), Kruskal Wallis test, and the Chi-squared test for categorical variables.
Repeated measure ANOVA was used to assess the magnitude of change in the muscle thickness at each time point (day 1, 3, and 7) and to show whether loss of muscle thickness was greater during the early or late part of the PICU stay. Pearson and Spearman’s correlations were used to measure the interdependence between different quantitative variables and the change in muscle thickness. P-value < 0.05 was considered significant.
Univariate linear regression models were used to test for the test the association of each effector on the percent of change in muscle thickness (dependent variable). The entered effectors are the predictors of mortality. Forward and backward multivariate linear regression models were also performed to select the independent effectors on the percent of change in muscle thickness. We entered the same predictors of mortality. Two-sided p values less than 0.05 were considered statistically significant.
Results
All patients admitted to the PICU at Cairo University Children’s Hospital from January 2021 to August 2022 were assessed, and 110 were found to fulfill the inclusion criteria. 55 patients’ guardians agreed to participate in this study. The cases had a median age of 14 (2–156) months, with 30 (54.5%) males and 25 (45.5%) females.
Some of our patients had more than one cause for PICU admission. Twenty-five (45.6%) of the study group were admitted for respiratory disorders, 21 (38.2%) had neurological disease, 9 (16.3%) sepsis, cardiac disorders comprised 5 (9.1%) of the study group, and other cases formed 10 (18.2%). History of chronic diseases, operations, and delayed developmental milestones were reported in 24 (43.6%), 6 (10.9%), and 14 (25.5%) cases respectively. Vitamins and minerals supplementation before admission were reported in about 17 cases (30.9%). Vitamin D, calcium, iron, folic acid, and L-carnitine were given to 52.9%, 29.4%, 29.4%, 17.6%, and 23.5% respectively. On admission, 67% of cases had normal random blood sugar levels, while hypoglycemia and hyperglycemia were reported in 22% and 11% of cases respectively. The most common metabolic disturbances revealed in arterial blood gases were respiratory alkalosis (38.5%) followed by metabolic acidosis, respiratory acidosis, and mixed acidosis in 27.3%, 18.2%, and 3.6% cases respectively. Sepsis screening in the form of cultures of various body fluids was performed. Positive results were obtained for blood, sputum, CSF, and urine specimens in 5 (9.1%), 25 (45.5%), 1 (1.8%), and 3 (5.5%) cases respectively. Sedatives were given to 42 (76.4%) cases with no muscle relaxants being utilized.
Using POCUS, the mean muscle thickness was 0.97 ± 0.40 (0.43–2.5) cm. The median age was 14 months which rationalizes the low mean muscle thickness. Within the first 24 h of admission, both PRISM and STRONGkids scores were calculated. The mean PRISM score was (13 ± 5 SD with a range of 3–24), while the mean STRONGkids score was (3 ± 1 SD with a range of 1–5) (Table 1).
Serial ultrasound measurements of muscle thickness were performed on the day of admission, day 3, and day 7. It decreased over the 1st week significantly (P < 0.001) (Table 2).
34 (61.8%) of the cases failed to achieve at least two-thirds of the goal requirements by the end of the first week and significantly had muscle wasting (P < 0.001) (Table 3).
During the PICU stay, muscle wasting correlated significantly to PRISM and STRONGkids scores, older age, use of inotropes, and different diagnoses on admission (P = 0.002, 0.019, 0.001, 0.007, and < 0.001 respectively). However, there was no significant correlation between muscle wasting and other clinical data including sex, anthropometric measurements, vital signs on admission, history of chronic diseases, operations, developmental pattern, supplements used before admission, dialysis, sedatives, and steroid use during PICU admission (Table 4).
The percentage of muscle wasting was significantly correlated to the low WBC count, low PLT count, prolonged PTT level, positive sputum culture, elevated CRP, and serum creatinine (P = 0.021, 0.018, 0.011, 0.045, 0.047, and 0.008respectively). No significant correlation was found between muscle wasting and other investigations (Table 5).
The mean duration of mechanical ventilation was 13 ± 13 (1–70) days, while the mean duration of PICU admission was 17 ± 14 (7–76) days. 33 (60%) children died. Muscle wasting was significantly correlated with mortality risk and duration of mechanical ventilation (P < 0.001 and 0.036 respectively). However, there was no correlation between muscle wasting and reintubation events or PICU length of stay (Table 6).
