Abstract
Understanding ecological succession is essential for managing complex ecosystems like coral reefs. This study investigates the successional dynamics of sessile cryptobenthic communities (SCC), a key component of coral reef biodiversity and functioning, focusing on deterministic and stochastic ecological processes. To assess changes in SCC richness and composition, we deployed five triplicates of Autonomous Reef Monitoring Structures (ARMS) at a coral reef slope site in Reunion Island (South-West Indian Ocean) during both hot and cool seasons, and recovered them after 6 months, 1 year, and 2 years. ARMS photo-analysis revealed that pioneer communities consisted of biofilms, hydrozoans, crustose coralline algae, and foraminifers, while sponges, macroalgae, and bivalves emerged as late colonisers. Seasonal effects were strongest in early succession but diminished over time. Mean morphospecies richness increased from 24.8 ± 1.9 at 6 months to 33.3 ± 5.8 at 2 years, with taxa turnover driving β-diversity. Null model analyses indicated that stochastic processes shaped community membership, while deterministic processes regulated taxa abundances throughout succession. SCC required more than 2 years to reach maturity, raising reef productivity and diversity concerns as extreme events like coral bleaching become more frequent. These findings, supported by metabarcoding, provide insights for reef monitoring and conservation amid increasing disturbances.
Similar content being viewed by others

Introduction
Understanding the processes driving community change and recovery after disturbances is a fundamental purpose in ecology1,2,3. These processes are central to ecosystem resilience4,5, and understanding them supports the development of effective conservation strategies. In marine environments, the establishment of pioneer communities following disturbances depends on factors such as the presence and reproductive status of source populations, the life-history traits of their larvae (e.g., motility, lifespan, feeding mode), local hydrodynamic regimes, and habitat availability6,7. Theories of community assembly and ecological succession provide valuable frameworks for interpreting these patterns8. From a deterministic perspective, succession leads to predictable, stable endpoints9, where community composition resists further colonisation and invasion. Conversely, a stochastic view considers succession as an unpredictable process shaped by random larval recruitment, ecological drift (i.e., the effect of fluctuations in births and deaths on community composition), species exclusion, and priority effects, resulting in multiple possible equilibria3,9.
Tropical coral reef ecosystems host exceptional biodiversity10,11,12 but face increasing pressure from anthropogenic disturbances13,14. Understanding the temporal dynamics and successional processes of reef communities is thus crucial for predicting their capacity for recovery. While successional patterns in scleractinian corals15,16,17,18 and reef fishes19 have been well-documented, less attention has been given to the cryptobiome—a hidden yet vital component of reef biodiversity20,21 consisting of sessile and mobile organisms inhabiting reef crevices. Indeed, the intricate three-dimensional structure of coral reefs provides extensive surface area within the reef matrix, surpassing that of exposed reef surfaces22. Sessile cryptobenthic communities (SCC) include taxa such as crustose coralline algae (CCA), sponges, annelids, bryozoans, foraminifers, and ascidians, which together represent substantial biomass that supports higher trophic levels. For example, sponges play a crucial role in nutrient cycling by converting dissolved organic matter into a form accessible to reef fauna through the release of filtering cells23.
Over the past decade, Autonomous Reef Monitoring Structures (ARMS)24,25,26 have emerged as a primary tool for sampling cryptobenthic communities. These artificial substrates mimic natural reef complexity, providing standardised and replicable habitats that facilitate cryptic biodiversity assessment and monitoring. Previous ARMS studies revealed that stochastic processes significantly influence cryptic communities at large spatial scales, such as across ecoregions27, while both deterministic and stochastic factors shape communities at smaller spatial scales, i.e., within ecoregions28. In a concurrent study, we also demonstrated intra-ARMS community structuring driven by micro-habitat conditions (i.e., presence or absence of crossbars between ARMS plates, plate face orientation, and their combinations)29. However, temporal variations in cryptic communities remain underexplored30,31,32. To date, only one recent metabarcoding-based study has investigated the potential roles of deterministic and stochastic drivers during succession and concluded that, although both season and immersion time influenced cryptic communities, their successional pattern was primarily driven by stochastic colonisation33.
