Abstract
About 60% of the etiological agents of human infections are of animal origin, and the microorganisms causing them can be isolated not only from farmed and domestic animals, but also from wildlife. Enterococcus spp. may exhibit intrinsic or acquired resistance to many antibiotic groups, posing significant therapeutic challenges. The aim of this study was to identify and assess the antibiotic resistance and virulence genes of Enterococcus strains isolated from fecal samples of wild animals. The 118 strains were obtained from deer (n = 38), wild boar (n = 29), hare (n = 19), roe deer (n = 12), fallow deer (n = 5), raccoon dog (n = 4), fox (n = 4), moose (n = 2), polecat (n = 2), rabbit (n = 1), wolf (n = 1) and marten (n = 1). Antibiotic resistance assessments were performed using the disk diffusion method following the recommendations of the European Committee on Antimicrobial Susceptibility Testing (EUCAST). The frequency of occurrence of vancomycin-resistant enterococci (VRE) phenotypes, high-level streptomycin resistance (HLSR), high-level gentamicin resistance (HLGR), and high-level aminoglycoside resistance (HLAR) was also determined. The PCR was used to detect virulence genes (VGs) (agg, gelE, EfaAfs, ace, pil, ebpA, ebpB, ebpC, srtA, hyl, asa, cylA and cylB). The study revealed a high species diversity of Enterococcus spp. Among the 118 strains collected, 70 were resistant to at least one antibiotic. The majority of strains exhibited resistance to eravacycline, while the least resistance was observed against ampicillin. Strains with VRE, HLSR, HLGR, and HLAR phenotypes were identified. Multidrug resistant (MDR) strains were detected. However, extensively drug-resistant (XDR) and pandrug-resistant (PDR) strains were not observed. The virulence factors were present in the tested strains, and the most frequently detected gene was agg encoding aggregation substance. We have provided evidence that healthy wild animals can be reservoirs of pathogenic Enterococcus strains, including MDR strains and with many VGs, which can be transmitted to humans.
Similar content being viewed by others
Introduction
Approximately 60% of human infectious diseases are zoonotic1,2. Pathogens causing infections in humans can be sourced from both wild and domesticated animals. In Poland, the most prevalent wild animals include roe deer, red deer, hares, and wild boars3,4. Various environmental changes resulting from human activities, such as habitat destruction, ongoing urbanization, and crossbreeding of domestic and wild animals, have led to an increased frequency of human contact with animals inhabiting wild areas, consequently elevating the importance of these animals in the spread of diseases5.
“One Health” is an initiative created and advocated by the World Health Organization (WHO). It is characterized by a balanced, integrated, and multisectoral approach to the health of humans, animals, and the environment. The main premise is the collaboration of public health, veterinary, ecological, and research sectors to predict, detect, and prevent global health threats. One of the primary actions defining the “One Health” initiative is the prevention, detection, and control of zoonotic diseases, as well as reducing the prevalence of Antimicrobial Resistance (AMR)6.
The Enterococcus genus comprises Gram-positive, non-spore-forming cocci characterized by low nutritional requirements7. They exhibit high adaptability, including resistance to desiccation, the ability to grow in the presence of high salt concentrations, and a wide range of pH and temperature tolerance, making them widespread in the natural environment. Enterococcus spp. tolerate high concentrations of bile salts, allowing them to colonize the digestive tracts of many animal species, including humans8. The most commonly isolated species from mammals are E. faecalis, E. faecium, E. hirae, and E. durans9. E. faecalis and E. faecium are of greatest clinical significance, often causing hospital-acquired infections such as endocarditis, urinary tract infections, and infections of soft tissues and postoperative wounds10. Infections with Enterococcus spp. have also been observed in animals, causing diarrhea in livestock species such as pigs and cattle and urinary tract infections in domestic cats and dogs11,12,13. In recent years, there has been an increase in microbial resistance to antibiotics. The resistance of Enterococcus spp. to antimicrobial agents is linked to the acquisition of antibiotic resistance genes by these microorganisms14.