Table 7 showed that the history of chronic diseases, operations, and developmental delay was significantly associated with mortality risk (P = 0.011, 0.034, and 0.005 respectively). Also, the presence of respiratory illness on admission, positive sputum culture, inotropic use, hypotension, duration on mechanical ventilation, low serum albumin, and prolonged PTT levels were significantly associated with mortality risk (P = 0.02, 0.032, < 0.001, 0.027, 0.001, 0.006 and 0.007 respectively). The inability to achieve goal nutritional requirements and STRONGkids scores were significantly associated with mortality risk (P < 0.001). The Muscle thickness on days 3 and 7 was significantly associated with mortality (P = 0.048 and 0.009 respectively).
Univariate analysis revealed that six factors significantly affected the percent of change in muscle thickness (inotropic use, positive sputum culture, achievement of nutritional requirement in the first week, duration on mechanical ventilation, PRISM score, and STRONGkids score) (Table 8). Forward analysis revealed that achievement of goal requirements in the 1st week and sputum culture are the only independent effectors, while backward analysis concluded that added PRISM score and calories as independent effectors on the percent of change in muscle thickness (Tables 9 and 10).
The percentage of muscle wasting was picked up during serial ultrasound assessment of muscle thickness during the first week of PICU admission. The mean percentage of muscle wasting was 16.41% over the first week of admission (Fig. 1).
The sensitivity and specificity of the percent of muscle wasting in discrimination between survivors and non-survivors were 100% and 41% respectively at a cutoff of 6.34% of muscle wasting. The Receiver-operating characteristic (ROC) curve showed that the percent of muscle wasting measurement is a significant tool in discrimination between survivors and non-survivors (AUC 81.2%, P < 0.001) (Fig. 2).
Discussion
In critically ill children, malnutrition increases the risk of mortality and prolongs PICU stay. It can be assessed by evaluating muscle wasting.9,27,28. A practical method for estimating muscle wasting during a PICU stay is measuring muscle thickness using POCUS13,29. During serial measurements of the quadriceps femoris muscle in this study, there was a mean loss in muscle thickness of 16.4% during the 1st week of admission. Several studies conducted on different criteria of the studied groups, both in adult and pediatric populations, have used ultrasound to monitor quadriceps muscle thickness. (11,13,16,17,27,28). In pediatrics, De Figueiredo et al. study included critically ill children aged between 28 days and 14 years whether they were ventilated or not. The US assessment noted a significant percentage (14%) of quadriceps muscle thickness loss over the first week of admission12. Likewise, Valla et al. reported a nearly 10% decrease in quadriceps muscle thickness on day 5 in PICU in mechanically ventilated children aged 15 years or younger whether they were fully sedated or cooperative during measurements by ultrasound17. Also, Johnson et al. observed that muscle thickness reduced by 1.5% per day within 5 to 7 days of admission in mechanically ventilated children16.
Subtle muscle contraction, even in sedated critically ill children may temporarily preserve muscle thickness during the early days of admission.17. Though 76.4% of our patients were sedated, they developed muscle wasting. In contrast to our study, Salinas et al. objected to quadriceps muscle wasting in the first 4 days of PICU admission30. Whereas, another study noticed that quadriceps muscle thickness was stable during the PICU stay31.
Critically ill children are in a catabolic state and it is a great challenge to achieve the targeted caloric intake for PICU patients32. 61.8% of our patients didn’t achieve minimal nutritional intake in the first week. Both added significant quadriceps muscle wasting. This was in line with other studies in critically ill children that found muscle wasting was associated with deficient caloric intake. They found that for each decrease in the goal of energy by 1%, the quadriceps muscle size declined by 0.14% and 0.22% respectively during PICU admission7,33. In contrast, two studies found no association between caloric intake and quadriceps muscle thickness12,17.
Malnutrition is a risk factor for muscle wasting in critically ill children28. Several tools were validated to assess nutritional status, even if lengthy and consisting of numerous parameters, we applied the STRONGkids score and anthropometric measurements to identify malnutrition on the day of admission. This study group scored from 1 to 5. Similarly, Aziz et al. applied the STRONGkids score which ranged from 0 to 534. In our study, the STRONGkids score was correlated with muscle wasting. Similar findings were reported in other research undertaken in the PICU10. Alternatively, a correlation between the NUTRIC score (to measure nutritional status in critically ill adults) and quadriceps muscle wasting was affirmed35.