In this study, we describe SCC composition and richness in ARMS and their contrasting micro-habitats, while assessing the influence of deterministic and stochastic processes during ecological succession. The effect of seasonal variation on succession was evaluated through an experimental design involving deployments of ARMS triplicates during both hot and cool seasons, with immersion times of 6 months, 1 year, and 2 years (hot season) and 6 months and 1 year (cool season) at a single outer reef slope site on Reunion Island. Following previous studies showing the effectiveness of visual assessments at the morpho-species (MSP) level for detecting variations in SCC28,34, we applied point-count photo-analysis of ARMS plate faces to analyse α- and β-diversity metrics, including turnover and nestedness (species replacement and species loss, respectively). We analysed the same 15 ARMS deployed in Couëdel et al.33, and, by comparing our results with those obtained using metabarcoding, offer a more comprehensive understanding of SCC successional dynamics within this compartment of coral reef biota.
Methods
ARMS characteristics, deployment and processing
Each ARMS unit consists of 9 stacked PVC plates (22.4 cm × 22.4 cm), separated alternately by 1 cm-high crossbars or spacers, resulting in a total volume of 4.5 L and a surface area of approximately 0.81 m2 (16 plate faces per ARMS). The crossbars create 4 semi-closed interstices, limiting water flow and light penetration. Consequently, plate faces within each ARMS experienced variable conditions of water flow, sedimentation, and light exposure (Fig. 1).
Five batches of three replicate ARMS units (designated A, B, and C) were deployed at a spur site on the outer coral reef slope of La Saline Reef (West coast of Reunion Island; 21.10401°S; 55.23598°E) at a depth of approximatively 11 m. To minimise the confounding effects of small-scale spatial variability29, all deployments were performed at a single site, located within a no-take zone of the marine reserve. The benthic community was dominated by dead coral substrates overgrown with algal turfs and crustose coralline algae, while live Scleractinia and Millepora cover did not exceed 0.32 ± 0.29% (mean ± standard deviation; Fig. S1), reflecting the degraded state of the reef due to repeated bleaching events and anthropogenic pressures such as nutrient enrichment and sedimentation35.
Between 17 December 2018 and 30 August 2021, three batches (CINA1, CINA2, and RUNA2; Fig. 2) were deployed during the hot season, with immersion times of 6 months, 1 year, and 2 years. Two additional batches (CINA3 and CINA4; Fig. 2) were deployed during the cool season, with immersion times of 6 months and 1 year. The 2-year ARMS were deployed one year earlier as part of a spatial survey29, but followed identical protocols. All batches (CINA3, CINA2, and RUNA2) were retrieved during the same hot season. This deployment schedule, along with the absence of 2-year ARMS triplicates during the cool season, could potentially introduce some bias. Nevertheless, we maintained experimental consistency by locating all ARMS CINA1-4 at the same site as RUNA2. All 15 ARMS units were randomly positioned within the same habitat, spaced 2 to 8 m apart, and firmly affixed to the reef substrate.
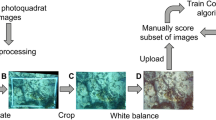
Upon retrieval, ARMS units were kept in aerated seawater until processing in the lab. Each plate face was photographed in high resolution under standardised lighting conditions. Morpho-species (MSP), i.e. categories grouping specimens with similar morphological characteristics, were photographed alive in-situ, and vouchers were preserved in ethanol for barcoding and identification by taxonomists.
The abundance of sessile MSPs on all ARMS plate faces (excluding the upward-facing surface of the top plate, which does not represent cryptic habitat) was quantified using Coral Point Count with Excel extension (CPCe)36 on the CoralNet website37. Each plate face was analysed by projecting 50 points in a stratified random manner onto each image. The CPCe software produced an abundance matrix comprising 240 plate faces colonised by 69 MSPs, including sponges, ascidians, bryozoans, and foraminifers. Taxonomists identified 21 MSPs to the genus level and 15 to the species level. Results and figures reflect the highest taxonomic resolution available. More details on ARMS characteristics, processing, taxonomic inventory, and SCC photo-analysis can be found in Frattini et al.29.