The Enterococcus genus is characterized by intrinsic resistance to a broad group of β-lactam antibiotics, including cephalosporins. This is related to two specific penicillin-binding proteins (PBPs), Pbp4(5) and PbpA(2b), exhibit low reactivity toward cephalosporins, allowing these PBPs to cross-link peptidoglycan in the presence of cephalosporins. Moreover, the CroS/R two-component signal transduction system (TCS) is also required for cephalosporin resistance. However, the specific genes regulated by CroS/R that are responsible for these resistance has not yet been fully characterized15,16,17. These bacteria also exhibit reduced susceptibility to penicillins18,19, especially among clinical E. faecium strains. Penicillin resistance in E. faecalis is due to Pbp4(5) overproduction and/or mutations20. Vancomycin-resistant enterococci (VRE) pose a particular threat by producing a different structure of peptidoglycan precursors, a component of bacterial cell walls14. High-level resistance to aminoglycosides (HLAR) is encoded by genes that modify the antibiotic. The HLGR phenotype (high-level gentamicin resistance) is characterized by a high level of resistance to all aminoglycosides, except streptomycin. High-level streptomycin resistance (HLSR) is reported as resistance only for streptomycin19,21,22.
Enterococcus virulence is associated with many virulence genes (VGs), of which two main groups can be distinguished: secreted virulence factors such as cytolysin (cylA), gelatinase (gelE) and hyaluronidase (hyl), which damage host tissues, and those associated with aggregation to the cell surface, such as aggregating substances (asa1), enterococcal surface protein (esp), endocarditis antigen (efaA) and collagen-binding protein (ace). Additionally, expression of pili (encoded by the ebpABC, srt, and pil locus) on the cell surface helps adhesion and biofilm formation23,24,25,26. In addition to antibiotic resistance, monitoring the occurrence of virulence factors among Enterococcus strains isolated from the natural environment is important in the context of the “One Health” trend.
The aim of this study was to identify and assess the antibiotic resistance and selected virulence genes of Enterococcus spp. strains isolated from fecal samples of wild animals.
Material and methods
Origin and collection of samples
Fecal samples from wild animals were collected from forested areas and ecotone zones in two forestry districts in the Kuyavian-Pomeranian Voivodeship, Poland (Table 1). Only fresh fecal samples were collected during the study. The freshness of the samples was assessed based on criteria such as color, sheen and consistency. The species identification of the animal from which the feces originated was determined by an experienced and long-serving forestry employee. Fecal fragments were collected manually and transferred to sterile, appropriately labeled containers, which were then transported to the laboratory within approximately 1 h of collection.
Isolation of Enterococcus spp. from samples
All samples were mechanically homogenized, and approximately 0.5 g aliquots were placed in tubes containing 4.5 ml Brain Heart Infusion (BHI) broth (Becton–Dickinson) and incubated at 37 °C for 24 h. After incubation in BHI (Becton–Dickinson), 0.5 ml of the mixture was transferred to a broth with sodium azide (Merck) (4.5 ml). The tubes were then incubated at 37 °C for 24 h. After incubation, 20 µl of the mixture was inoculated onto Enterococcosel Agar (Becton–Dickinson). The samples were incubated at 37 °C for 24 h. All presumptive Enterococcus colonies were select for identification.
Isolates identification
Species identification was performed using the Matrix-Assisted Laser Desorption/Ionization, Time of Flight (MALDI-TOF) system—Microflex (Bruker) according to the manufacturer’s instructions. To preserve the research material, single colonies of identified microorganisms were transferred to Eppendorf tubes with BHI broth (Becton–Dickinson) and 15.0% glycerol (Avantor) and frozen at − 80 °C.
Assessment of Enterococcus spp. strains susceptibility to antibiotics
The antibiotic susceptibility was assessed using the disk diffusion method with 14 antibiotics: streptomycin (300 µg), ampicillin (2 µg), tigecycline (15 µg), norfloxacin (10 µg), gentamicin (30 µg), teicoplanin (30 µg), eravacycline (20 µg), dalfopristin-quinupristin (15 µg), vancomycin (5 µg), nitrofurantoin (100 µg), levofloxacin (5 µg), ciprofloxacin (5 µg), linezolid (10 µg) and imipenem (10 µg) (Argenta). Susceptibility assessments for nitrofurantoin were only performed for E. faecalis, for dalfopristin-quinupristin only for E. faecium. The zones of growth inhibition, the presence of VRE, HLGR (high-level gentamicin resistance), HLSR (high-level streptomycin resistance), and HLAR (high-level aminoglycoside resistance) phenotypes were determined according to the EUCAST (European Committee on Antimicrobial Susceptibility Testing) v. 13.0 recommendations27. Vancomycin resistance was confirmed by determining the minimum inhibitory concentration (MIC) using MIC Test Strips (Liofilchem). The HLSR phenotype was identified when resistance to streptomycin was detected, HLGR in the case of gentamicin resistance, and the HLAR phenotype was identified in strains resistant to both gentamicin and streptomycin.
