Abstract
Alterations in plasma levels of the components of the mineral metabolism (MM) system are related to cardiovascular diseases. However, gender differences of the whole MM system in patients with acute coronary syndrome (ACS) have not been reported. Our objective was to analyse the potential differences on the prognostic role of MM in women suffering an ACS as compared to men. We included 1,230 patients with ACS and collected clinical data and plasma levels of MM components. Primary outcome was a composite of acute ischaemic events, heart failure and all-cause mortality. Secondary outcomes included each component separately. 282 patients (22.9%) were female. After 5.44 years of follow-up, primary outcome occurred in 28.0% women and 23.5% men, and death in 10.6% and 9.4% respectively. FGF23 was associated with primary outcome in both sexes, and calcidiol only in men (HR 1.04, CI95%1.00-1.03). Klotho levels are inversely related to all-cause mortality only in women (HR 0.80, CI95% 0.67–0.96), while calcidiol (HR 0.84, CI95%0.72–0.98) and FGF23 levels (HR 1.02 CI95%1.00-1.03) were predictors in men, highlighting a possible gender-specific prognostic biomarker. These results underline the importance of considering MM biomarkers in risk stratification and management of patients with acute coronary syndromes, with attention to gender differences.
Similar content being viewed by others
Introduction
The main role of the mineral metabolism (MM) system (calcidiol, fibroblast growth factor-23 [FGF23], phosphate, parathormone [PTH] and klotho) is to maintain mineral homeostasis. Specifically, FGF23 helps the failing kidneys to eliminate phosphorus and calcium1. It also promotes a reduction of the concentration of 1-25-dihydroxyvitamin D, that leads to a decrease in intestinal calcium absorption, stimulating PTH secretion. However, these compensatory changes of the different components of MM may promote the development of cardiovascular disorders (CVD), and their plasma levels may also have a prognostic role in certain CVD2,3,4,5. In the case of klotho, it works as a co-receptor of FGFR1 for the canonical actions of FGF236. The non-canonical actions of FGF23, such as cytokine production and the development of cardiac hypertrophy and fibrosis, are mediated mainly by FGFR2-4 receptors without the participation of klotho. Then, a decrease of klotho levels favours an increase of the deleterious, non-canonical effects of FGF237, and it has been said that this molecule possesses protective effects8,9,10.
In addition, these MM abnormalities are not limited to patients with chronic kidney disease (CKD), because they have been observed in patients with preserved renal function11. Furthermore, a prognostic role of abnormal plasma levels of MM has been demonstrated in subjects with average renal function4,12,13,14.
Moreover, CVD is the most frequent cause of mortality worldwide. In this setting, there are important gender differences in acute coronary syndromes (ACS): different comorbidities, cardiovascular risk factors, differences in clinical presentation and in the quality of diagnostic and therapeutic medical management15. Even more, the risk of death is known to be higher in women, especially in the context of younger populations16,17,18.
To our knowledge, there are no previous publications in the literature assessing the prognostic role of the whole MM after an ACS focusing on gender differences. In this work we have analysed the potential differences on the prognostic role of MM in women suffering an ACS as compared to men.
Methods
Patients and study design
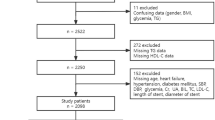
We analysed the BACS & BAMI (Biomarkers in Acute Coronary Syndrome & Biomarkers in Acute Myocardial Infarction) study population, which included patients admitted to five hospitals in Madrid with ST-segment elevation myocardial infarction (STEMI) or non-ST-segment elevation acute coronary syndrome (NSTEACS, including non-STEMI and unstable angina). Inclusion and exclusion criteria have been detailed previously19,20. Between July 2006 and June 2014, a total of 2,740 patients were discharged from the study hospitals with a diagnosis of NSTEACS or STEMI. Of these, 1,483 patients were excluded based on the following predefined criteria: presence of survival-limiting toxic conditions or habits (29.8%), age > 85 years (16.4%),inability to complete follow-up (16.3%), clinical instability beyond day six after the index event (10.9%),inability of the investigators to include them (9.8%), inability to undergo cardiac revascularisation (9.6%), presence of other significant cardiac conditions (5.7%), and refusal to participate in the study (1.5%). Of the 1,257 patients included, 1,230 completed the follow-up (Fig. 1).
