Abstract
Seed germination and seedling growth are crucial for the successful establishment and reproduction of plants in heterogeneous environments, especially in the ecologically fragile karst regions. Despite the ecological importance of perennial ryegrass (Lolium perenne L.) as a forage resource and its role in mitigating rocky desertification, studies addressing the effects of karst-specific environmental factors on its early growth stages are limited. This study is the first to simulate karst soil conditions to evaluate the impacts of drought (0–0.53 MPa), salinity (0–150 mM), and pH (pH 3–9) on seed germination and seedling growth of perennial ryegrass. The results showed that under different drought stresses, water potentials ranging from 0 to − 0.32 MPa had no significant effect on seed germination. However, water potentials of − 0.06 MPa and − 0.17 MPa significantly promoted root and shoot growth, as well as increased biomass. In the salt stress experiment, CaCl2 concentrations of 5–10 mM favored seed germination; specifically, 5 mM CaCl2 increased the germination rate to 96.5%, and root and shoot lengths exceeded those of the control. pH levels ranging from 3 to 9 had little effect on germination, but extremely acidic conditions (pH 3) significantly inhibited root and shoot elongation. Therefore, optimal growth conditions were determined to be drought stress from 0 to − 0.17 MPa, calcium salt stress from 0 to 25 mM, and a pH of 4 to 9. These findings identify optimal growth conditions for perennial ryegrass, providing a scientific basis for seed cultivation, pasture management, and ecological restoration in karst regions. Our study contributes to the understanding of plant responses to environmental stresses in karst systems and supports sustainable agricultural and conservation practices.
Similar content being viewed by others
Introduction
Karst regions account for approximately 15% of the global land area and possess unique ecosystems that are widely distributed worldwide. The karst region in southwestern China covers over 500,000 square kilometers and represents the most densely distributed karst area globally, characterized by extremely fragile ecosystems1,2,3. Like other fragile ecosystems, their biodiversity and ecological stability face dual challenges from biotic and abiotic stresses1,4. Biotic stresses mainly arise from herbivores and human activities such as grazing, trampling, mowing, and plowing, while abiotic stresses are related to environmental and climatic factors including drought, rocky desertification, soil erosion, and salinization5,6. In karst areas, the intensification of rocky desertification has led to soil erosion, resulting in the loss of clay and silt particles and making the soil texture coarser. This degradation decreases soil and water conservation capacity, exacerbating soil drought7, which poses a serious threat to seed germination and seedling growth8,9. Additionally, rocky desertification exacerbates the accumulation of soil Ca2+ and Mg2+ ions, leading to soil pH imbalance and further challenging plant survival10,11. Therefore, in karst areas, drought, high salinity, and unsuitable pH are key factors limiting plant growth, especially during the seed germination stage12,13.
Drought stress affects seed germination and seedling growth by increasing osmotic pressure14. Morphologically, drought causes decreased germination rates, hindered hypocotyl elongation, reduced fresh weight of stems and roots, increased root length, curled and tightly closed leaves, and premature flowering15. At the physiological level, drought stress leads to impaired cell expansion, decreased water potential, and reduced stomatal conductance, resulting in lower intracellular CO2 concentration and reduced net photosynthetic rates. These changes ultimately result in decreased plant growth rate and biomass16,17. Drought stress significantly impacts carbon assimilation and plant phenology18,19, especially during key germination and emergence stages of crops such as maize20, rice21, and wheat22. To resist drought, plants adopt various adaptive strategies, including enhancing root systems, reducing leaf area and stomatal conductance, osmotic regulation, activating antioxidant systems, and inducing drought-responsive genes and transcription factors23,24,25.
Salt stress adversely affects plant physiology and growth at various developmental stages21,26, causing significant losses in agricultural production27,28. Salinity impacts plants by reducing soil water potential, causing ion toxicity and osmotic shock, and interfering with the uptake and transport of essential nutrients29. During the seedling stage plants are particularly sensitive to soil salinity. Salt may interfere with seed germination, limit plant growth, and ultimately lead to decreased crop yields27,30. Salt-induced osmotic stress and ion toxicity are two key factors that hinder seed germination. When salinity disrupts the solute balance inside seeds, osmotic stress occurs, leading to higher osmotic potential, hindering normal water absorption, and making it difficult for radicle to break through the seed coat, thereby inhibiting successful germination. Harmful ion accumulation in seed cells leads to a series of physiological disorders, such as changes in metabolic pathways, disruption of cell membrane integrity, and reduced cell division and expansion31,32. Therefore, studying the salt tolerance limits of plants is crucial for maintaining their normal growth and development.
Another key soil factor influencing seed germination is the concentration of hydrogen ions, reflected by soil pH. Soil pH affects plant growth in two ways: directly, through the toxic effects of hydrogen ions under extreme pH conditions, and indirectly, by influencing the absorption and assimilation of multiple nutrients and altering the solubility of certain harmful elements33,34. Soil pH not only affects seed germination but also influences nutrient availability, with tolerance ranges varying among plant species35. According to previous research, many plant species can germinate within a wide pH range36,37; however, for certain species, pH may become a limiting factor for their growth38.
Perennial ryegrass (Lolium perenne L.) is a grass species native to Europe, Asia, and North Africa, widely distributed in eastern, central, and southwestern China39,40. Perennial ryegrass has important nutritional and ecological values. It is nutritious and has good palatability, making it a high-quality feed suitable for introduction as an economic crop41. In addition, ryegrass has a well-developed fibrous root system that can deepen the active soil layer, stabilize the soil during growth, enhance soil erosion resistance, reduce runoff, and control soil erosion42. In karst areas, drought, high salinity, and soil pH imbalance have been identified as key abiotic factors limiting plant establishment and growth in karst ecosystems43. Although perennial ryegrass has important ecological and economic significance as a feed resource and a key plant for preventing and controlling rocky desertification in karst areas44,45, but there is a lack of comprehensive research to evaluate the specific impact of these environmental factors on their early developmental stages. This study simulated karst soil conditions to investigate the effects of drought, calcium salt stress, and pH on the germination and early growth of perennial ryegrass seeds. For the first time, a systematic evaluation was conducted on how specific abiotic stresses characteristic of Karst areas affect the germination and early growth of perennial ryegrass.
Therefore, this study used perennial ryegrass seeds as materials to investigate the effects of abiotic stress on seed germination indicators (germination rate, germination potential, germination index, vitality index), seedling morphology indicators (shoot length and root length), and biomass indicators (fresh and dry weight of roots and shoots). The aim of this study is (1) to investigate the effects of drought stress, calcium salt stress, and pH stress on the germination ability of perennial ryegrass seeds; (2) Compare the changes in plant traits during the seedling stage under different stress conditions; (3) Determine the optimal growth conditions under these stresses. By evaluating the correlation between these stress factors and seed germination and seedling growth, it is determined whether these correlations change due to changes in stress conditions, providing valuable insights for ecological protection, pasture management, and sustainable use of perennial ryegrass in karst areas.