As our patients presented with a high PRISM score, they encountered a high mortality rate (60%).
Also, an increased PRISM score was associated with quadriceps muscle wasting. Likewise, the STRONGkids score was correlated to mortality and muscle wasting. A systematic and meta-analysis review perceived that under-nourished cases had a higher risk of PICU mortality36. Field-Ridley et al. found that muscle weakness was also related to elevated mortality risk in PICU10. Individual and prompt nutritional support is recommended as malnourishment increases muscle breakdown and the risk of mortality in PICU37.
The duration of PICU stay had no significant correlation with muscle wasting or mortality risk in our study as reported by others12,38. Opposingly, Field-Ridley et al. found that muscle weakness in PICU was associated with prolonged PICU length of stay10. Nevertheless, the duration of mechanical ventilation was correlated with increased quadriceps muscle wasting and mortality risk in our cases. This was in line with Field-Ridley et al.10.
In our study, we observed the duration of mechanical ventilation, prolonged PICU length of stay, and the inability to achieve goal nutritional requirements mounted the risk of mortality. Similar findings were observed in several studies in developing countries3940. It is advocated that adequate caloric intake during PICU admission significantly reduces the duration of mechanical ventilation and hospital stay19,27,41.
Limitations of the current study; First, we didn’t measure the cross-sectional area or echogenicity of the quadriceps femoris muscle, however, Jain et al. found that quadriceps muscle echogenicity changed in critically ill children by 16% over the 1st week of PICU admission with no significant change in quadriceps muscle thickness31. Second, we didn’t consider or assess the effect of the cumulative fluid balance on muscle thickness, since moderate fluid imbalance had no significant impact on muscle thickness42. Third, our study did not assess reproducibility, intra-observer reliability, and inter-observer reliability using ultrasound as the radiologist measured each time.
Conclusion and recommendations
During the first week of PICU admission, POCUS exposed a considerable loss of quadriceps muscle thickness, which was linked to insufficient nutritional intake. Muscle wasting was correlated to increased mortality risk and the duration of mechanical ventilation. We emphasize the importance of prompt achievement of nutritional requirements, in conjunction with serial quadriceps muscle thickness measurement using POCUS to assess nutritional status.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Joosten, K. F. M., Kerklaan, D. & Verbruggen, S. C. A. T. Nutritional support and the role of the stress response in critically ill children. Curr. Opin. Clin. Nutr. Metab. Care 19, 226–233. https://doi.org/10.1097/MCO.0000000000000268 (2016).
Sharma, K., Mogensen, K. M. & Robinson, M. K. Pathophysiology of critical illness and role of nutrition. Nutr. Clin. Pract. 34, 12–22. https://doi.org/10.1002/NCP.10232 (2019).
Wischmeyer, P. E. & San-Millan, I. Winning the war against ICU-acquired weakness: New innovations in nutrition and exercise physiology. Crit. Care https://doi.org/10.1186/CC14724 (2015).
Kawahara, K. et al. Evaluation of the site specificity of acute disuse muscle atrophy developed during a relatively short period in critically ill patients according to the activities of daily living level: A prospective observational study. Aust. Crit. Care 30, 29–36. https://doi.org/10.1016/J.AUCC.2016.01.003 (2017).
Bury, C. et al. Use of bedside ultrasound to assess muscle changes in the critically ill surgical patient. J. Parenter. Enter. Nutr. 45, 394–402. https://doi.org/10.1002/jpen.1840 (2021).
Katari, Y., Srinivasan, R., Arvind, P. & Hiremathada, S. Point-of-care ultrasound to evaluate thickness of rectus femoris, vastus intermedius muscle, and fat as an indicator of muscle and fat wasting in critically ill patients in a multidisciplinary intensive care unit. Indian J. Crit. Care Med. 22, 781–788. https://doi.org/10.4103/IJCCM.IJCCM_394_18 (2018).
Hoffmann, R. M. et al. Ultrasound assessment of quadriceps femoris muscle thickness in critically ill children. Pediatr. Crit. Care Med. 22, 889–897. https://doi.org/10.1097/PCC.0000000000002747 (2021).
Tume, L. N. et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med. 46, 411–425. https://doi.org/10.1007/S00134-019-05922-5 (2020).