On admission, baseline clinical variables were documented, and 12-hour fasting venous blood samples were collected in EDTA tubes. These samples were centrifuged at 2500 g for 10 min, and the plasma was stored at -80 °C.
After discharge from hospital, all patients underwent annual assessments at their respective medical centres. At the conclusion of the follow-up period, medical records were reviewed, and patient status was confirmed through telephone contact. The last follow-up visits were conducted in June 2016.
The research protocol suited the ethical guidelines of the 1975 Declaration of Helsinki. This protocol was approved by the Ethics Committee of Fundación Jiménez Díaz University Hospital. The date of approval by this Ethics Committee was 24 April 2007 (act number 05-07). In addition, this protocol was also approved by the Ethics Committees of the other institutions participating in the study: Fundación Alcorcón Hospital, Fuenlabrada Hospital, Puerta de Hierro Majadahonda University Hospital, and Móstoles University Hospital. All participants were provided informed consent during the study.
Outcomes
The primary outcome was a composite of acute ischemic events (including non-STEMI, STEMI, unstable angina, ischaemic stroke, and transient ischemic attack), heart failure, and all-cause mortality. Secondary outcomes included each component of the primary outcome: acute ischemic events, heart failure, and death. NSTEACS was defined as rest angina lasting more than 20 min within the preceding 24 h, or new-onset class III-IV angina, accompanied by transient ST depression or T wave inversion on the electrocardiogram, as interpreted by the attending cardiologist, and/or elevated troponin levels. STEMI was defined by angina-like symptoms persisting for more than 20 min, ST elevation in at least two contiguous leads on the electrocardiogram, lack of response to nitroglycerin, and elevated troponin levels. A previous acute myocardial infarction was diagnosed in the presence of new pathological Q waves on the electrocardiogram, along with corresponding new myocardial scarring identified via echocardiography or nuclear magnetic resonance imaging. Heart failure was defined as the presence of typical symptoms, with or without signs, associated with left ventricular systolic dysfunction (< 50%) or, in case of preserved systolic function, associated with objective evidence of cardiac structural and/or functional abnormalities, including elevated natriuretic peptide levels. Ischaemic stroke was defined as the rapid onset of a neurological deficit attributable to a specific vascular territory, lasting more than 24 h, or confirmed by new ischemic lesions on imaging studies. A transient ischemic attack was characterized by transient neurological signs and symptoms of cerebral ischemia that resolved within 24 h, without acute ischemic lesions on imaging studies.
Although all events were recorded for each patient, only the first event was included in the Cox regression analysis. Therefore, while the total number of events is reported, patients who experienced multiple events were counted only once in these analyses.
Biochemical analysis
Plasma analyses were conducted at the Mineral Metabolism laboratory of La Paz Hospital and at the Vascular Pathology and Biochemistry laboratories of Fundación Jiménez Díaz University Hospital. The investigators responsible for these laboratory studies were blinded to the clinical data. Soluble-α-klotho levels (here in after referred to as “klotho”) were assessed by ELISA (Human Soluble Alpha Klotho Assay Kit, Immuno-Biological Laboratories Co., Hokkaido, Japan). FGF23 was measured through an enzyme-linked immunosorbent assay (ELISA) that targets epitopes within the carboxyl-terminal region of FGF23 (Human FGF23, C-Term, Immutopics Inc, San Clemente, CA). Calcidiol plasma levels were quantified using a chemiluminescent immunoassay on the LIAISON XL analyzer (LIAISON 25OH-Vitamin D Total Assay, DiaSorin, Saluggia, Italy). Intact parathyroid hormone (PTH) was analyzed using a second-generation automated chemiluminescent method (Elecsys 2010 platform, Roche Diagnostics, Mannheim, Germany), and phosphate levels were determined by an enzymatic method (Integra 400 analyzer, Roche Diagnostics, Mannheim, Germany). N-terminal pro-brain natriuretic peptide (NT-proBNP) levels were measured via immunoassay (VITROS, Ortho Clinical Diagnostics, Raritan, NJ, USA), troponin I through an immunometric immunoassay using a biotinylated monoclonal mouse antibody and a luminescent reaction (Ortho Clinical Diagnostics Vitros XT 7600, Raritan, NJ, USA), and high-sensitivity C-reactive protein (hs-CRP) by latex-enhanced immunoturbidimetry (ADVIA 2400 Chemistry System, Siemens, Munich, Germany). Lipid, glucose, and creatinine levels were determined using standard methods (ADVIA 2400 Chemistry System, Siemens, Munich, Germany). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
Statistical analysis
Quantitative data following a normal distribution are presented as mean ± standard deviation, and those with a not normal distribution are displayed as median (interquartile range). Categorical variables are expressed using frequency measurements (absolute frequencies and percentages).