Materials and methods
Germination experiment
Seed material and preparation
Seeds of the perennial ryegrass (Lolium perenne L.) cultivar “Green Spirit” were provided by Bailu China Grassland Development Company. Prior to the experiment, seeds uniform in size, plump, and free from pests and diseases were selected. These seeds were surface-sterilized by soaking in a 1% sodium hypochlorite (NaClO) solution for 5 min, followed by five rinses with distilled water to remove any residual disinfectant.
Germination setup
Fifty treated seeds were uniformly placed in 9 cm diameter petri dishes lined with Whatman No. 10 filter paper. Each treatment was replicated four times and each petri dish was used as an experimental unit. The filter paper was moistened with 5 ml of distilled water or specific treatment solution. To minimize water evaporation, the dishes were sealed with cling film. All experiments were carried out at a constant temperature of 25 °C with a photoperiod of 12 h light/12 hours dark, a relative humidity of 55%, and a light intensity of 1500 lx, using fluorescent lamps as the light source46.
Seeds were considered germinated when the radicle reached approximately 2 mm in length. Germinated seeds were counted daily over a period of 15 days.
Drought treatment
Osmotic solutions with potentials of 0 (distilled water/control), − 0.06, − 0.17, − 0.32, and − 0.53 MPa were prepared by dissolving appropriate amounts of polyethylene glycol (PEG) 6000 in deionized water.
Salt treatment
To assess the effects of salt stress, calcium chloride (CaCl2) solutions at concentrations of 0 (distilled water/control), 5, 10, 25, 50, 100, and 150 mM were prepared.
pH treatment
Buffer solutions at pH values of 3, 4, 5, 6, 7 (distilled water/control), 8, and 9 were prepared by adjusting distilled water using NaOH or HCl. The pH was monitored using pH test strips. Each culture dish received 5 mL of the corresponding buffer solution, replenished daily to maintain pH stability47.
Germination test
Seed germination indicators were determined according to the International Rules for Seed Testing (International Seed Testing Association)48. The germination potential was calculated on day 5, while the germination rate, germination index, vigor index, root length, shoot length, inhibition rates, fresh weight, and dry weight were measured on day 14. A seed was considered germinated when the radicle reached approximately 2 mm in length49.
On day 14, ten seedlings were randomly selected from each treatment group for measurement. Shoot length was measured from the cotyledonary node to the tip of the longest leaf, and root length was measured from the cotyledonary node to the tip of the longest root. The inhibition rates of root and shoot lengths were calculated based on comparisons with the control group. Roots and shoots were then separated, washed, blotted dry, and weighed to obtain fresh weight. Samples were first oven-dried at 105 °C for 20 min to terminate metabolic activity, and subsequently dried at 75 °C until reaching a constant weight for dry mass determination50.
In the formula: n1 is the number of seeds germinated during the peak germination period, N is the number of tested seeds, n2 is the number of seeds germinated on day 14, and GT is the number of seeds germinated on day T; DT is the corresponding germination days; G1, G2,…, Gn are the number of germinated seeds on days 1, 2,…, n; N1, N2,…, Nn are the corresponding germination days.
Data analysis
Data were processed and statistically analyzed using Microsoft Excel 2022 and SPSS 25.0 software respectively. One-way analysis of variance (ANOVA) was performed and means were compared using Tukey’s multiple range test at 5% level of significance (P ≤ 0.05). Graphs were generated using Origin 10.1 software. Pearson’s correlation analysis and heatmap generation were conducted using Origin 10.1 software to evaluate relationships between seed germination and seedling growth parameters under different treatments.
Results
Effects of different treatments on seed germination
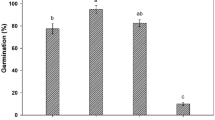
Analysis of variance showed that drought, salinity, and pH had varying degrees of effects on the germination potential, germination rate, germination index, and vitality index of perennial ryegrass seeds (Fig. 1). Among them, the impact of drought on seed germination exhibits different trends under varying levels of water stress (Fig. 1A, D). Under PEG-6000 treatment at − 0.17 MPa, there was no significant difference in germination potential (74.25%) and germination rate (89.75%) compared to the control group (distilled water). However, at − 0.53 MPa, both germination potential (52.75%) and germination rate (75.9%) were significantly lower than those of the control group (Fig. 1A). Similarly, the germination index at − 0.17 MPa (27.12) was comparable to that of the control group, while at − 0.53 MPa, it was significantly reduced (16.73) (Fig. 1D). Additionally, the vitality index was significantly reduced even at − 0.06 MPa PEG-induced drought stress. Overall, under drought stress, the final germination rate remained high across all osmotic potentials, ranging from 79.5 to 91.75% (Fig. 1A), but did not reach the germination rate of the control group (96.25%). Significant responses to drought stress were observed only at higher stress levels (–0.32 MPa and below).
Salt stress significantly affected the germination potential, germination rate, germination index, and vitality index of perennial ryegrass seeds (Fig. 1B, E). Analysis under different salt concentrations showed that at 50 mM CaCl2, the germination rate (90.00%) was comparable to that of the control group, while at 100 mM, the germination rate slightly decreased to 80.25% (Fig. 1B). At 10 mM CaCl2, the germination potential (83.25%) was similar to the control, but it significantly decreased at 25 mM. There was no significant difference in germination index and vitality index between the 0–10 mM salt treatments and the control group (Fig. 1E). These results indicate that within the salt concentration range of 0–50 mM, although some effects on the germination potential, germination index, and germination index were observed, the final germination rate of perennial ryegrass seeds was not significantly altered.
pH had no significant effect on the germination of perennial ryegrass seeds (Fig. 1C, F). Within the pH range of 3 to 9, the germination rate remained above 92%. At pH 3, the germination potential (74.5%) was comparable to that of the control group (Fig. 1C). When pH was 8 or 9, the germination index was 30.18 and 29.22, respectively, similar to the control group. However, at pH 6, the germination index was significantly lower than the control. Additionally, at pH 3 and 9, the vitality index significantly decreased (Fig. 1F). These results indicate that the germination of perennial ryegrass seeds exhibits a wide tolerance range to pH values.