Environmental data
We recorded seawater temperature hourly in-situ from 23 October 2019 to 19 February 2021 using a HOBO Water Temp Pro v2 (ONSET) logger attached to the base plate of one ARMS, and we obtained sea surface temperatures (SST) from the NOAA model (virtual station Reunion-Tromelin) over the entire 2.5-year deployment period. We used these data to delineate 4 seasonal phases, following Conand et al.38: hot season (January to April), cooling season (May and June), cool season (July to October), and warming season (November and December). Additional environmental data, including radiation and rainfall, were sourced from the Météo France meteorological station at Trois-Bassins (21.09°S, 55.25°E), located near sea level, 2.3 km from the study site. Particulate organic carbon (POC) and chlorophyll concentrations were retrieved from the NASA Ocean Color database39.
Hot seasons were characterised by higher average SST, higher maximum monthly rainfall, and lower chlorophyll and POC levels compared to cool seasons33. Mean SSTs in 2019 and 2020 were similar, except for a slightly higher peak during the hot season of 2019 (28.9 °C vs. 28.3 °C). Environmental data are provided for context and seasonal characterisation only, as the effects of environmental parameters were not directly analysed in this study.
Data analysis
The experimental design (Fig. 1) enabled testing the combined effects of immersion time and deployment/retrieval season on SCC composition within ARMS. Analyses focused on immersion time and deployment season, which showed stronger ecological signals than retrieval season (Figs. S5, S6).
α diversity and taxa abundance
The α diversity metrics, including species richness (S) and the Chao estimator (estimated total MSP), were calculated using the ‘vegan’ package40,41. Differences in mean MSP richness in ARMS were tested using permutational ANOVA and pairwise permutation tests with Bonferroni correction, implemented via the ‘rstatix’ package42. Permutation-based approaches were used due to the low number of replicates (N = 3) for 2-year ARMS (RUNA2). We examined taxa abundance across immersion times and seasons, focusing on dominant taxonomic groups and abiotic categories (i.e., sediment or bare PVC plate).
β -diversity analysis among ARMS batches
The effects of immersion time, deployment season, and their interactions on SCC composition were tested with a permutational analysis of variance (PERMANOVA; adonis function from the ‘vegan’ package41) using abundance-based Bray–Curtis (BC) dissimilarity. Principal Coordinate Analysis (PCoA) was conducted using two community matrices corresponding to different levels of taxonomic resolution: one with 13 main categories and one with all 69 MSPs. The contribution of each MSP to dissimilarity among the ARMS units was computed using the simper function from the ‘vegan’ package41.
β-diversity metrics—BC dissimilarity, Jaccard dissimilarity, turnover and nestedness—were computed using the ‘beta.pair’ function from the ‘betapart’ package43. BC dissimilarity considers taxa abundances, while Jaccard dissimilarity focuses on community membership. In this context, turnover reflects dissimilarity not caused by α-diversity differences44. These β-diversity metrics were computed within and across batches, and compared using ANOVA and pairwise Student’s t-tests with Bonferroni correction when parametric conditions were met, or Kruskal–Wallis tests with Wilcoxon pairwise comparisons when these assumptions were unmet.
Null model analysis
Ecological succession involves ongoing community assembly, where species composition changes over time. Null models provide a statistical framework to distinguish between deterministic (non-random) and stochastic (random) processes driving community changes by comparing observed communities at different successional stages to randomised communities8. We generated 999 randomised abundance matrices using the ‘quasiswap count’ algorithm from the ‘vegan’ package41. This algorithm preserves both row and column totals, ensuring that species abundances remain consistent across permutations. Additionally, 999 presence-absence matrices were created using the ‘EcoSimR’ package45. This algorithm fixed column sums (species occurrences), while allowing row sums (community sizes) to vary equiprobably. The β-diversity metrics were evaluated by comparing observed values against null distributions. Since the β-diversity distributions were skewed but approximately normal, we applied non-parametric statistical tests to detect significant deviations. This allowed inference of the relative influence of random recruitment versus deterministic successional processes.