A baseline comparative analysis of variables was conducted based on gender. Categorical data were evaluated using the χ² test or Fisher’s exact test. For continuous variables, a Student’s t-test was applied to those with a normal distribution, while the Mann–Whitney U test was employed for those not normally distributed. A p-value of less than 0.05 was considered indicative of statistical significance.
A univariate Cox regression analysis was conducted to determine which variables were associated with the development of various outcomes, separately for men and women. Subsequently, a multivariate regression analysis was performed to identify significant predictors of clinical outcomes in both genders. The selection criteria for variables included in the multivariate analysis were based on clinical and biological plausibility, as well as statistical significance observed in the univariate analyses. The magnitude of the effects of the variables was expressed in the form of hazard ratios (HRs) and 95% confidence intervals (CIs).
All analyses were conducted using the Statistical Package for the Social Sciences (SPSS v.26.0, IBM, Armonk, NY, USA), the R statistical language version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria) and the statistical package for the biomedical sciences (MedCalc v.23.0.2, Ostend, Belgium; https://www.medcalc.org).
Results
Baseline characteristics
1,230 patients were included in our study. Of these, 282 (22.9%) were women. Women were older than men (65.7 vs. 60.5 years, p < 0.001), with a higher percentage of hypertension (70.6% vs. 53.2%), and of CKD prior to admission (28.7% vs. 15.9%) (Table 1). On the other hand, men had a significantly higher rate of smokers (46.6% vs. 28.0%), with more coronary heart disease and peripheral arterial disease prior to inclusion in the study. In both genders, the percentage of STEMI included in our population was around 50%. On average, men had a greater number of affected vessels (1.51 vs. 1.22, p < 0.001), and a higher percentage of them received revascularization treatment. This translated into a lower proportion of women with P2Y12 inhibitors at discharge (85.5% vs. 91.6%, p 0.004), with no other difference in treatment at discharge between men and women. Regarding the biomarkers analyzed, women generally presented significantly higher levels of PTH, FGF23, klotho, phosphorus, and NT-proBNP. Median time for blood extraction from admission was 4 (2–5) days.
Primary outcome
Median follow-up was 5.44 (3.03–7.46) years. During follow-up, 79 women (28.0%) and 223 men (23.5%) developed a primary event (a composite of acute ischemic events, heart failure, and all-cause mortality).
We performed a multivariate Cox regression analysis of our study population to identify independent predictors of primary outcomes separately for men and women, as described previously. This analysis revealed that FGF23 levels were directly and significantly related to the occurrence of a primary outcome in both sexes (Table 2). PTH also showed a relationship with primary outcome in the male group, but not in the female group. Other variables (clinical or treatment) also showed a significant relationship with outcomes in both groups, although no other biomarker showed a significant relationship.
Secondary outcomes
At the end of follow-up, 55 women (19.5%) and 133 men (14%) presented an acute ischemic event. For each of these groups, we performed a multivariate Cox regression analysis to identify significant predictors of this outcome (Table 3). Variables such as eGFR, heart failure (prior to inclusion), hypertension, etc. were shown to be a predictor of acute ischemic events. However, none of the analyzed biomarkers showed a statistically significant relationship in either group.