Effects of different treatments on the germination of perennial ryegrass seeds (mean ± SE; n = 4). (A) Effects of PEG-6000 treatments on germination potential and germination rate. (B) Effects of CaCl2 treatments on germination potential and germination rate. (C) Effects of pH treatments on germination potential and germination rate. (D) Effects of PEG-6000 treatments on germination index and vitality index. (E) Effects of CaCl2 treatments on germination index and vitality index. (F) Effects of pH treatments on germination index and vitality index. Different lowercase letters indicate significant differences among treatments at P ≤ 0.05 level.
Effects of different treatments on seedling growth
Analysis of variance showed that drought, salinity, and pH significantly affected the aboveground and belowground characteristics of perennial ryegrass seedlings (Fig. 2). Under simulated drought stress using PEG-6000 solutions, PEG-6000 treatments at − 0.06 MPa and − 0.17 MPa promoted root (10.98 cm and 9.58 cm, respectively) and shoot growth (9.15 cm and 8.47 cm, respectively) compared to the control. However, as the PEG-6000 concentration increased, the growth rate of seedlings gradually decreased (Fig. 2A). Additionally, with intensifying drought stress, the inhibition rates of root and shoot lengths initially increased and then decreased, with drought stress inhibiting shoot growth more significantly than root growth (Fig. 2D).
Under salt stress, the growth trend of perennial ryegrass seedlings was similar to that observed under drought stress (Fig. 2B, E). Specifically, at salt concentrations of 5 mM, 10 mM, and 25 mM, shoot growth was promoted, with shoot lengths of 9.16 cm, 8.99 cm, and 8.87 cm, respectively. Root lengths at 5 mM and 10 mM (8.55 cm and 7.86 cm, respectively) were similar to those of the control group (Fig. 2B). Within the salt concentration range of 5–25 mM, the inhibition rate of shoot length was lower than that of the control. At 10 mM salt concentration, the root length inhibition rate (4.01%) was higher compared to the control, indicating that the inhibitory effect of salt stress on root growth was more pronounced than on shoot growth (Fig. 2E).
Figure 2C shows that perennial ryegrass seedlings exhibit high adaptability to pH changes, with no significant growth differences observed as pH increased or decreased. Within the pH range of 4 to 9, shoot and root growth were not significantly different from the control group. Notably, shoot growth was slightly promoted at pH 5 and 6 (8.38 cm and 8.43 cm, respectively), while root growth was more favorable at pH 8 and 9 (8.56 cm and 8.40 cm, respectively). Additionally, the data in Fig. 2F indicate that under acidic conditions, the inhibitory effect on root growth was more pronounced than on shoot growth. Conversely, in alkaline environments, the inhibition of shoot growth was more significant, while the effect on root growth was relatively small.
Effects of different treatments on the growth of perennial ryegrass seedlings (mean ± SE; n = 4). (A) Effects of PEG-6000 treatments on root and shoot lengths. (B) Effects of CaCl2 treatments on root and shoot lengths. (C) Effects of pH treatments on root and shoot lengths. (D) Effects of PEG-6000 treatments on root length inhibition rate and shoot length inhibition rate. (E) Effects of CaCl2 treatments on root length inhibition rate and shoot length inhibition rate. (F) Effects of pH treatments on root length inhibition rate and shoot length inhibition rate. Different lowercase letters indicate significant differences among treatments at P ≤ 0.05 level.
Effects of different treatments on seedling biomass
Figure 3 illustrates the differential effects of various environmental factors on the biomass allocation of perennial ryegrass. With increasing PEG-6000 concentration, the fresh and dry weights of seedlings initially increased and then decreased (Fig. 3A, D). Under PEG-6000 treatments at − 0.06 MPa and − 0.17 MPa, there was no significant difference in shoot fresh weight compared to the control group (0.10 g and 0.09 g, respectively), and an increase in root fresh weight was observed (0.06 g and 0.05 g, respectively) (Fig. 3A). Within the PEG-6000 treatment range of − 0.06 MPa to − 0.32 MPa, no significant differences in shoot and root dry weights were found compared to the control. However, at − 0.53 MPa, the dry weights were significantly lower than those of the control group (Fig. 3D).
Salt stress significantly affected the biomass accumulation in perennial ryegrass seedlings (Fig. 3B, E). At salt concentrations of 5 mM and 10 mM, shoot fresh weight was promoted (0.13 g and 0.11 g, respectively). Within the salt concentration range of 5–50 mM, shoot fresh weights were similar to those of the control group. However, at a salt concentration of 150 mM, root fresh weight was significantly lower than that of the control group (0.02 g, Fig. 3B). Within the salt concentration range of 5–50 mM, no significant changes in shoot and root dry weights were observed. However, at 100 mM salt concentration, both shoot and root dry weights were significantly lower than those of the control group (Fig. 3E).
Under different pH treatments, the fresh weight of perennial ryegrass shoots showed minimal differences overall. However, at pH 3, root fresh weight was significantly lower than that of the control group (0.02 g, Fig. 3C). Within the pH range of 3 to 9, seedling fresh and dry weights showed varying degrees of promotion. Nonetheless, at pH 3, root dry weight was significantly lower than that of the control group (Fig. 3F).
Effects of different treatments on the biomass accumulation of perennial ryegrass seedlings (mean ± SE; n = 4). (A) Effects of PEG-6000 treatments on root fresh weight and shoot fresh weight. (B) Effects of CaCl2 treatments on root fresh weight and shoot fresh weight. (C) Effects of pH treatments on root fresh weight and shoot fresh weight. (D) Effects of PEG-6000 treatments on root dry weight and shoot dry weight. (E) Effects of CaCl2 treatments on root dry weight and shoot dry weight. (F) Effects of pH treatments on root dry weight and shoot dry weight. Different lowercase letters indicate significant differences among treatments at P ≤ 0.05 level.
Correlation analysis between seed germination and seedling growth indicators under different treatments
Under drought stress (Fig. 4), the PEG-6000 concentration was significantly positively correlated with root length inhibition rate, and shoot length inhibition rate, and highly significantly negatively correlated with germination potential, germination rate, germination index, vitality index, root length, shoot length, root fresh weight, shoot fresh weight, root dry weight, and shoot dry weight. A positive correlation was observed between various indicators of seed germination and various indicators of seedling growth. Under salt stress (Fig. 5), the correlations among various indicators showed a consistent trend with those under drought stress. Under pH stress (Fig. 6), pH was positively correlated with germination index, vitality index, root length, and root fresh weight, but negatively correlated with root length inhibition rate. It was not significantly correlated with germination potential, germination rate, shoot length, shoot length inhibition rate, shoot fresh weight and shoot dry weight. Overall, both drought and salt stresses had significant negative effects on the germination and seedling growth of perennial ryegrass seeds, while the negative impact of pH on seed germination was relatively low. In addition, there is a strong correlation between the germination indicators of perennial ryegrass seeds and the seedling growth parameters, suggesting that multiple indicators should be comprehensively considered when evaluating the adaptability of this species to abiotic stress conditions.