The plates were incubated at 35 °C for 18 ± 2 h. After the incubation period, the zone of inhibition around the antibiotic disks were measured. E. faecalis ATCC 29212 was used as a control.
Classification of strains (multidrug-resistant, extensively drug-resistant, pandrug-resistant)
The classification of each strain into three groups: multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR), and thus the determination of the degree of resistance, was based on the guidelines established by Sweeney et al.28. Briefly, a strain was considered MDR when it was resistant to at least one antibiotic in three or more classes. Strain was XDR, when it was resistance to at least one antibiotics in all classes, expected for one or two. Finally, strain was PDR, when it was resistant to all available antibiotics from all chemical groups28.
Detection of virulence genes (VGs)
To detect VGs, isolation of DNA and multiplex PCR reactions were performed. DNA from the Enterococcus spp. strains was isolated using thermal method29. A single colony was suspended in 100 μl of 1 × Tris–EDTA buffer (pH 8.0) (Sigma-Aldrich). Next, it was incubated at 90 °C for 10 min. After this time, the mixture was cooled on ice for 2 min, and then the samples were centrifuged for 5 min (16,000×g, 4 °C). Purified DNA was placed in new tubes and stored at − 20 °C until further studies. The presence of genes encoding the following VFs: endocarditis and biofilm-associated pili (ebpA, ebpB, ebpC), pili (pil), pilus-associated sortase (srt), collagen-binding protein (ace), aggregation substance (agg, asa1), gelatinase (gelE), hyaluronidase (hyl), E. faecalis specific endocarditis antigen (EfaAfs), cytolysin activator (cylA), transport of cytolysin (cylB) was detected using a multiplex PCR, according to the Stępień-Pyśniak et al.30 with some modification (Supplementary Table S1 online). The E. faecalis ATCC 29212 strain was used as positive control. As a negative control, a reaction mixture without DNA was used.
Results
The occurrence of Enterococcus spp. in fecal samples of wild animals
A total of 98 fecal samples from 12 species of animals were examined (Table 1). Enterococci were isolated from 92 (93.9%) samples, resulting in 118 strains belonging to nine species (Table 2). Some samples contained multiple isolates. Among the species, E. faecalis was the most frequently identified (38 strains; 38.2%), followed by the clinically significant E. faecium (7 strains; 7.6%). The least commonly detected species were E. avium and E. thailandicus, with one strain each (0.9%) (Table 2). The samples from deer showed the highest diversity of Enterococcus species, likely reflecting the larger number of fecal samples analyzed from this group (n = 31). Seven species were identified from deer, with E. hirae being the most common (26.0%) and E. avium the least frequent (2.0%). From rabbit feces (n = 1), only E. mundtii was identified, while E. faecium was the sole isolate from wolf feces (n = 1). Polecat feces (n = 1) yielded two species: E. mundtii and E. casseliflavus. For wild boars (n = 17), E. hirae was the most prevalent species (21.0%) (Table 1).
Assessment of Enterococcus spp. strains susceptibility to antibiotics
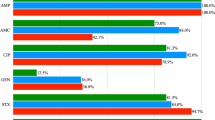
Among the 118 tested strains, 70 (59.3%) were resistant to at least one antibiotic (Fig. 1). Resistance to eravacycline was most common (33.1%; 39 strains), whereas resistance to ampicillin was rare (0.8%; 1 strain). The highest percentage of strains resistant to antibiotics from different chemical groups was observed among E. faecalis and E. faecium, and the lowest among E. avium and E. thailandicus.
Susceptibility of Enterococcus spp. (n = 118) strains to antibiotics. AMP—ampiciline, CIP—ciprofloxacin, ERV—eravacycline, F—nitrofurantoin, IMP—imipenem, LEV—levofloxacin, LZD—linezolid, NOR—norfloxacin, QD—quinupristin-dalfopristin, TEC—teicoplanin, TGC—tigecycline, VA—vancomycin, I- susceptible, increased exposure, R—resistant, S—susceptible.
For E. faecalis (n = 38), most strains were resistant to eravacycline (50.0%), while resistance to nitrofurantoin, imipenem, and norfloxacin was low (5.3% each). In E. hirae (n = 22), resistance to eravacycline was also dominant (31.8%), with resistance to imipenem and norfloxacin limited to one strain each (4.6%). For E. mundtii (n = 19) and E. casseliflavus (n = 17), also most strains were resistance to eravacycline. Notably, E. thailandicus showed no resistance to any of the antibiotics, while the one strain of E. avium showed resistance only to eravacycline.