Twenty women (7.1%) and 48 men (5.1%) developed heart failure during follow-up. After multivariate Cox regression analysis, again FGF23 was an independent risk factor for the development of heart failure in both men and women (Table 4). PTH also showed a statistically significant relationship with this event but as observed for the primary outcome, only in the male population.
Finally, we analyzed the variables related to all-cause mortality in both genders. By the end of follow-up, 30 women (10.6%) and 89 men (9.4%) had died. We observed that klotho was an independent protective factor in the female population for all-cause mortality (HR 0.80, CI95% 0.67–0.96; p = 0.019) (Table 5). Phosphate levels were also independently but positively associated with this outcome in this population (HR 2.24 (1.11–4.50; p = 0.025). In contrast, in men the MM components associated with this outcome included only FGF23 (HR 1.02 (1.00-1.03); p = 0.048) and calcidiol (HR 0.84 (0.72–0.98); p = 0.024) but not klotho and phosphate plasma levels.
Discussion
Many differences have been described between men and women regarding coronary artery disease. Women have less coronary atherosclerosis21, and a lower risk of suffering ACS than men22. In addition, the age of onset is higher in women than in men, with a higher prevalence of comorbidities23. The impact of the different risk factors according to gender also presents differences; with the greater importance in women of diabetes, hypertension and smoking24,25,26. Thus, the risk of death is known to be higher in women. This higher mortality in women is described mainly in the earlier phases after ACS, with a worse prognosis and higher mortality having been observed immediately after percutaneous intervention23,27. The possible causes of this worse prognosis are diverse, and they may include a delay in the diagnosis of ACS favored by the higher likelihood of atypical symptoms28and differences in high-sensitivity troponin levels as compared to men29. Furthermore, women with ACS are less likely to receive optimal therapy than men30,31,32,33,34.
Changes in plasma levels of the different components of MM have been related to different cardiovascular abnormalities. Low calcidiol and high PTH levels have been associated with left ventricular hypertrophy, hypertension, coronary heart disease and increased cardiovascular risk2,20,35. Increased FGF23 has been related with left ventricular hypertrophy3,36,37, heart failure38,39, atrial fibrillation40and coronary heart disease4and is associated with worse prognoses both in the general population and in patients with CVD5,41,42,43,44. Furthermore, MM abnormalities are not restricted to patients with CKD, but they are also present in patients with normal renal function11, where they may also have prognostic value4,12,13,14. In spite of this, there is no information regarding the behaviour of MM components in women with ACS.
In this paper we demonstrate that women with ACS present a worse MM profile than men, with higher FGF23, PTH, and phosphate levels. Regarding prognosis, FGF23 levels were independent predictors of the primary outcome in both genders, while PTH added independent predictive value only in men. Similar results were obtained in the prediction of heart failure, where PTH showed significant predictive value in the multivariate study only in males (in agreement with the observations of other authors where PTH level was related to clinical and subclinical markers of congestion45, but not in females. However, for the prediction of all-cause mortality there were marked differences between both groups, with low klotho levels being predictive in women, along with phosphate levels, while in men high FGF23 and low calcidiol levels were the independent predictors for this outcome.
Klotho is the co-receptor of FGFR1 for FGF23, which exerts its beneficial actions through this canonical pathway, such as helping the failing kidneys to eliminate phosphate. However, in the absence of klotho, FGF23 binds to other receptors promoting cardiac hypertrophy and fibrosis and stimulating the production of pro-inflammatory cytokines7. Then, it has been said to have protective effects. Klotho suppression in animal models is associated with early ageing, shorter life expectancy, multi-organ dysfunction and marked alterations in mineral homeostasis (hyperphosphataemia, hypercalcaemia, among others.)46,47. More recently, an independent association between low klotho levels with left ventricular hypertrophy48, heart failure49,50,51,52, atrial fibrillation53and myocardial infarction50 has been described in humans. Klotho has also demonstrated a prognostic role in heart failure49,54, with low levels also being a marker of total and cardiovascular mortality in CKD patients55and in the general population56,57.