Discussion
The adaptability of seed germination and seedling growth is a key factor for plants to successfully establish populations and reproduce in heterogeneous environments. The aim of this study was to investigate the effects of different stress conditions on seed germination and seedling growth of perennial ryegrass, to provide a scientific basis for seed germination and seedling management strategies in karst areas. Our results indicate that drought, salinity, and pH significantly affect the germination and seedling growth of perennial ryegrass seeds.
Effects of drought stress on seed germination and seedling growth
Karst landscapes are characterized by high bedrock exposure, shallow soils, poor water retention capacity, strong surface evaporation, and abundant but unstable precipitation. Therefore, even in areas with ample rainfall, the phenomenon of “karst drought” can occur51,52. Numerous studies on plants in karst areas have shown that limited water resources inhibit seed germination and plant growth8,9,53, making seed germination critically important for vegetation restoration in degraded karst ecosystems.
The results of this study indicate that perennial ryegrass can tolerate moderate drought conditions. When its water potential is within the range of − 0.06 to − 0.17 MPa, there is no significant difference in seed germination rate compared to the control group, and it can significantly promote root growth and biomass accumulation. This finding is similar to the conclusion drawn by Wang et al. in their study comparing the drought resistance of 10 herbaceous plants during germination54. This indicates that controlling the level of drought stress can optimize root development, enhance soil stability and water absorption efficiency in karst areas55. These insights are valuable for ecological restoration projects, as water scarcity often limits the establishment of plants.
However, under extreme drought conditions (water potential of − 0.53 MPa), seed germination and seedling growth are significantly inhibited. For perennial ryegrass, the availability of water is the initial key factor affecting seed germination. Lack of water can interfere with the activity of enzymes, cell division, and other physiological metabolic processes within the seeds. The decrease in germination rate may be due to insufficient seed hydration caused by high osmotic potential, which inhibits the mechanism that promotes seed germination and leads to delayed germination56,57,58. In addition, it may also be due to changes in enzymes and hormones in the seeds59. Moreover, the softening rate of endosperm is extremely sensitive to seed water potential. Similarly, under water stress, a decrease in seed water potential can inhibit embryonic root growth and germination60. This may be because the seeds extend the preparation period for germination to resist drought stress, requiring sufficient water accumulation before germination. This mechanism helps to reduce the risk of plant survival in arid regions, while the embryonic roots continue to elongate to obtain more water to meet metabolic activities and growth needs61. Under drought stress, perennial ryegrass seedlings prioritize resource allocation to the underground parts, reflecting the plant’s resource allocation strategy in response to stress. These results are consistent with studies on other species such as alfalfa62 and sorghum63, which suggest that mild drought stress can promote root elongation and resource allocation to underground structures. This emphasizes the importance of considering rainy season time management or timely irrigation management during critical stages such as seed germination and seedling growth in areas prone to long-term drought. In practice, these findings can guide time management (rainy season) or irrigation time and intensity management in the establishment of ryegrass grasslands in karst areas, which is a basic strategy for seasonal water scarce karst areas and can also be considered in similar semi-arid ecosystems worldwide.
Effects of salt stress on seed germination and seedling growth
Salt stress is one of the main limiting factors for plant development, especially in the context of livestock feed production. Seed germination and seedling growth are critical stages in the plant life cycle, and plant salt tolerance is crucial for survival. In karst habitats, most of the bedrock is composed of carbonate rocks, with CaCO3 and MgCO3 as the main components; calcium-rich soils are a significant feature of these areas. Calcium is an essential element for the growth of higher plants; Ca2+ is a signaling substance involved in plant growth, development, and seed germination64. However, high concentrations of salt can inhibit seed germination65. Therefore, understanding the response of plants during germination is crucial for a deeper understanding of salt tolerance, sensitivity, and survival mechanisms.
This study found that low salt concentrations (5–10 mM CaCl₂) can promote seed germination and seedling growth, while high salt concentrations (≥ 50 mM) can inhibit these processes. This result is similar to the study by Tanveer et al.66, indicating that the response of melon seed germination to salt stress exhibits a “low promotion, high inhibition” characteristic. Similar salt stress inhibition effects have also been observed in the growth process of rice and wheat seedlings67,68. This biphasic reaction highlights the complex role of calcium ions in plant physiological processes. Low concentration salt stress promotes germination and seedling growth, possibly due to the ability of low concentration salt solutions to trigger seeds, stimulate increased enzyme activity and membrane metabolism within the seeds69. In addition, although CaCl₂ can provide calcium and promote root growth at low concentrations, it also increases Cl⁻ content. The increase in salinity can inhibit seed germination and root elongation by slowing down seed water absorption70. At high concentrations, CaCl₂ can cause ion toxicity71, leading to root shortening, root tip softening, and gradual seedling death, thereby posing a threat to seedling growth. When plants are subjected to salt stress, the roots are the first organ to sense the stress and gradually transmit the stress signal to the aboveground parts. The normal growth, development, physiological and biochemical metabolic processes of plants are severely disrupted72. This study also found that with the increase of salt concentration, the inhibitory effect of salt stress on root growth was significantly greater than that on aboveground growth, which further confirms that the root system is indeed the primary organ affected by salt stress. Meanwhile, the growth and biomass of roots may determine energy allocation, which in turn affects plant tolerance to adversity73.
Our research findings suggest that if calcium salinity is controlled within a tolerable range, it can naturally provide optimal conditions for the germination of perennial ryegrass. The soil calcium content in karst areas is generally high74. The soil exchangeable calcium content in areas affected by mild rocky desertification is 0.02 g/kg, while in areas affected by severe rocky desertification, the soil exchangeable calcium content is as high as 3.92 g/kg75. Soil with high calcium content has become one of the key environmental factors affecting the physiological characteristics and distribution of plants in rocky desertification areas. Excessive calcium ions not only inhibit plant growth, but may also reduce crop yields76. From the perspective of ecological restoration, these results emphasize the importance of monitoring soil calcium concentration and applying low concentration calcium treatment to improve the germination rate and successful establishment of plants in degraded or calcium saline soils. On a global scale, this study emphasizes the potential application of perennial ryegrass in other calcium rich or saline ecosystems, such as the Mediterranean and semi-arid pastures, where soil salinity often limits plant growth77.