Resistance was identified in strains from 9 of the 12 animal species examined. No resistant strains were found in moose, rabbit, or wolf samples (Table 3).
Detection of phenotypes: VRE, HLAR, HLSR, HLGR
The VRE phenotype was detected in two (1.7%) strains of E. faecalis isolated from roe deer and wild boar feces. The HLSR phenotype was detected in six (5.1%) of all strains (three E. hirae, one each of E. durans, E. casseliflavus, E. faecalis). Meanwhile, HLGR was observed in 10 strains (five E. durans, three E. hirae, one each of E. casseliflavus and E. faecalis). The highest percentage of strains resistant to streptomycin occurred in hares—two (10.5%), wild boars—two (6.9%), and deer—two (5.3%).
HLAR phenotype was demonstrated in five (4.2%) of all strains. This phenotype was detected in one (20.0%) strain of E. durans, three (13.6%) strains of E. hirae, and one (2.6%) strains of E. faecalis. The presence of the HLAR phenotype was detected in two (5.3%) strains from deer, two (6.9%) strains from wild boars, and one (5.3%) strain isolated from a hare.
Antibiotic resistance profiles
A total of 41 different resistance profiles were observed (Fig. 2). The most common profiles indicated susceptibility to imipenem with increased exposure (profile I, 41.5%), resistant to gentamicin, susceptible, increased exposure to imipenem (profile II, 6.8%), and III resistant to eravacycline; susceptible, increased exposure to imipenem (profile III, 5.1%). These profiles were most common in Enterococcus spp. isolates from deer, hare, and wild boar samples. The strain of E. faecalis isolated from roe deer feces exhibited resistance to seven antibiotics (profile XXXII). Overall, MDR was detected in 22.9% of strains, of which most strains were isolated from deer (18.4%). No PDR or XDR strains were found.
Prevalence of virulence genes (VGs)
Among the virulence genes (VGs) tested, agg, encoding aggregation substance, was the most common, found in 64.7% of strains (Table 4). Also, hyl and gelE genes were frequently detected in tested strains, at 43.7% and 42.0%, respectively. Other VGs, including ebpC, srt, EfaAfs ace, ebpB, ebpA, were present in 16.0–30.0% of strains, whereas cylA and cylB were absent in all isolates (Table 4).
The greatest number of VGs were found in E. faecalis strains. The tested strains were classified into 35 different virulence profiles (Table 5). The largest number of strains had the agg and hyl genes (20; 16.8%) and the agg gene (18; 15.1%). Only in nine (7.6%) strains none of the tested VGs were detected.
Discussion
Wild animals serve as a natural reservoir of microorganisms, some of which may prove to be potentially pathogenic not only for themselves but also for domestic animals and humans. The significant increase in the transfer of microorganisms, including pathogens, between these two niches has been observed due to human economic activities and the urbanization of forested areas. There are several diseases that can be transmitted between animals and humans through direct contact with an infected individual, as well as through contact with their feces or consumption of animal products31,32,33. The increasing prevalence of bacterial resistance to antibiotics is also becoming a significant problem34,35.
The aim of the study was to identify and evaluate the antibiotic resistance of Enterococcus spp. strains that could be a zoonotic pathogenic factor. A total of 12 species of animals (98 collected samples) were examined, resulting in the isolation of nine species of enterococci (118 strains). The occurrence of enterococci in wild animals is not well-described in the literature, as it is in the case of farm animals such as poultry. There is limited research on Enterococcus spp. strains isolated from wild animals.
In this study, the number of fecal samples from which Enterococcus spp. strains were isolated was 92 (93.9%). In a study conducted by Cagnoli et al.36 on Italian wild avifauna species, Enterococcus spp. were isolated from all 103 fecal samples. In a study conducted by Kemper et al.37 on semi-domesticated cervid populations, enterococci were isolated from 92.9% (2224 out of 2243) of samples. Similar results were obtained by Dias et al.34, who recovered Enterococcus spp. isolates from 89.0% of the Red Fox (Vulpes vulpes) fecal samples.