Basic research studies report results that may explain in part a potential benefit of klotho in ACS. The administration of klotho in animal models protects the myocardium from ischaemic and reperfusion damage through the activation of different pathways that reduce oxidative stress and restore autophagy levels58,59. Klotho has been described to modulate platelet activity60. Klotho therapy improves cardiac remodelling in a murine model of myocardial infarction61 and it also ameliorates diastolic function62, although there are not clinical studies confirming these findings. In several other studies, authors have also observed a beneficial effect of klotho on the vascular endothelium, reducing oxidative stress at this level or attenuating cell apoptosis, among other mechanisms63,64,65,66.
However, there are no published data on the prognostic role of klotho after an ACS. To our knowledge, our work is the first to describe an independent protective role of klotho after ACS. According to the present findings, we have recently shown that cardiac rehabilitation after ACS is associated with increases of klotho levels67, suggesting an increase in klotho could explain, at least in part, the benefits of cardiac rehabilitation. Of interest, the protective role of klotho is limited to women. It is possible that the higher cardiovascular risk profile of men, with more extensive coronary disease and a greater number of affected vessels, may interfere with the prognostic role of klotho, attenuating its protective effect after ACS. It is also striking that our results show that other MM biomarkers have a prognostic value in men but not in women. Nevertheless, we have demonstrated previously that it is common that several MM biomarkers have independent prognostic value14,41, even after adjusting for established biomarkers such as NT-proBNP. This underlines the need to make a complete assessment of MM to investigate the prognostic value of its components.
Limitations
First, the percentage of women in our study population is relatively low. This is in line with many other published studies. There are several factors that may influence the lower recruitment of women, among them, a higher prevalence of ACS in men could partly account for this difference. Second, the design of the study required the collection of plasma for analysis at discharge no later than 6 days after admission, to achieve homogeneous results. This led to the exclusion of ACS patients who did not meet this condition and that fact may explain the low number of cases with LVEF < 40% that were included. Therefore, these results should not be extrapolated to populations with a high percentage of patients with moderate or severe LV systolic dysfunction. Third, the inclusion period ended in 2014. Since then, new pharmacological and non-pharmacological treatments (stents, catheters, etc.) have emerged that could influence the results of similar studies in current populations. Fourth, almost all the women included in our study population were in the postmenopausal period. However, the variables collected in our study did not include the presence of osteoporosis or the use of specific gynaecological therapies. It is possible that hormonal or osteoporosis treatments may influence some of the parameters and/or biomarkers obtained.
Conclusions
In conclusion, our results show that klotho levels are inversely related to all-cause mortality in women after an ACS, highlighting a possible gender-specific prognostic biomarker. These results underline the importance of considering MM biomarkers in the risk stratification and management of ACS patients, with attention to gender differences. Future research should explore the underlying mechanisms of these associations.
Data availability
Data is provided within the manuscript. Further information and requests for resources and data base should be directed to and will be fulfilled by the lead contact, Dr.Marcelino Cortés (mcortesg@quironsalud.es).
Change history
29 August 2025
The original online version of this Article was revised: The Funding section was incorrect in the original version of this Article. The correct information now accompanies the original Article.
References
Wolf, M. Forging forward with 10 burning questions on FGF23 in kidney disease. J. Am. Soc. Nephrol. 21, 1427–1435 (2010).
Michos, E. D., Cainzos-Achirica, M., Heravi, A. S., Appel, L. J. & Vitamin, D. Calcium supplements, and implications for cardiovascular health. J. Am. Coll. Cardiol. 77, 437–449 (2021).
Falkner, B., Keith, S. W., Gidding, S. S. & Langman, C. B. Fibroblast growth factor-23 is independently associated with cardiac mass in African-American adolescent males. J. Am. Soc. Hypertens. 11, 480–487 (2017).
Panwar, B. et al. Association of fibroblast growth factor 23 with risk of incident coronary heart disease in Community-Living adults. JAMA Cardiol. 3, 318–325 (2018).
Liu, M. et al. Fibroblast growth factor-23 and the risk of cardiovascular diseases and mortality in the general population: A systematic review and dose-response meta-analysis. Front. Cardiovasc. Med. 9, 989574 (2022).