β-diversity analysis among micro-habitats within ARMS
The effects of micro-habitat and immersion time, as well as their interaction, on community composition were assessed using PERMANOVA. Permutations were restricted within ARMS using the strata argument in the ‘adonis’ function41. This analysis evaluated whether SCCs were spatially structured within ARMS units and if this structuring changes over time. To quantify these interactions, we generated boxplots comparing β-diversity between micro-habitats and within the same micro-habitat for each ARMS. Mean values were compared using the same approach as described previously. To visualise community dissimilarity between contrasting micro-habitats, we generated PCoA plots and applied simper (‘vegan’ package41) analysis to identify key contributing taxa.
All data analyses were performed using R v.4.3.046.
Results
α-diversity of SCC and the relative abundance of taxa in ARMS
A total of 69 MSPs, encompassing 12 phyla, were identified within the sessile cryptobenthic communities that settled in the 15 ARMS at the La Saline site. The Chao estimator predicted a potential species pool of 116.9 MSPs (ChaoSE: ± 6.8). Accumulation curves of MSP detection, plotted against the number of plate faces analysed across immersion times, deployment, and retrieval seasons, approached a plateau (Fig. S2). This suggests that the sampling effort conducted using Coral Point Count was sufficient to capture most species present and allowed reliable comparisons of SCC diversity across immersion times and seasons.
Species richness (α-diversity) increased with immersion time (Fig. S3). On average, ARMS immersed for 6 months supported 24.8 ± 1.9 (mean ± standard deviation) morphospecies, while those immersed for 1 year harboured 27.8 ± 3.4 species. ARMS retrieved after 2 years exhibited the highest richness, with 33.3 ± 5.8 species. Permutational ANOVA revealed a statistically significant effect of immersion time on species richness (Niterations = 5000, p = 0.007). However, pairwise comparisons using permutational tests with Bonferroni correction did not confirm significant differences between immersion times (p6months-1year = 0.276, p1year-2years = 0.326, p6months-2years = 0.077). The observed increase in mean richness between 6-month and 2-year immersions approached but did not reach statistical significance. MSP richness did not vary significantly between ARMS deployed or retrieved during the hot or cool seasons.
The biotic categories occupying the largest surface areas on ARMS plates, in decreasing order of relative abundance, were CCA, foraminifers, annelids, prokaryotic biota (such as biofilms and cyanobacteria), bryozoans, hydrozoans, and ascidians (Fig. 3). While certain categories, such as prokaryotic biota, maintained relatively stable coverage over time, others varied notably with immersion time and deployment season. Sponges, for example, only became a prominent category after 2 years of immersion (RUNA2, Fig. S4a). Among abiotic categories, which accounted for approximately one-third of total surface area, sediment increased after 2 years of immersion, while the proportion of bare plate declined.
Mean percent cover of main benthic categories across ARMS plate faces. Stacked column chart representing the mean percent cover of the 13 main benthic categories across all ARMS plate faces. Categories are ordered by decreasing relative abundance (except for bare plate and sediment). The chart illustrates the distribution of both biotic and abiotic cover types within the ARMS communities. The labels ‘6 mo’, ‘1 yr’, ‘2 yr’ appears in red for triplicates deployed in the hot season and in blue for those deployed in the cool season.
Influence of immersion time and deployment season
PERMANOVA revealed that immersion time was the dominant factor driving variation in SCC composition among ARMS (R2 = 0.45, F = 6.77, p < 0.001). Deployment season also contributed to community differences, though to a lesser extent (R2 = 0.12, F = 3.51, p = 0.013). A significant interaction between immersion time and deployment season (R2 = 0.10, F = 3.15, p = 0.021) indicated that seasonal influence diminished with longer immersion time.
Principal Coordinate Analysis (PCoA) of biotic categories revealed clustering of SCC by immersion time (Fig. 4a). ARMS immersed for 6 months and deployed during the hot season formed a distinct cluster from those deployed in the cool season. Ascidians (both solitary and colonial) and prokaryotic biotas were more abundant in the 6-month ARMS deployed during the hot season, while 6-month ARMS deployed in the cool season (CINA3) exhibited significantly greater colonised surface area, driven by higher abundances of annelids, foraminifers, and bryozoans (Figs. S4a, b). Notably, SCC in CINA3 resembled those from ARMS immersed for 1 year.