The most clinically relevant species of the Enterococcus genus are E. faecalis and E. faecium24,38. In our study, E. faecalis was the most frequently isolated species among the obtained enterococci. On the other hand, E. faecium was isolated from 7.6% of samples. In a similar study conducted by García et al.39, E. faecalis was isolated in 37.6%, and E. faecium in 17.5%. These authors also used MALDI TOF MS for identification similarly to ours. We obtained the highest number of strains from deer, which is related to the largest number of deer fecal samples included in our study. Nine species of Enterococcus bacteria were isolated from deer fecal samples, with E. hirae (26.0%) and E. mundtii (21.0%) being the most prevalent. In comparison, Liliehaug et al.40 isolated only five strains of E. faecalis and three strains of E. faecium from 50 fecal samples from red deer. For wild boar feces, they reported a much higher percentage of E. faecalis at 93.8%. But they identified enterococci based on PCR. Concerning samples from foxes in our study, the presence of three Enterococcus spp. was detected, with E. faecium constituting 50.0% of all strains of this genus and E. faecalis 25.0%. In a study by Dias et al.34, E. faecalis constituted 49.0% of all Enterococcus species, which is a higher percentage than in our study. In the same study, E. faecium was isolated less frequently at 39.0%, but this result is lower than in our study34. The differences may be related to the different identification method used by the authors who used PCR. In another study conducted in Brazil with 50 strains isolated from wild foxes, identified using MALDI-TOF MS 64.0% E. faecalis and 22.0% E. faecium41. For other animals, species diversity was lower than for deer or foxes in our study.
In our study, it was demonstrated that 59.3% (70/118) of the tested Enterococcus spp. strains were resistant to at least one antibiotic. In a study Lillehaug et al.40, resistance to one or more antibiotics was found in all Enterococcus spp. strains isolated from cervids. Similarly, in a study Oliveira de Araujo et al.41, 98.0% of all Enterococcus spp. strains isolated from foxes were resistant to at least one antibiotic. In our study, enterococci were least resistant to ampicillin (0.8%), a result that is low compared to the literature. In a study by Nocera et al.33, as much as 75.0% of the tested strains were resistant to ampicillin. In another study, 7.2% of Enterococcus spp. strains were resistant to ampicillin, which is still a high result compared to our findings39. In our study, the highest percentage of strains resistant to antibiotics from different chemical groups was found among E. faecalis and E. faecium, and the lowest among E. avium and E. thailandicus.
The detection of resistance phenotypes (VRE, HLAR, HLGR, and HLSR) in Enterococcus spp. isolated from the feces of wild animals is a poorly understood issue, and there is a lack of relevant data in the available literature. However, databases contain studies related to farm animals. In an experiment conducted by Kim et al.21, out of 345 strains of Enterococcus bacteria, 8.7% of the strains exhibited the HLAR phenotype. In our study, this percentage was 4.2% of the tested strains. In this study, the VRE phenotype was detected in only two out of 38 E. faecalis samples (5.3%), which constitutes 1.7% of all Enterococcus spp. strains. Dec et al.38 obtained three out of 52 isolates with high level of resistance (minimal inhibitory concentration ≥ 1,024 μg/mL) to vancomycin and teicoplanin. Other authors39,41 did not detect vancomycin-resistant strains derived from wild animals.
Strains that are resistant to multiple antibiotics (MDR, PDR, and XDR) pose a particular danger and therapeutic challenge. Bacteria are increasingly acquiring resistance not only to one group of antibiotics but to several or even all groups. In our study, multidrug-resistance was detected in 22.9% of Enterococcus spp. strains. No PDR or XDR strains were found, but an E. faecalis strain from deer was resistant to at least one antibiotic from 5 different chemical groups. For comparison, in a study on wild birds conducted in Italy by Cagnoli et al.36, as much as 77.0% of Enterococcus spp. strains exhibited multidrug-resistance, almost 20.0% were PDR, and about 3.0% were XDR. In our study, the percentage of MDR strains in Enterococcus spp. isolated from wild deer feces was 20.69% (6/29). In a study by Oliveira de Araujo et al.41 on foxes in Brazil, 66.0% of strains exhibited multidrug-resistance. In the present study 13 different VGs were detected. The most frequently detected genes were agg, hyl and gelE, which play an important role in the pathogenesis process. Similar results were obtained by Pillay et al.42, who detected the presence of the gelE gene in more than 50% of Enterococcus strains isolated from chicken cloacal samples. Until now, the presence of VGs in strains isolated from wild animals has not been well known. Our study significantly expands the state of knowledge in this area and proves that Enterococcus strains, especially E. faecalis, isolated from this material may contain a number of VGs.