Wong Vega, M., Beer, S., Juarez, M. & Srivaths, P. R. Malnutrition risk in hospitalized children: A descriptive study of malnutrition-related characteristics and development of a pilot pediatric risk-assessment tool. Nutr. Clin. Pract. 34, 406–413. https://doi.org/10.1002/NCP.10200 (2019).
Field-Ridley, A. et al. ICU-acquired weakness is associated with differences in clinical outcomes in critically ill children. Pediatr. Crit. Care Med. 17, 53–57. https://doi.org/10.1097/PCC.0000000000000538 (2016).
Sato, J. N. Book review: Pediatric critical care nutrition, Goday PS, Mehta NM, eds. Nutr. Clin. Pract. 30, 581–581. https://doi.org/10.1177/0884533615584003 (2015).
de Figueiredo, R. S. et al. Sarcopenia in critically ill children: A bedside assessment using point-of-care ultrasound and anthropometry. Clin. Nutr. 40, 4871–4877. https://doi.org/10.1016/j.clnu.2021.07.014 (2021).
Mourtzakis, M., Parry, S., Connolly, B. & Puthucheary, Z. Skeletal muscle ultrasound in critical care: A tool in need of translation. Ann. Am. Thorac. Soc. 14, 1495–1503. https://doi.org/10.1513/ANNALSATS.201612-967PS (2017).
McLeod, G. et al. Feasibility of using ultrasound to measure preterm body composition and to assess macronutrient influences on tissue accretion rates. Early Hum. Dev. 89, 577–582. https://doi.org/10.1016/J.EARLHUMDEV.2013.02.007 (2013).
Fivez, T. et al. An analysis of reliability and accuracy of muscle thickness ultrasonography in critically ill children and adults. J. Parenter. Enter. Nutr. 40, 944–949. https://doi.org/10.1177/0148607115575033 (2016).
Johnson, R. W. et al. Muscle atrophy in mechanically-ventilated critically ill children. PLoS ONE https://doi.org/10.1371/JOURNAL.PONE.0207720 (2018).
Valla, F. V. et al. Thigh ultrasound monitoring identifies decreases in quadriceps femoris thickness as a frequent observation in critically ill children. Pediatr. Crit. Care Med. 18, e339–e347. https://doi.org/10.1097/PCC.0000000000001235 (2017).
Ng, K. W. P. et al. Reliability of bedside ultrasound of limb and diaphragm muscle thickness in critically ill children. Muscle Nerve 59, 88–94. https://doi.org/10.1002/MUS.26327 (2019).
Mehta, N. M. et al. Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enteral Nutr. 41, 706–742. https://doi.org/10.1177/0148607117711387 (2017).
Pollack, M. M., Ruttimann, U. E. & Getson, P. R. Pediatric risk of mortality (PRISM) score. Crit. Care Med. 16, 1110–1116. https://doi.org/10.1097/00003246-198811000-00006 (1988).
Pollack, M. M. et al. The pediatric risk of mortality score: update 2015. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 17, 2–9. https://doi.org/10.1097/PCC.0000000000000558 (2016).
Hulst, J. M., Zwart, H., Hop, W. C. & Joosten, K. F. M. Dutch national survey to test the STRONGkids nutritional risk screening tool in hospitalized children. Clin. Nutr. 29, 106–111. https://doi.org/10.1016/j.clnu.2009.07.006 (2010).
Schofield, W. N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 39(Suppl 1), 5–41 (1985).
Mahmoud, N. M. S. Predicting successful extubation rate using modified spontaneous breathing trial in PICUs. J. Compr. Pediatr. 12, 0–7. https://doi.org/10.5812/compreped.116602 (2021).
Frankel, L. R. et al. Criteria for critical care infants and children: PICU admission, discharge, and triage practice statement and levels of care guidance. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 20, 847–887. https://doi.org/10.1097/PCC.0000000000001963 (2019).
Sabatino, A. et al. Reliability of bedside ultrasound for measurement of quadriceps muscle thickness in critically ill patients with acute kidney injury. Clin. Nutr. 36, 1710–1715. https://doi.org/10.1016/J.CLNU.2016.09.029 (2017).
Ventura, J. C. et al. Undernutrition at PICU admission is predictor of 60-day mortality and PICU length of stay in critically ill children. J. Acad. Nutr. Diet 120, 219–229. https://doi.org/10.1016/j.jand.2019.06.250 (2020).
Ong, C., Lee, J. H. & Puthucheary, Z. A. Narrative review of muscle weakness and wasting in pediatric critical illness. Pediatr. Med. https://doi.org/10.21037/pm-20-83 (2021).