Quarles, L. D. Endocrine functions of bone in mineral metabolism regulation. J. Clin. Invest. 118, 3820–3828 (2008).
Stöhr, R., Schuh, A., Heine, G. H. & Brandenburg, V. FGF23 in cardiovascular disease: Innocent bystander or active mediator?? Front. Endocrinol. 9, 351 (2018).
Xiao, Z. et al. FGF23 expression is stimulated in Transgenic α-Klotho longevity mouse model. JCI Insight 4, e132820–e132820 (2019).
Ding, J. et al. Klotho inhibits angiotensin II-induced cardiac hypertrophy, fibrosis, and dysfunction in mice through suppression of transforming growth factor-β1 signaling pathway. Eur. J. Pharmacol. 859, 172549 (2019).
Xie, J., Yoon, J., An, S. W., Kuro-o, M. & Huang, C. L. Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J. Am. Soc. Nephrol. 26, 1150–1160 (2015).
Gonzalez-Parra, E. et al. Important abnormalities of bone mineral metabolism are present in patients with coronary artery disease with a mild decrease of the estimated glomerular filtration rate. J. Bone Min. Metab. 34, 587–598 (2016).
Wohlfahrt, P. et al. Association of fibroblast growth Factor-23 levels and Angiotensin-Converting enzyme Inhibition in chronic systolic heart failure. JACC Heart Fail. 3, 829–839 (2015).
Poelzl, G. et al. FGF23 is associated with disease severity and prognosis in chronic heart failure. Eur. J. Clin. Invest. 44, 1150–1158 (2014).
Tunon, J. et al. Coexistence of low vitamin D and high fibroblast growth factor-23 plasma levels predicts an adverse outcome in patients with coronary artery disease. PLoS One 9, e95402 (2014).
Mehilli, J. & Presbitero, P. Coronary artery disease and acute coronary syndrome in women. Heart 106, 487–492 (2020).
Udell, J. A. et al. Sustained sex-based treatment differences in acute coronary syndrome care: Insights from the American heart association get with the guidelines coronary artery disease registry. Clin. Cardiol. 41, 758–768 (2018).
Cenko, E. et al. Sex differences in outcomes after STEMI: effect modification by treatment strategy and age. JAMA Intern. Med. 178, 632–639 (2018).
Sabbag, A. et al. Sex differences in the management and 5-Year outcome of young patients (< 55 Years) with acute coronary syndromes. Am. J. Med. 130, 1324e15–1324e22 (2017).
Tunon, J. et al. Usefulness of a combination of monocyte chemoattractant protein-1, galectin-3, and N-terminal probrain natriuretic peptide to predict cardiovascular events in patients with coronary artery disease. Am. J. Cardiol. 113, 434–440 (2014).
Gutiérrez-Landaluce, C. et al. Parathormone levels add prognostic ability to N‐terminal pro‐brain natriuretic peptide in stable coronary patients. ESC Heart Fail. 8, 2713–2722 (2021).
Fernández-Friera, L. et al. Vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a Middle-Aged cohort: The PESA (Progression of early subclinical atherosclerosis) study. Circulation 131, 2104–2113 (2015). Prevalence.
Akhter, N. et al. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American college of Cardiology-National cardiovascular data registry (ACC-NCDR). Am. Heart J. 157, 141–148 (2009).
Potts, J. et al. Persistent sex disparities in clinical outcomes with percutaneous coronary intervention: Insights from 6.6 million PCI procedures in the united States. PLoS One 13, e0203325 (2018).
Yusuf, S. et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 364, 937–952 (2004).
Njølstad, I., Arnesen, E. & Lund-Larsen, P. G. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark study. Circulation 93, 450–456 (1996).
Young, L. & Cho, L. Unique cardiovascular risk factors in women. Heart 105, 1656–1660 (2019).
Pancholy, S. B., Shantha, G. P. S., Patel, T. & Cheskin, L. J. Sex differences in short-term and long-term all-cause mortality among patients with ST-segment elevation myocardial infarction treated by primary percutaneous intervention: A meta-analysis. JAMA Intern. Med. 174, 1822 (2014).