Principal Coordinate Analysis (PCoA) based on Bray–Curtis dissimilarity of sessile cryptobenthic communities for: (a) the 13 main cryptobenthic categories (dimensions 1 and 2 explaining 54% and 26% of the variance, respectively); (b) morpho-species (MSPs) contributing up to 95% of dissimilarities among ARMS (dimensions 1 and 2 explaining 19% and 11% of the variance, respectively). Arrows indicate the relative contribution of categories and MSPs to the dissimilarity between ARMS triplicates. Colours represent different immersion times (purple: 6 months, green: 1 year, orange: 2 years) while shapes denote deployment seasons (triangles: hot, circles: cool). Prefixes indicate taxonomic groups: Ascc = Colonial ascidian; Ascs = Solitary ascidian; Bry = Bryozoan; Cni = Cnidarian; For = Foraminiferan; Por = Poriferan.
All ARMS immersed for 1 year (CINA2 and CINA4) clustered in the lower central region of the PCoA projection, indicating reduced seasonal influence compared to 6-month immersions. This aligns with the significant interaction between immersion time and deployment season detected by PERMANOVA, where seasonal effects were pronounced early in succession but diminished over time. After 1 year, SCC were characterised by increased abundances of annelids, bryozoans, and algae such as red erect macroalgae (Fig. 4b).
After 2 years of immersion, ARMS displayed greater coverage of sponges and CCA, along with sediment accumulation. Though bivalves remained rare, they also appeared later in succession. Boxplots of relative category abundances (Figs. S4a, b), supported by ANOVA and t-tests, confirmed the patterns observed in the PCoA analysis.
The finer taxonomic resolution (MSP level, Fig. 4b) detailed the taxa contributions to dissimilarities. ARMS immersed for 6 months and deployed in the hot season (CINA1) were characterised by two colonial ascidian species of the genus Botryllus (B. gregalis and B. tuberatus), a solitary ascidian MSP5, and green biofilms. In contrast, ARMS deployed for 6 months in the cool season (CINA3) hosted two colonial ascidian species of the family Didemnidae (sp2 and sp3) and juvenile foraminifers of the genus Miniacina.
We observed that communities from 1-year ARMS deployed in the cool season (CINA4) tended to exhibit higher abundances of calcareous worm tubes, red erect algae, cyanobacteria, and a dominant crust-forming bryozoan (Watersiporidae sp., Fig. 4b).
ARMS immersed for 2 years (RUNA2) formed a distinct cluster divergent from shorter immersions. PCoA at MSP level revealed compositional differences among the three RUNA2 replicates, a pattern less apparent when analysing broader biotic categories. Key contributors included 5 sponge MSPs, and 4 ascidians (including a solitary species of the genus Polycarpa, three colonial ascidians Eusynstyela sp., Aplidium sp. and an unidentified MSP). These ARMS also showed increased coverage of brown erect algae, CCA, and adult foraminifers of the genus Miniacina.
Effect of immersion time and deployment season on β-diversity components
Analysis of β-diversity indicated that ARMS from the same batch (triplicates) were significantly less dissimilar in SCC composition than ARMS from different batches (with different immersion times and/or deployment seasons, p-value < 0.001, Fig. 5). Jaccard dissimilarity was primarily driven by MSPs turnover (Fig. 5a), reflecting species replacement rather than nestedness.
β-diversity indices and components among ARMS units. Boxplots of β-diversity indices (Bray–Curtis and Jaccard dissimilarity and its components turnover and nestedness) for comparisons between ARMS (a) from the same batch versus different batches; (b) immersed for different time periods; and (c) deployed in the same season versus different seasons. N indicates the number of pairwise comparisons used to compute each index. Asterisks denote significance levels (ns: p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
BC dissimilarity showed no significant change between ARMS immersed for 6 months and 1 year, nor between 1 and 2 years (Fig. 5b), suggesting gradual changes in relative species abundances. In contrast, Jaccard dissimilarity was significantly higher between 1- and 2-year ARMS than between 6-month and 1–year ARMS (Fig. 5b), indicating increased species turnover during later successional stages.