To sum up, it can be stated that Enterococcus spp. strains identified in this study demonstrated a moderate level of antibiotic resistance and virulence compared to findings from other studies on wild animals. Approximately 59.3% of the strains were resistant to at least one antibiotic, which is lower than some studies, such as Lillehaug et al.40 and Oliveira de Araujo et al.41, where nearly all tested strains exhibited resistance to multiple antibiotics. Additionally, the prevalence of MDR strains in our study was 22.9%, considerably lower than the 66–77% MDR rates reported in other research. Virulence factors were detected in a significant proportion of strains, but the diversity and frequency were generally comparable or slightly lower than in studies focusing on wild avifauna or other mammals. Overall, while this study highlights important public health risks, the strains studied appear less resistant and virulent than those reported in certain other contexts, particularly in environments with higher antibiotic pressure.
The findings of this study align with the One Health framework by illustrating the interconnectedness of human, animal, and environmental health through the investigation of antibiotic-resistant and virulent Enterococcus spp. strains in wild animals. They provide valuable insights into how wildlife serves as reservoirs of multidrug-resistant pathogens with zoonotic potential, thus emphasizing the need for integrated surveillance to prevent the spread of AMR. These results are critical for understanding the environmental reservoirs of AMR and for developing strategies to control zoonotic risks, directly supporting One Health’s goal of collaborative, cross-sectoral approaches to global health challenges.
Conclusion
In all fecal samples from wild animals, the presence of potentially pathogenic bacteria from the Enterococcus genus was detected. This study revealed that 23.0% constituted MDR strains. The assessment of resistance to the applied antibiotics, as well as the low proportion of strains with VRE and HLAR phenotypes, indicates overall low antibiotic resistance among Enterococcus spp. strains isolated from fecal samples of wild animals. This work has contributed to enhancing the current understanding of the complexity and antibiotic resistance of the gut microbiota of wild animals. The results, particularly the surprisingly high percentage of strains resistant to eravacycline, may serve as a basis for further comprehensive analyses. The study shows the occurrence of many VGs among the tested strains, of which the gene encoding the aggregation substance was detected most frequently.
Data availability
The data sets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Ghai, R. & Behravesh, C. B. Zoonoses—One Health Approach. In: Centers for Disease Control and Prevention (CDC), Jeffrey B., Nemhauser, C. CDC Yellow Book 2024: Health Information for International Travel. Eds: New York, 2023; online edn, Oxford Academic (2023).
Taylor, L. H., Latham, S. M. & Woolhouse, M. E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356, 983–989. https://doi.org/10.1098/rstb.2001.0888 (2001).
Ssaki - Lasy Państwowe 2018–2023. Available at: https://www.lasy.gov.pl/pl/edukacja/lesnoteka-1/ssaki
WWF. Edukacja WWF | WWF Polska. (2020). Available at: https://www.wwf.pl/edukacja-wwf
Chowdhury, S. et al. Major zoonotic diseases of public health importance in Bangladesh. Vet. Med. Sci. 7(4), 1199–1210. https://doi.org/10.1002/vms3.465 (2021).
World Health Organization (WHO). One Health. (2017). Available at: https://www.who.int/news-room/questions-and-answers/item/one-health
Fisher, K. & Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 155(6), 1749–1757. https://doi.org/10.1099/mic.0.026385-0 (2009).
García-Solache, M. & Rice, L. B. The Enterococcus: A model of adaptability to its environment. Clin. Microbiol. Rev. 32(2), 1–28. https://doi.org/10.1128/CMR.00058-18 (2019).
Ben Braïek, O. & Smaoui, S. Enterococci: Between emerging pathogens and potential probiotics. Biomed. Res. Int. https://doi.org/10.1155/2019/5938210 (2019).
Fiore, E., Van Tyne, D. & Gilmore, M. S. Pathogenicity of Enterococci. Microbiol. Spectr. 7(4). https://doi.org/10.1128/microbiolspec.gpp3-0053-2018. https://doi.org/10.1128/microbiolspec (2019)
Jackson, C. R., Fedorka-Cray, P. J., Barrett, J. B. & Ladely, S. R. High-level aminoglycoside resistant enterococci isolated from swine. Epidemiol. Infect. 133(2), 367–371 (2005).
Smoglica, C. et al. Evidence of linezolid resistance and virulence factors in Enterococcus spp. isolates from wild and domestic ruminants, Italy. Antibiotics (Basel) 11(2), 223. https://doi.org/10.3390/antibiotics11020223 (2022).