Ferrie, S. & Tsang, E. Monitoring nutrition in critical illness: What can we use?. Nutr. Clin. Pract. 33, 133–146. https://doi.org/10.1177/0884533617706312 (2018).
Godoy Salinas, S. et al. Ultrasound assessment of thickness decreases of femoris quadriceps and his relation with functional outcomes in pediatric critical subjects under invasive mechanical ventilation. Pediatr. Crit. Care Med. 22, 237–237. https://doi.org/10.1097/01.PCC.0000740204.99955.9B (2021).
Jain, A. et al. Evaluation of changes in quadriceps femoris muscle in critically iii children using ultrasonography. Indian J. Pediatr. 90, 541–547. https://doi.org/10.1007/s12098-022-04220-1 (2023).
Taylor, R. M., Preedy, V. R., Baker, A. J. & Grimble, G. Nutritional support in critically ill children. Clin. Nutr. 22, 365–369. https://doi.org/10.1016/S0261-5614(03)00033-5 (2003).
Ong, C. et al. Skeletal muscle changes, function, and health-related quality of life in survivors of pediatric critical illness. Crit. Care Med. 49, 1547–1557. https://doi.org/10.1097/CCM.0000000000004970 (2021).
Aziz, M. M., Haroun, M. & Gad, O. M. STRONGKIDS nutritional risk score and body mass index in malnourishment risk assessment in critically ill children. Int. J. Pharm. Phytopharm. Res. 9, 5–97 (2019).
Lee, Z. Y. et al. Association between ultrasound quadriceps muscle status with premorbid functional status and 60-day mortality in mechanically ventilated critically ill patient: A single-center prospective observational study. Clin. Nutr. 40, 1338–1347. https://doi.org/10.1016/J.CLNU.2020.08.022 (2021).
Albadi, M. S. & Bookari, K. Is undernutrition associated with deterioration of outcomes in the Pediatric Intensive Care Unit (PICU): Systematic and meta-analysis review. Front. Pediatr. 10, 769401 (2022).
Kratochvíl, M. et al. Nutrition in pediatric intensive care: A narrative review. Child https://doi.org/10.3390/children9071031 (2022).
Hariharan, S., Krishnamurthy, K. & Grannum, D. Validation of pediatric index of mortality-2 scoring system in a pediatric intensive care unit, Barbados. J. Trop. Pediatr. 57, 9–13. https://doi.org/10.1093/TROPEJ/FMQ031 (2011).
Rady, H. I., Mohamed, S. A., Mohssen, N. A. & ElBaz, M. Application of different scoring systems and their value in pediatric intensive care unit. Egypt Pediatr. Assoc. Gaz 62, 59–64. https://doi.org/10.1016/J.EPAG.2014.10.003 (2014).
Teka, S. G., Kebede, R. A. & Sherman, C. The prevalence of malnutrition during admission to the pediatric intensive care unit, a retrospective cross-sectional study at, Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Pan Afr. Med. J. https://doi.org/10.11604/pamj.2022.41.77.31284 (2022).
Solana, M. J. et al. Prevalence, risk factors and impact of nutrition interruptions in critically ill children. Nutrients 15, 1–11. https://doi.org/10.3390/nu15040855 (2023).
Puthucheary, Z. et al. Structure to function: muscle failure in critically ill patients. J. Physiol. 588, 4641–4648. https://doi.org/10.1113/JPHYSIOL.2010.197632 (2010).
Acknowledgements
The authors thank the parents and the patients for participating in the study and sincerely appreciate the dedicated efforts of the attending physicians and nursing staff.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
The Egyptian Knowledge Bank Agreement with Springer Nature will fund open-access publication.
Author information
Authors and Affiliations
Contributions
S.A.M., H.M.E., H.M.B., M.H.G., & M.A.M. made the study design. S.A.M. & H.M.E. wrote the manuscript. H.M.B. did the statistics and wrote the results. M.H.G. did the ultrasonography and revised the data collection. M.A.M. shared in writing the results. All authors revised the final draft of the manuscript and accepted its publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, S.A., Elsawy, H.M., Bazaraa, H.M. et al. Point of care ultrasonography of quadriceps femoris muscle thickness for assessing nutritional status in critically ill children. Sci Rep 15, 16946 (2025). https://doi.org/10.1038/s41598-025-01205-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01205-w