Effects of pH stress on seed germination and seedling growth
Soil pH is an important factor affecting weed seed germination78,79. It is also the subject of many studies on grasslands80,81 and tree species82. Additionally, the germination response to different pH buffer solutions has been used to identify the adaptability or potential ability of plant species to establish in acidic, neutral, or alkaline soils. The effect of pH on seed germination and subsequent seedling growth may be mediated by affecting enzyme activity, metabolic activity, and weakening of the cell wall83,84.
Under extreme acidic conditions, the synthesis and activity of enzymes required for seed germination are inhibited, which not only affects the integrity of the seeds but may also lead to perforation of the seed coat by promoting the growth of certain pathogens or directly dissolving the seed coat, thereby affecting normal seed germination85. In highly alkaline environments, the germination time of seeds may be delayed86.
The results of this study reveal that perennial ryegrass has a wide range of pH tolerance, with a tolerance range of pH 4–9. The optimal growth was observed in a slightly acidic to slightly alkaline environment, i.e. pH 6–9. This is similar to the findings of Florentine et al. regarding the response of seed germination of the agricultural weed Plantago asiatica to pH levels87. In an acidic environment, when the pH value is 3, the germination potential, germination rate, germination index, and vigor index of seeds are all at their lowest values. This may be due to changes in seed permeability caused by strong acid stress, ion imbalance, and disruption of normal physiological functions within the seed, resulting in hindered seed germination. In alkaline environments, the germination rate of seeds is higher than that of the control. This adaptability makes it suitable for various soil types, especially in karst areas where changes in soil pH are common due to the presence of carbonate rocks. The effect of pH value on plant growth varies depending on the plant species. Silva et al.88 found that after 40 days of simulated acid rain treatment at pH 3, the seedlings of two trees (Eugenia uniflora and Clusia hilariana) suffered significant damage. Our research found that compared to pH 7 conditions, pH 3 had a significant inhibitory effect on the growth of perennial ryegrass. When the pH value is 4–9, there is no significant difference in root length of perennial ryegrass seedlings. The alkaline conditions of pH 8–9 are conducive to biomass accumulation. The ability of perennial ryegrass to germinate within a wide pH range indicates its potential for use in soil protection and ecological restoration in various environments, including acidic or alkaline soils in degraded landscapes. This broad pH adaptability also suggests that soil pH is unlikely to be a significant limiting factor for perennial ryegrass cultivation, allowing it to be applied in global environments where soil pH is extremely prevalent.
A comprehensive analysis of the effects of various stresses on the germination of perennial ryegrass seeds and the growth of seedlings shows that different stresses have different directions and intensities on the above ground and underground characteristics of perennial ryegrass seeds and seedlings, with negative effects usually being more common than positive effects. Drought stress has the greatest impact on seed germination and seedling growth. This suggests that drought stress can reduce enzyme activity, disrupt cellular water balance, and alter the hormonal content and balance within seeds89. This combination has adverse effects on seed germination ability and overall growth and development of seedlings. In the context of global change, drought has become the deadliest abiotic stress, interfering with the physical and biochemical pathways of seed germination and plant growth, severely damaging their germination, growth, and final yield90. Therefore, ensuring appropriate soil moisture management during the cultivation of plant seeds is crucial for supporting successful seed germination, promoting healthy seedling growth, and ensuring the healthy development of the entire plant life cycle. On the contrary, the impact of pH stress is relatively small, indicating that perennial ryegrass seedlings may have better adaptability to acidic or alkaline environments. In addition, in karst areas, drought stress and calcium salt stress often occur simultaneously91. Drought stress and salt are physiologically related, as both induce osmotic stress and most metabolic responses in affected plants are somewhat similar92. However, in complex field environments containing multiple abiotic and biotic stress factors, the generalizability and determinacy of the conclusions obtained from single factor control experiments are limited. Therefore, future research should focus on conducting long-term field experiments to control multiple factors of stress, in order to more accurately reveal the response and adaptation mechanisms of plants in natural environments.
Conclusion
In this study, we simulated various environmental conditions typical of karst landscapes to thoroughly investigate the germination characteristics of perennial ryegrass (Lolium perenne L.) seeds. Our research shows that drought, salinity, and pH significantly affect seed germination and seedling growth in perennial ryegrass. Moderate drought (− 0.06 MPa and − 0.17 MPa) promotes root and shoot growth, while salt stress at 5–10 mM CaCl₂ enhances germination and seedling development. Within the pH range of 3–9, germination remains relatively stable, though extreme acidity (pH 3) significantly inhibits root and shoot elongation. Therefore, optimal growth conditions were determined to be drought stress from 0 to − 0.17 MPa, calcium salt stress from 0 to 25 mM, and a pH of 4 to 9. These findings identify optimal growth conditions for perennial ryegrass, providing a scientific basis for seed cultivation, pasture management, and ecological restoration in karst regions. Our study contributes to the understanding of plant responses to environmental stresses in karst systems and supports sustainable agricultural and conservation practices.
Data availability
Data availabilityThe original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
References
Goldscheider, N. et al. Global distribution of carbonate rocks and karst water resources. Hydrogeol. J. 28, 1661–1677. https://doi.org/10.1007/s10040-020-02139-5 (2020).
Canedoli, C. et al. Integrating landscape ecology and the assessment of ecosystem services in the study of karst areas. Landsc. Ecol. 37, 347–365. https://doi.org/10.1007/s10980-021-01351-2 (2022).
Wang, K. et al. Karst landscapes of China: patterns, ecosystem processes and services. Landsc. Ecol. 34, 2743–2763. https://doi.org/10.1007/s10980-019-00912-w (2019).
Xu, Y. et al. Adapting the RUSLE and GIS to model soil erosion risk in a mountains karst watershed, Guizhou Province, China. Environ. Monit. Assess. 141, 275–286. https://doi.org/10.1007/s10661-007-9894-9 (2008).
Grime, J. P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111, 1169–1194. https://doi.org/10.1086/283244 (1977).
Zandalinas, S. I. et al. The impact of multifactorial stress combination on plant growth and survival. New. Phytol. 230, 1034–1048. https://doi.org/10.1111/nph.17232 (2021).
He, J. Y. et al. Climate characteristics of the extreme drought events in Southwest China during recent 50 years. Acta Geogr. Sin. 66, 1179–1190. https://doi.org/10.11821/xb201109003 (2011).
Zhao, Y. et al. Do shallow soil, low water availability, or their combination increase the competition between grasses with different root systems in karst soil? Environ. Sci. Pollut R. 24, 10640–10651. https://doi.org/10.1007/s11356-017-8675-4 (2017).
Liu, J. T. et al. Seed and fruiting phenology plasticity and offspring seed germination rate in two Asteraceae herbs growing in karst soils with varying thickness and water availability. J. Resour. Ecol. 13, 319–327. https://doi.org/10.5814/j.issn.1674-764x.2022.02.014 (2022).