Clark, H., Lasarev, M. & Wood, M. Risk factors of enterococcal bacteriuria in cats: A retrospective study. Can. Vet. J. 64(1), 40–44 (2023).
Li, G., Walker, M. J. & De Oliveira, D. M. P. Vancomycin resistance in Enterococcus and Staphylococcus aureus. Microorganisms 11(1), 24. https://doi.org/10.3390/microorganisms11010024 (2023).
Arbeloa, A. et al. Role of class A penicillin-binding proteins in PBP5-mediated β-lactam resistance in Enterococcus faecalis. J. Bacteriol. 186, 1221–1228 (2004).
Comenge, Y. et al. The CroRS two-component regulatory system is required for intrinsic beta-lactam resistance in Enterococcus faecalis. J. Bacteriol. 185(24), 7184–7192. https://doi.org/10.1128/JB.185.24.7184-7192.2003 (2003).
Timmler, S. B., Kellogg, S. L., Atkinson, S. N., Little, J. L., Djorić, D. & Kristich, C. J. CroR regulates expression of pbp4(5) to promote cephalosporin resistance in Enterococcus faecalis. mBio. 30; 13(4), e0111922. https://doi.org/10.1128/mbio.01119-22 (2022)
Miller, W. R., Munita, J. M. & Arias, C. A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti Infect. Ther. 12(10), 1221–1236. https://doi.org/10.1586/14787210.2014.956092 (2014).
Torres, C. et al. Antimicrobial resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.arba-0032-2018 (2018).
Gawryszewska, I., Żabicka, D., Hryniewicz, W. & Sadowy, E. Penicillin-Resistant, Ampicillin-Susceptible Enterococcus faecalis in Polish Hospitals. Microb. Drug Resist. 27(3), 291–300. https://doi.org/10.1089/mdr.2019.0504 (2021).
Kim, Y. B., Seo, K. W., Son, S. H., Noh, E. B. & Lee, Y. J. Genetic characterization of high-level aminoglycoside-resistant Enterococcus faecalis and Enterococcus faecium isolated from retail chicken meat. Poult. Sci. 98(11), 5981–5988. https://doi.org/10.3382/ps/pez403 (2019).
Padmasini, E., Padmaraj, R. & Ramesh, S. S. High level aminoglycoside resistance and distribution of aminoglycoside resistant genes among clinical isolates of Enterococcus species in Chennai, India. Sci. World J. https://doi.org/10.1155/2014/329157 (2014).
Chajęcka-Wierzchowska, W., Zadernowska, A. & Łaniewska-Trokenheim, Ł. Virulence factors of Enterococcus spp. presented in food. LWT 75, 670–676 (2017).
Eaton, T. J. & Gasson, M. J. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl. Environ. Microbiol. 67(4), 1628–1635 (2001).
Kraszewska, Z., Skuczyńska, I., Bogiel, T. & Gospodarek-Komkowska, E. Rola wybranych czynników wirulencji w zakażeniach wywoływanych przez szczepy Enterococcus spp. Adv. Microbiol. 62, 157–171 (2023).
Süßmuth, S. D. et al. Aggregation Substane Promotes Adherence, Phagocytosis and Intracellular Survival of Enterococcus faecalis within Human Macrophages and Suppresses Respiratory Burst. Infect. Immun. 68(9), 4900–4906 (2000).
European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoints tables for interpretation of MICs and zones diameters. Version 13.0 (2023).
Sweeney, M. T., Lubbers, B. V., Schwarz, S. & Watts, J. L. Applying definitions for multidrug resistance, extensive drug resistance and pandrug resistance to clinically significant livestock and companion animal bacterial pathogens. J. Antimicrob. Chemother. 73(6), 1460–1463. https://doi.org/10.1093/jac/dky043 (2018).
Kovačević, J., Mesak, L. R. & Allen, K. J. Occurrence and characterization of Listeria spp. in ready-to-eat retail foods from Vancouver, British Columbia. Food Microbiol. 30(2), 372–378 (2012).
Stępień-Pyśniak, D., Hauschild, T., Kosikowska, U., Dec, M. & Urban-Chmiel, R. Biofilm formation capacity and presence of virulence factors among commensal Enterococcus spp. from wild birds. Sci. Rep. 9(1), 1–7 (2019).
Gliński, Z. & Żmuda, A. Jelenie i sarny rezerwuarem patogenów dla zwierząt hodowlanych i ludzi. Życie Wet. 96(9), 631–635 (2021).