β-diversity decomposition showed that composition differences were largely attributable to species turnover. Therefore, while MSP richness increased over time (Fig. S2), communities from 6-month and 1-year ARMS were not merely subsets of the more diverse communities found in 2-year ARMS.
Consistent with the abundance-based PCoA and PERMANOVA results, BC dissimilarity was significantly higher between ARMS deployed in different seasons than within the same season (Fig. 5c). However, Jaccard dissimilarity did not differ significantly between or within deployment seasons (Fig. 5c), suggesting that seasonality primarily influenced relative abundance of MSPs rather than community membership.
Testing for deterministic and stochastic processes
Distinct patterns in β-diversity were revealed through null models analyses, providing insights into the processes driving SCC assembly. BC dissimilarity was consistently lower than expected under the null model for comparisons between ARMS within the same batch. Most BC values fell within the first quantile range (0%—2.5%), below the red line in Fig. 6. This marked β-diversity shortfall suggests that deterministic processes, such as environmental filtering and competition, played a dominant role in shaping MSPs relative abundances during succession.
Null model projections of β-diversity within ARMS batches. Projection of β-diversity values (Bray–Curtis and Jaccard dissimilarity, and its components turnover and nestedness) for ARMS pairs within the same batch (purple: 6 months, green: 6 months, orange: 2 years) onto the quantile distribution of β-diversity values from 999 null models. Red lines represent significance thresholds at the 2.5% and 97.5% quantiles.
In contrast, Jaccard dissimilarity and species turnover did not significantly deviate from null expectations. Most values were distributed between the 2.5th and 97.5th percentiles (Fig. 6), closely matching the null distribution. This indicates that MSP membership was primarily governed by stochastic processes, including dispersal limitation, random larval settlement, and survival, throughout the successional sequence.
β-diversity among micro-habitats within ARMS along succession
PERMANOVA revealed that microhabitat type was the primary driver of SCC composition at the plate face level (R2 = 0.23, F = 29.21, p < 0.001). Immersion time also contributed to variation in composition, albeit to a lesser extent (R2 = 0.10, F = 19.04, p < 0.001). The interaction between microhabitat and immersion time was statistically significant (R2 = 0.04, F = 2.93, p < 0.001), suggesting that the effect of microhabitat on SCC differentiation changes throughout succession.
Mean BC dissimilarity values between different micro-habitats were significantly higher than those between same micro-habitat types, except for one comparison after 2 years of immersion (Upward/Closed vs. Upward/Open, Fig. 7). This pattern supports the PERMANOVA results, reinforcing the structuring role of micro-habitat characteristics. Community differentiation across micro-habitats was evident at all successional stages. The most pronounced differences in β-diversity were observed between downward-oriented closed plate faces and upward-oriented open plate faces. PCoA indicated that the taxa driving these differences remained relatively consistent between the 6-month and 1-year immersion times (Fig. 7b). Upward-oriented open plate faces were predominantly colonised by foraminifers, CCA and cyanobacteria, reflecting higher light availability and greater water flow. Conversely, downward-oriented closed plate faces were dominated by Didemnidae ascidians and bryozoans.
Micro-habitat β-diversity within ARMS plate faces after 6 months (purple), 1 year (green), and 2 years (orange). (a) Boxplots of Bray–Curtis dissimilarity between ARMS plate faces. D: Downward, U: Upward, O: Open, C: Closed. Thus, ‘DC_UO’ indicates comparisons between micro-habitats DOWNWARD/CLOSED and UPWARD/OPEN); ‘same’ indicates comparisons between the same micro-habitat types. (b) Principal Coordinate Analysis (PCoA) of DC and UO plate faces. Triangles represent UO plate faces, circles represent DC plate faces, while arrows indicate the relative contribution of MSPs to the dissimilarity between these plate faces. Prefixes indicate taxonomic groups: Ascc = Colonial ascidian; Ascs = Solitary ascidian; Bry = Bryozoan; Cni = Cnidarian; For = Foraminiferan; Por = Poriferan.