Kaiser, M. et al. Effects of land use and mineral characteristics on the organic carbon content, and the amount and composition of Na-pyrophosphate-soluble organic matter, in subsurface soils. Eur. J. Soil. Sci. 62, 226–236. https://doi.org/10.1111/j.1365-2389.2010.01340.x (2011).
Tang, J. et al. Karst Rocky desertification progress: soil calcium as a possible driving force. Sci. Total Environ. 649, 1250–1259. https://doi.org/10.1016/j.scitotenv.2018.08.242 (2019).
Guo, F. et al. Evolution of major environmental geological problems in karst areas of Southwestern China. Environ. Earth Sci. 69, 2427–2435. https://doi.org/10.1007/s12665-012-2070-8 (2013).
Bu, X. L. et al. Effect of Biochar on seed germination and seedling growth of Robinia pseudoacacia L. in karst calcareous soils. Commun. Soil. Sci. Plan. 51, 352–363. https://doi.org/10.1080/00103624.2019.1709484 (2020).
Al-Taisan, W. A. Comparative effects of drought and salt stress on germination and seedling growth of Pennisetum divisum (Gmel) Henr. Am. J. Appl. Sci. 7, 640–646. https://doi.org/10.3844/ajassp.2010.640.646 (2010).
Sadeghipour, O. & Abbasi, S. Soybean response to drought and seed inoculation. World Appl. Sci. J. 17, 55–60 (2012).
Zhang, H. & Sonnewald, U. Differences and commonalities of plant responses to single and combined stresses. Plant. J. 90, 839–855. https://doi.org/10.1111/tpj.13557 (2017).
Zhou, Z. J. et al. Research on drought stress in Medicago sativa L. from 1998 to 2023: a bibliometric analysis. Front. Plant. Sci. 15, 1406256. https://doi.org/10.3389/fpls.2024.1406256 (2024).
Zeid, I. M. & Shedeed, Z. A. Response of alfalfa to Putrescine treatment under drought stress. Biol. Plant. 50, 635–640. https://doi.org/10.1007/s10535-006-0099-9 (2006).
Huang, H. et al. Trends and directions in Oats research under drought and salt stresses: a bibliometric analysis (1993–2023). Plants 13, 902. https://doi.org/10.3390/plants13141902 (2024).
Seetharam, K. et al. Genomic regions associated with heat stress tolerance in tropical maize (Zea mays L). Sci. Rep. U K. 11, 13730. https://doi.org/10.1038/s41598-021-93061-7 (2021).
Nayyeripasand, L., Garoosi, G. A. & Ahmadikhah, A. Genome-wide association study (GWAS) to identify salt-tolerance QTLs carrying novel candidate genes in rice during early vegetative stage. Rice 14, 1–21. https://doi.org/10.1186/s12284-020-00433-0 (2021).
Ahmed, A. A. et al. Genomic regions associated with leaf wilting traits under drought stress in spring wheat at the seedling stage revealed by GWAS. Environ. Exp. Bot. 184, 104393. https://doi.org/10.1016/j.envexpbot.2021.104393 (2021).
Kavar, T. et al. Identification of genes involved in the response of leaves of Phaseolus vulgaris to drought stress. Mol. Breed. 21, 159–172. https://doi.org/10.1007/s11032-007-9116-8 (2008).
Lacerda, J. S. et al. Importance of zinc for Arabica coffee and its effects on the chemical composition of Raw grain and beverage quality. Crop Sci. 58, 1360–1370. https://doi.org/10.2135/cropsci2017.06.0373 (2018).
Rao, X. et al. Influence of exogenous abscisic acid on germination and physiological traits of Sophora viciifolia seedlings under drought conditions. Appl. Sci. 14, 4359. https://doi.org/10.3390/app14114359 (2024).
Khan, M. A. & Gulzar, S. Germination responses of Sporobolus Ioclados: a saline desert grass. J. Arid Environ. 53, 387–394. https://doi.org/10.1006/jare.2002.1045 (2003).
Sun, J., He, L. & Li, T. Response of seedling growth and physiology of Sorghum bicolor (L.) Moench to saline-alkali stress. PLoS ONE. 14, e0220340. https://doi.org/10.1371/journal.pone.0220340 (2019).
Hameed, A. et al. Salinity inhibits seed germination of perennial halophytes Limonium Stocksii and Suaeda fruticosa by reducing water uptake and ascorbate dependent antioxidant system. Environ. Exp. Bot. 107, 32–38. https://doi.org/10.1016/j.envexpbot.2014.04.005 (2014).
Foti, C., Khah, E. M. & Pavli, O. I. Germination profiling of lentil genotypes subjected to salinity stress. Plant Biol. 21, 480–486. https://doi.org/10.1111/plb.12714 (2019).
Zhang, K. et al. Advances and future research in ecological stoichiometry under saline-alkali stress. Environ. Sci. Pollut R. 30, 5475–5486. https://doi.org/10.1007/s11356-022-24293-x (2023).
Hasegawa, P. M. et al. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant. Biol. 51, 463–499 (2000).
Munns, R. Comparative physiology of salt and water stress. New. Phytol. 25, 239–250. https://doi.org/10.1046/j.0016-8025.2001.00808.x (2002).
Ortiz, T. A., Urbano, M. R. & Takahashi, L. S. A. Effects of water deficit and pH on seed germination and seedling development in Cereus jamacaru. Semin-Cienc Agrar. 40, 1379–1392. https://doi.org/10.5433/1679-0359.2019v40n4p1379 (2019).
Rocha, S. P. D. et al. Soil physical properties and eucalyptus growth planted after different tillage methods. Scientia Forestalis. 43, 965–977. https://doi.org/10.18671/scifor.v43n108.20 (2015).
Tanveer, A. et al. Effect of ecological factors on germination of horse purslane (Trianthema portulacastrum). Planta Daninha. 31, 587–597. https://doi.org/10.1590/s0100-83582013000300011 (2013).
Vahabinia, F., Pirdashti, H. & Bakhshandeh, E. Environmental factors’ effect on seed germination and seedling growth of Chicory (Cichorium intybus L.) as an important medicinal plant. Acta Physiol. Plant. 41, 27. https://doi.org/10.1007/s11738-019-2820-2 (2019).
Rezvani, M. & Fani Yazdi, S. Factors affecting seed germination of black nightshade (Solanum nigrum). Acta Bot. Hung. 55, 397–408 (2013).