Khan, N. A. et al. Sex differences in prodromal symptoms in acute coronary syndrome in patients aged 55 years or younger. Heart 103, 863–869 (2017).
Kimenai, D. M. et al. Direct comparison of clinical decision limits for cardiac troponin T and I. Heart 102, 610–616 (2016).
Jneid, H. et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation 118, 2803–2810 (2008).
Redfors, B. et al. Trends in gender differences in cardiac care and outcome after acute myocardial infarction in Western Sweden: a report from the Swedish web system for enhancement of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). JAHA 4, e001995 (2015).
Bugiardini, R. et al. Factors influencing underutilization of evidence-based therapies in women. Eur. Heart J. 32, 1337–1344 (2011).
Mehta, R. et al. Association of fibroblast growth factor 23 with atrial fibrillation in chronic kidney disease, from the chronic renal insufficiency cohort study. JAMA Cardiol. 1, 548–556 (2016).
Samayoa, L. et al. Sex differences in cardiac rehabilitation enrollment: A Meta-analysis. Can. J. Cardiol. 30, 793–800 (2014).
Acena, A. et al. Parathormone levels are independently associated with the presence of left ventricular hypertrophy in patients with coronary artery disease. J. Nutr. Health Aging 20, 659–664 (2016).
Agarwal, I. et al. Fibroblast growth factor-23 and cardiac structure and function. J. Am. Heart Assoc. 3, e000584 (2014).
Faul, C. et al. FGF23 induces left ventricular hypertrophy. J. Clin. Invest. 121, 4393–4408 (2011).
Binnenmars, S. H. et al. Fibroblast growth factor 23 and risk of new onset heart failure with preserved or reduced ejection fraction: The PREVEND study. J. Am. Heart Assoc. 11, e024952 (2022).
Janus, S. E. et al. Multi-variable biomarker approach in identifying incident heart failure in chronic kidney disease: Results from the chronic renal insufficiency cohort study. Eur. J. Heart Fail. 24, 988–995 (2022).
Tan, Z. et al. Relationship between serum growth differentiation factor 15, fibroblast growth factor-23 and risk of atrial fibrillation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9, 899667 (2022).
Kallmeyer, A. et al. Fibroblast growth factor 23 independently predicts adverse outcomes after an acute coronary syndrome. ESC Heart Fail. 11, 240–250 (2024).
Eggers, K. M. et al. Predicting outcome in acute myocardial infarction: An analysis investigating 175 Circulating biomarkers. Eur. Heart J. Acute Cardiovasc. Care 10, 806–812 (2021).
Bergmark, B. A. et al. Association of fibroblast growth factor 23 with recurrent cardiovascular events in patients after an acute coronary syndrome: A secondary analysis of a randomized clinical trial. JAMA Cardiol. 3, 473–480 (2018).
Sharma, S. et al. FGF23 and cause-specific mortality in community-living individuals-the health, aging, and body composition study. J. Am. Geriatr. Soc. 69, 711–717 (2021).
Scicchitano, P. et al. Plasma levels of intact parathyroid hormone and congestion burden in heart failure: Clinical correlations and prognostic role. J. Cardiovasc. Dev. Dis. 9, 334 (2022).
Kuro-o, M. et al. Mutation of the mouse Klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
Tanaka, S., Fujita, S. I., Kizawa, S., Morita, H. & Ishizaka, N. Association between FGF23, α-Klotho, and cardiac abnormalities among patients with various chronic kidney disease stages. PLoS One 11, e0156860 (2016).
Taneike, M. et al. Alpha-Klotho is a novel predictor of treatment responsiveness in patients with heart failure. Sci. Rep. 11, 2058 (2021).
Xu, J. P. et al. Associations between serum soluble α-Klotho and the prevalence of specific cardiovascular disease. Front. Cardiovasc. Med. 9, 899307 (2022).
Cai, J. et al. Association between serum Klotho concentration and heart failure in adults, a cross-sectional study from NHANES 2007–2016. Int. J. Cardiol. 370, 236–243 (2023).