After 2 years of immersion, SCC composition became more diverse, and certain Porifera species arose as key contributors to micro-habitat differentiation. Sponge MSP4 and MSP15 exhibited higher abundances on downward-oriented closed plate faces, while MSP12 and MSP3 were more prevalent on upward-oriented open plate faces. These observations underscore both the increasing role of sponges in structuring mature SCCs and the importance of micro-habitat complexity in fostering cryptic reef diversity and structuring their communities over time.
Discussion
Pioneer communities in ecological succession are composed of opportunistic and generalist species capable of colonising a variety of (micro)habitats, regardless of environmental conditions47. These organisms—such as foraminifers, hydrozoans, CCA, and biofilms—exhibit high fecundity and rapid growth rates, facilitating early settlement and colonisation of available substrates48,49,50. Our results confirm these patterns, with foraminifers and CCA arising as dominant taxa after 6 months of immersion, consistent with findings by Choi51, who identified Homotrema foraminifers and CCA as early colonisers of coral rubble.
The observed increase in MSP richness over time likely reflects the continuous influx of propagules. Early colonisers may enhance substrate suitability for subsequent settlers by creating complex structures that diversify available niches, as suggested in previous studies52. The accumulation of sediment over time (visible in Fig. S4b) may further promote niche diversification by providing resources for species that feed on sediment-associated organic matter53 or by offering habitat for burrowing and tube-building organisms, such as Terebellidae polychaetes53. The progressive decline in bare plate surface area, coupled with increasing α-diversity, underscores ongoing community development. However, the persistence of approximately 10% bare surface area after 2 years of immersion suggests that factors beyond substrate availability may limit settlement. These likely include environmental parameters such as light availability, nutrients54, dissolved oxygen55, and interspecific competition, with allelopathic biochemicals from certain species of ascidians or sponges potentially inhibiting larval settlement56. Additionally, CCA did not colonise the inner, central areas of plates (Fig. S5), possibly limiting their capacity to promote larval recruitment57.
The persistence of bare substrate also indicates that the SCC had not yet reached full maturity by the end of the 2-year immersion time. Previous research showed that SCC on coral reefs may require up to three years to attain mature, stable assemblages30,51. This protracted successional trajectory raises concerns regarding the recovery potential of SCC31 following large-scale disturbances such as thermal anomalies, which are expected to become more frequent and severe in the coming decades58. The delayed development of late-successional taxa, particularly sponges, is critical given their essential ecological role in coral reefs23,30. Although pioneering sponge groups (e.g., Haplosclerida or Calcarea)30 may colonise early, sponges are typically considered secondary colonisers51. Frequent disturbances may favour early-successional species59, limiting the establishment of sponges and other late colonisers, ultimately compromising ecosystem functions. A better understanding of ecological succession could help develop indicators of ecosystem health and guide conservation strategies59.
The role of the larval pool during ARMS deployment appears critical in shaping community composition, especially in early succession. The dominance of Botryllus spp. (B. gregaris and B. tuberatus) in ARMS deployed during the hot season and retrieved after 6 months (CINA1) aligns with the reproductive patterns of ascidians, many of which release larvae during warmer months60. Ascidian larvae settle rapidly and exhibit high growth61, contributing to the strong seasonal signal observed in early stages of immersion. However, the influence of deployment season diminished with longer immersion times. After 1 year, community richness reflected the cumulative arrival of multiple propagule cohorts, overshadowing seasonal effects on early settlers.
Our observations support the hypothesis that deterministic processes primarily shape MSPs abundances, while stochastic processes govern community membership throughout succession. This aligns with findings from a larger-scale study of SCC composition along Reunion Island’s coral reefs29. Notably, MSP turnover increased between 1 and 2 years of immersion, compared to the period between 6 months and 1 year. This may reflect complex dynamics such as competitive exclusion among secondary colonisers or ecological drift62. Although community membership remained variable across ARMS replicates, null model analyses revealed consistently lower BC dissimilarity than expected under random models. This suggests a deterministic pattern in species abundance distribution, likely driven by the repeated dominance of a few taxa63. For instance, CCA maintained a dominant role across all successional stages, while most MSPs exhibited low overall abundances, reinforcing the common structure in biological communities where multiple rare taxa coexist with a few dominant ones63. However, species successions within the very diverse category CCA are to be expected.