Amini, V., Zaefarian, F. & Rezvani, M. Interspecific variations in seed germination and seedling emergence of three Setaria species. Braz Bot. 38, 539–545. https://doi.org/10.1007/s40415-015-0158-6 (2015).
Jonaviciene, K. et al. Genetic and phenotypic diversity for drought tolerance in perennial ryegrass (Lolium Perenne L). Zemdirbyste-Agriculture 101, 411–418. https://doi.org/10.13080/z-a.2014.101.052 (2014).
Bothe, A. et al. Drought tolerance in perennial ryegrass (Lolium Perenne L.) as assessed by two contrasting phenotyping systems. J. Agron. Crop Sci. 204, 375–389. https://doi.org/10.1111/jac.12269 (2018).
Byrne, S. L. et al. A synteny-based draft genome sequence of the forage grass Lolium perenne. Plant. J. 84, 816–826. https://doi.org/10.1111/tpj.13037 (2015).
Ding, Z. et al. Purification of eutrophic water by ryegrass. Water Sci. Technol. 66, 2138–2145. https://doi.org/10.2166/wst.2012.424 (2012).
Zhang, Z., Hu, B. & Hu, G. Spatial heterogeneity of soil chemical properties in a subtropical karst forest, Southwest China. Sci. World J. 9, 651. https://doi.org/10.1155/2014/473651 (2014).
Xin, L. I. et al. Advances in research on the function of artificial grassland in karst rock desertification control. Acta Prataculturae Sinica. 20, 279–286 (2011).
Xu, C. Q. & Wu, Q. Research progress on application of ryegrass and its enlightenment to Rocky desertification control. Chin. Wild Plant. Resour. 40, 66–72. https://doi.org/10.3969/j.issn.1006-9690.2021.10.011 (2021).
Amora-Lazcano, E. et al. Plant growth-promoting bacteria belonging to the genera pseudomonas and Bacillus improve the growth of sorghum seedings in a low-nutrient soil. Bot. Sci. 100, 56–66. https://doi.org/10.17129/botsci.2841 (2022).
Chachalis, D. & Reddy, K. N. Factors affecting Campsis radicans seed germination and seedling emergence. Weed Sci. 48, 212–216 (2000).
International Seed Testing Association. The international rules for seed testing. Seed Sci. Technol. 4, 3–49 (1999).
Ferrari, F. N. & Parera, C. A. Germination of six native perennial grasses that can be used as potential soil cover crops in drip-irrigated vineyards in semiarid environs of Argentina. J. Arid Environ. 113, 1–5. https://doi.org/10.1016/j.jaridenv.2014.09.002 (2015).
Ma, L. Y. et al. Seed osmopriming with polyethylene glycol (PEG) enhances seed germination and seedling physiological traits of Coronilla varia L. under water stress. PLoS ONE. 19, 3145. https://doi.org/10.1371/journal.pone.0303145 (2024).
Zhou, J., Huang, Y. & Mo, M. Phylogenetic analysis on the soil bacteria distributed in karst forest. Braz J. Microbiol. 40, 827–837. https://doi.org/10.1590/s1517-83822009000400013 (2009).
Zeng, F. et al. Changes in vegetation after 22 years’ natural restoration in the karst disturbed area in Northwestern Guangxi, China. Acta Ecol. Sin. 27, 5110–5119. https://doi.org/10.1016/S1872-2032(08)60016-5 (2007).
Zhao, X. et al. Differential physiological, transcriptomic, and metabolomic responses of paspalum wettsteinii under high-temperature stress. Front. Plant. Sci. 13, 865608. https://doi.org/10.3389/fpls.2022.865608 (2022).
Wang, Y., Zhao, S. & Cao, B. Study on drought resistance of ten herbaceous plants under PEG-6000 simulated drought stress. Acta Agrestia Sinica. 28, 983 (2020).
Wang, J. et al. Ponse strategies of Lolium perenne L. to karst heterogeneous habitats under drought stress. Acta Ecol. Sin. 40, 4566–4572. https://doi.org/10.5846/stxb201911162471 (2020).
He, Y. et al. Rice CENTRORADIALIS 2 regulates seed germination and salt tolerance via ABA-mediated pathway. Theor. Appl. Genet. 135, 4245–4259. https://doi.org/10.1007/s00122-022-04215-8 (2022).
Li, M. Q. et al. Transcriptome pro files identify the common responsive genes to drought stress in two Elymus species. J. Plant. Physiol. 250, 153183. https://doi.org/10.1016/j.jplph.2020.153183 (2020).
Dodd, G. L. & Donovan, L. A. Water potential and ionic effects on germination and seedling growth of two cold desert shrubs. Am. J. Bot. 86, 1146–1153. https://doi.org/10.2307/2656978 (1999).
Botía, P. et al. Response of eight Cucumis melo cultivars to salinity during germination and early vegetative growth. Agronomie 18, 503–513. https://doi.org/10.1051/agro:19980801 (1998).
Xu, W. et al. Endosperm cellularization failure induces a dehydration-stress response leading to embryo arrest. Plant. Cell. 35, 874–888 (2023).
Brunner, I. et al. How tree roots respond to drought. Front. Plant. Sci. 6, 547. https://doi.org/10.3389/fpls.2015.00547 (2015).
Feng, J. K. et al. Effects of cold plasma treatment on alfalfa seed growth under simulated drought stress. Plasma Sci. Technol. 20. https://doi.org/10.1088/2058-6272/aa9b27 (2018).
Singh, S. & Singh, M. Effect of temperature and water potential on germination of twelve weed species. Indian J. Weed Sci. 41, 134–145 (2009).
Kudla, J., Batistič, O. & Hashimoto, K. Calcium signals: the lead currency of plant information processing. Plant. Cell. 22, 541–563. https://doi.org/10.1105/tpc.109.072686 (2010).
Kim, S. G. & Park, C. M. Gibberellic acid-mediated salt signaling in seed germination. Plant. Signal. Behav. 3, 877–879. https://doi.org/10.4161/psb.3.10.6247 (2008).
Tanveer, A. et al. Effect of temperature, light, salinity, drought stress and seeding depth on germination of Cucumis melo Var. Agrestis. Pak J. Weed Sci. Res. 18, 445–459 (2012).
Bhattacharjee, S. & Mukherjee, A. K. Salt stress-induced cytosolute accumulation, antioxidant response and membrane deterioration in three rice cultivars during early germination. Seed Sci. Technol. 30, 279–287 (2002).
Farooq, S. & Azam, F. The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. J. Plant. Physiol. 163, 629–637. https://doi.org/10.1016/j.jplph.2005.06.006 (2006).
Yu, Z. et al. Effect of salt stress on seed germination of different sunflower. Seed 32, 29–33 (2013).