Kruse, H., Kirkemo, A. M. & Handeland, K. Wildlife as source of zoonotic infections. Emerg. Infect. Dis. 10(12), 2067–2072. https://doi.org/10.3201/eid1012.040707 (2004).
Nocera, F. P. et al. A preliminary study on antimicrobial susceptibility of Staphylococcus spp. and Enterococcus spp. grown on mannitol salt agar in European wild boar (susscrofa) hunted in campania region—Italy. Animals https://doi.org/10.3390/ani12010085 (2022).
Dias, D. et al. Unravelling the diversity and abundance of the red fox (Vulpesvulpes) faecal resistome and the phenotypic antibiotic susceptibility of indicator bacteria. Animals https://doi.org/10.3390/ani12192572 (2022).
World Health Organization (WHO). Antibiotic resistance (2020). Available at: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance
Cagnoli, G. et al. Antimicrobial Resistant Enterococcus spp. in Wild Avifauna from Central Italy. Antibiotics 11(7), 852. https://doi.org/10.3390/antibiotics11070852 (2022).
Kemper, N., Aschfalk, A. & Höller, C. Campylobacter spp., Enterococcus spp., Escherichia coli, Salmonella spp., Yersinia spp., and Cryptosporidium oocysts in semi-domesticated reindeer (Rangifer tarandustarandus) in Northern Finland and Norway. Acta Vet. Scand. https://doi.org/10.1186/1751-0147-48-S1-S7 (2006).
Dec, M. et al. Antibiotic susceptibility and virulence genes in Enterococcus isolates from wild mammals living in Tuscany, Italy. Microb. Drug Resist. 26(5), 505–519. https://doi.org/10.1089/mdr.2019.0052 (2020).
García, L. A., Torres, C., López, A. R., Rodríguez, C. O. & Valencia, C. S. Antimicrobial resistance of Enterococcus species isolated from wild mammals in Aragón, Spain. J. Vet. Res. 66(2), 151–159. https://doi.org/10.2478/jvetres-2022-0020 (2022).
Lillehaug, A. et al. Campylobacter spp., Salmonella spp., Verocytotoxic Escherichia coli, and Antibiotic Resistance in Indicator Organisms in Wild Cervids. Acta. Vet. Scand. 46(1–2), 23–32. https://doi.org/10.1186/1751-0147-46-23 (2005).
Oliveira de Araujo, G. et al. Multidrug Resistance in Enterococci Isolated From Wild Pampas Foxes (Lycalopexgymnocercus) and Geoffroy’s Cats (Leopardusgeoffroyi) in the Brazilian Pampa Biome. Front. Vet. Sci. https://doi.org/10.3389/fvets.2020.606377 (2020).
Pillay, S., Zishiri, O. T. & Adeleke, M. A. Prevalence of virulence genes in Enterococcus species isolated from companion animals and livestock. Onderstepoort J. Vet. Res. 27;85(1), e1–e8. https://doi.org/10.4102/ojvr.v85i1.1583 (2018)
Acknowledgements
We sincerely thank the employees of the Forest Districts for granting permission for our research and assisting in sample collection.
Funding
The research was financed with founds of Emerging Field One Health—antimicrobial stewardship in human and veterinary medicine (Excellence Initiative—Research University, Nicolaus Copernicus University in Toruń). The article is financed with funding of publication costs under the "Excellence Initiative—Research University" programme in Nicolaus Copernicus University in Toruń.
Author information
Authors and Affiliations
Contributions
Skowron Krzysztof: Conceptualization, Funding acquisition, Methodology, Supervision, Writing—review & editing. Borkowski Wiktor: Conceptualization, Investigation, Validation, Writing—original draft. Wiktorczyk-Kapischke Natalia: Formal analysis, Methodology, Visualization, Writing—original draft. Budzyńska Anna: Formal analysis, Project administration, Writing—original draft. Wilk Monika: Investigation, Visualization. Czuba Julia: Investigation, Validation. Fiderewicz Jan: Resources. Skonieczna-Kurpiel Joanna: Investigation. Grudlewska-Buda Katarzyna: Conceptualization, Funding acquisition, Methodology, Project administration, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Skowron, K., Borkowski, W., Wiktorczyk-Kapischke, N. et al. Occurrence and assessment of antibiotic resistance and virulence of Enterococcus spp. strains isolated from fecal samples of wild animals. Sci Rep 15, 16957 (2025). https://doi.org/10.1038/s41598-025-01492-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01492-3