Luo, W., Wei, N., Sun, Z. & Gong, Y. Association between serum α-klotho level and the prevalence of heart failure in the general population. Cardiovasc. J. Afr. 34, 1–6 (2023).
Nowak, A. et al. Prognostic value and link to atrial fibrillation of soluble Klotho and FGF23 in Hemodialysis patients. PLoS One 9, e100688 (2014).
Bergmark, B. A. et al. Klotho, fibroblast growth factor-23, and the renin-angiotensin system—an analysis from the PEACE trial. Eur. J. Heart Fail. 21, 462–470 (2019).
Memmos, E. et al. Soluble Klotho is associated with mortality and cardiovascular events in Hemodialysis. BMC Nephrol. 20, 217 (2019).
Kresovich, J. K. & Bulka, C. M. Low serum Klotho associated with All-cause mortality among a nationally representative sample of American adults. J. Gerontol. Ser. A 77, 452–456 (2022).
Yang, Z. et al. The prognostic value of serum α-klotho in age-related diseases among the US population: A prospective population-based cohort study. Prev. Med. Rep. 42, 102730 (2024).
Olejnik, A., Radajewska, A., Krzywonos-Zawadzka, A. & Bil-Lula, I. Klotho inhibits IGF1R/PI3K/AKT signalling pathway and protects the heart from oxidative stress during ischemia/reperfusion injury. Sci. Rep. 13, 20312 (2023).
Qiu, Z. et al. Activation of Klotho/SIRT1 signaling pathway attenuates myocardial ischemia reperfusion injury in diabetic rats. Shock. https://doi.org/10.1097/SHK.0000000000002418 (2024).
Yang, K. et al. Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease-associated thrombosis in mice. Blood 129, 2667–2679 (2017).
Yue, C. et al. Ultrasound–targeted microbubble destruction technology delivering β–klotho to the heart enhances FGF21 sensitivity and attenuates heart remodeling post–myocardial infarction. Int. J. Mol. Med. 53, 54 (2024).
Daneshgar, N. et al. Klotho enhances diastolic function in aged hearts through Sirt1-mediated pathways. GeroScience 46, 4729–4741 (2024).
Ikushima, M. et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem. Biophys. Res. Commun. 339, 827–832 (2006).
Cui, W., Leng, B., Liu, W. & Wang, G. Suppression of apoptosis in human umbilical vein endothelial cells (HUVECs) by Klotho protein is associated with reduced Endoplasmic reticulum oxidative stress and activation of the PI3K/AKT pathway. Med. Sci. Monit. 24, 8489–8499 (2018).
Maltese, G. et al. The anti-ageing hormone Klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J. Cell. Mol. Med. 21, 621–627 (2017).
Kawarazaki, W. et al. Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency. J. Clin. Invest. 130, 4152–4166 (2020).
Pello Lázaro, A. M. et al. Cardiac rehabilitation increases plasma Klotho levels. J. Clin. Med. 13, 1664 (2024).
Funding
This work was supported by grants from Carlos III Health Institute (ISCIII) (PI17/01495; PI20/00923; PI23/00119; PI24/00978), co-funded by the European Union; Spain’s Ministry of Science and Innovation (RTC2019-006826-1), Spanish Society of Cardiology and Carlos III Health Institute FEDER (FJD biobank: RD09/0076/00101).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.C. and J.T.; Methodology, N.T., C.C., C.G.L., A.H., J.A., L.L.B., M.L.G.C., J.E. and J.T.; Formal Analysis, I.M.F.; Investigation, M.C., A.K.M., A.M., A.A. and O.L.; Resources, N.T., C.C., C.G.L., A.H., J.A., L.L.B., M.L.G.C., J.E. and J.T.; Writing-Original Draft, M.C.; Writing-Review & Editing, J.T.; Supervision, J.T.; Funding Acquisition, J.T. and J.E.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cortés, M., Kallmeyer, A., Tarín, N. et al. Klotho plasma levels are an independent predictor of mortality in women with acute coronary syndrome. Sci Rep 15, 16744 (2025). https://doi.org/10.1038/s41598-025-01334-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01334-2