Werner, J. E. & Finkelstein, R. R. Arabidopsis mutants with reduced response to NaCl and osmotic stress. Physiol. Plant. 93, 659–666. https://doi.org/10.1034/j.1399-3054.1995.930412.x (1995).
Long, J. et al. Effects of drought and salt stress on seed germination and seedling growth of Elymus nutans. Peerj 11, e15968. https://doi.org/10.7717/peerj.15968 (2023).
Fang, S., Hou, X. & Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant. Sci. 12, 458. https://doi.org/10.3389/fpls.2021.667458 (2021).
Cramer, G. R., Epstein, E. & Läuchli, A. Kinetics of root elongation of maize in response to short-term exposure to NaCl and elevated calcium concentration. J. Exp. Bot. 39, 1513–1522. https://doi.org/10.1093/jxb/39.11.1513 (1988).
Yuan, D. X. World correlation of karst ecosystem: objectives and implementation plant. Adv. Earth Sci. 16, 461–466 (2001).
Wei, X. et al. Calcium content and high calcium adaptation of plants in karst areas of Southwestern Hunan. China Biogeosciences. 15, 2991–3002. https://doi.org/10.5194/bg-15-2991-2018 (2018).
Ji, F. T., Li, N. & Deng, X. Calcium contents and high calcium adaptation of plants in karst areas of China. Chin. J. Plant. Ecol. 33, 926–935. https://doi.org/10.3773/j.issn.1005-264x.2009.05.012 (2009).
Navarro-Torre, S. et al. Sustainable agricultural management of saline soils in arid and semi-arid mediterranean regions through halophytes, microbial and soil-based technologies. Environ. Exp. Bot. 212, 397. https://doi.org/10.1016/j.envexpbot.2023.105397 (2023).
Nakamura, I. & Hossain, M. A. Factors affecting the seed germination and seedling emergence of Redflower ragleaf (Crassocephalum crepidioides). Weed Biol. Manag. 9, 315–322. https://doi.org/10.1111/j.1445-6664.2009.00356.x (2009).
Ebrahimi, E. & Eslami, S. Effect of environmental factors on seed germination and seedling emergence of invasive Ceratocarpus arenarius. Weed Res. 52, 50–59. https://doi.org/10.1111/j.1365-3180.2011.00896.x (2012).
Li, R., Shi, F. & Fukuda, K. Interactive effects of salt and alkali stresses on seed germination, germination recovery, and seedling growth of a halophyte Spartina alterniflora (Poaceae). S Afr. J. Bot. 76, 380–387. https://doi.org/10.1016/j.sajb.2010.01.004 (2010).
Basto, S. et al. Effect of pH buffer solutions on seed germination of Hypericum pulchrum, Campanula rotundifolia and Scabiosa columbaria. Seed Sci. Technol. 41, 298–302. https://doi.org/10.15258/sst.2013.41.2.12 (2013).
Redmann, R. & Abouguendia, Z. Germination and seedling growth on substrates with extreme pH-laboratory evaluation of buffers. J. Appl. Ecol. 16, 901–907. https://doi.org/10.2307/2402863 (1979).
Sliwinska, E., Bassel, G. W. & Bewley, J. D. Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocotyl. J. Exp. Bot. 60, 3587–3594. https://doi.org/10.1093/jxb/erp203 (2009).
Krahmer, J. & Fankhauser, C. Environmental control of hypocotyl elongation. Annu. Rev. Plant. Biol. 75, 489–519. https://doi.org/10.1146/annurev-arplant-062923-023852 (2024).
Vleeshouwers, L. M., Bouwmeester, H. J. & Karssen, C. M. Redefining seed dormancy: an attempt to integrate physiology and ecology. J. Ecol. 83, 1031–1037. https://doi.org/10.2307/2261184 (1995).
Omar, B. et al. Effect of light, temperature, salt stress and pH on seed germination of medicinal plant Origanum elongatum (Bonnet) Emb. Maire Biocatal. Agric. Biotechnol. 16, 126–131. https://doi.org/10.1016/j.bcab.2018.07.032 (2018).
Florentine, S. et al. Seed germination response of a noxious agricultural weed Echium plantagineum to temperature, light, pH, drought stress, salinity, heat and smoke. Crop Pasture Sci. 69, 326–333. https://doi.org/10.1071/cp17308 (2018).
Silva, L. C. D. et al. Micromorphological and anatomical alterations caused by simulated acid rain in restinga plants: Eugenia uniflora and Clusia hilariana. Water Air Soil. Pollut. 168, 129–143. https://doi.org/10.1007/s11270-005-0941-2 (2005).
Liu, Y. et al. ABA-insensitivity of alfalfa (Medicago sativa L.) during seed germination associated with plant drought tolerance. Environ. Exp. Bot. 203, 105069. https://doi.org/10.1016/j.envexpbot.2022.105069 (2022).
Hameed, A. et al. Curcumin-based priming agent confers drought tolerance in wheat seedlings: A climate-smart approach. Ind. Crops Prod. 1, 119451. https://doi.org/10.1016/j.indcrop.2024.119451 (2025).
Liu, C. et al. Plant adaptability in karst regions. J. Plant. Res. 134, 889–906. https://doi.org/10.1007/s10265-021-01330-3 (2021).
Al-Taisan, W. Comparative effects of drought and salt stress on germination and seedling growth of Pennisetum divisum (Gmel.) Henr. Am. J. Appl. Sci. 7, 640–646. https://doi.org/10.3844/ajassp.2010.640.646 (2010).
Funding
This study was supported by the Natural Science Foundation of China (32260351), State Key Laboratory of Microbial Technology Open Projects Fund (Project NO. M2022-14) and Guizhou high-level innovative talents project (Qiankehepingtairencai-GCC [2022]022-1).
Author information
Authors and Affiliations
Contributions
J.L. Writing—review & editing, Supervision, Conceptualization. L.Z. Writing—review & editing, Supervision, Conceptualization. P.W. Methodology, Writing—review & editing, Funding acquisition. R.W. Writing—review & editing, Writing—original draft, Methodology. X.W. Writing—review & editing, Supervision, Conceptualization. Y.G. Formal analysis, Writing—review & editing. Y.Y. Writing—review & editing, Supervision, Conceptualization. H.H. Writing—review & editing, Supervision, Conceptualization. Z.Z. Writing—review & editing, Supervision, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, R., Yang, Y., Wang, X. et al. Response of seed germination and seedling growth of perennial ryegrass (Lolium perenne L.) to drought, salinity, and pH in Karst regions. Sci Rep 15, 16874 (2025). https://doi.org/10.1038/s41598-025-01539-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01539-5