Dominant taxa have a strong influence on abundance-based β-diversity metrics such as BC dissimilarity. These taxa thrive under certain biotic and abiotic conditions that favour their survival and growth, typically associated with niche-based (deterministic) processes. In contrast, occurrence-based β-diversity metrics, such as Jaccard dissimilarity, are more influenced by the numerous rare species, which typically arise from random colonisation events by weakly competitive species (e.g., species living near the limits of their ecological tolerance). Micro-habitat heterogeneity exerted a significant influence on SCC composition29, even at early immersion stages, likely due to the short generation times of many species. After 6 months of immersion, distinct community structuring had already emerged between different micro-habitats, shaped by factors such as plate face orientation, light availability, and water flow. These selective pressures persisted throughout the 2-year immersion time, although the taxa contributing to community differentiation shifted over time. The consistent influence of micro-habitat characteristics highlights the importance of small-scale environmental variability in shaping cryptic community composition, a contributing factor to the overwhelming diversity of cryptic organisms in coral reefs.
To our knowledge, only two other experiments have examined SCC succession in ARMS30,33, one of which used metabarcoding to analyse the sessile fraction of the cryptobiome on the same 15 ARMS units studied here33. Our findings corroborate Couëdel et al., highlighting the importance of immersion time and deployment season in shaping SCC composition. Both methods revealed the consistent role of stochastic processes in driving sessile community membership during succession. Interestingly, the metabarcoding-based study reported higher eukaryotic α-diversity in the sessile fraction of ARMS immersed for 1 year compared to ARMS immersed for 6 months or 2 years, based on the 18S ribosomal RNA gene marker. Moreover, the Cytochrome c oxidase subunit I (COI) marker detected no significant difference in α-diversity with immersion time, in contrast to our results based on photo-analysis. This discrepancy may stem from limitations inherent to visual assessments, as small MSPs may go undetected at 1-year immersion stages. Additionally, DNA from mobile organisms living within sessile ones (e.g. arthropods) may influence metabarcoding results, artificially inflating perceived diversity of SCC64. Despite these limitations, there was notable congruence between molecular abundance estimates read counts) and visual estimates (plate cover) for main taxa (e.g., red algae, sponges, ascidians, and hydrozoans), supporting the reliability and complementarity of both techniques.
After two years of immersion, SCC may form multiple overlapping layers, and erect organisms can sometimes obscure other MSPs, potentially misrepresenting cryptobenthic communities. Photo-analysis provides abundance estimates based on projected surface area, which may underestimate the abundance of three-dimensional organisms22. However, the 1 cm spacing between ARMS plates likely mitigates this bias. Lastly, the experiment was conducted at a single site, limiting its generalisability. Similar studies conducted at broader spatial scales would help clarify whether the observed patterns of SCC succession apply across different reef habitats and environmental settings.
Conclusions
Through photo-analysis of sessile cryptic communities in ARMS, we provide valuable insights into ecological succession within a coral reef ecosystem. We demonstrate that immersion time significantly influences SCC structure, while the effect of deployment season diminishes over time. Community membership appears shaped by stochastic processes such as ecological drift, dispersal, and larval recruitment, whereas species abundance distributions are driven by deterministic, niche-based mechanisms.
Several key takeaway messages arise for future research. First, taxonomic resolution critically affects the interpretation of cryptobenthic succession, as previously highlighted30. We recommend prioritising MSP-level identification over broader taxonomic categories, even when species-level identification is not attainable. Second, standardising immersion time and deployment season in future cryptobenthic monitoring programmes is essential to minimise confounding factors and improve data comparability33. Finally, longitudinal studies incorporating sequential photographs at shorter intervals (e.g., < 6 months) and extending beyond 2 years would provide deeper insights into SCC dynamics. Such an approach would enable more precise tracking of settlement timing and community establishment, ultimately improving our understanding of cryptobenthic successional trajectories and informing coral reef conservation strategies.
Data availability
Datasets used in this study and all code used for the analysis and figures will be available from the GitHub repository: https://github.com/BaptisteFrattini/cinarms_pipeline.
References
Gleason, H. A. Further views on the succession-concept. Ecology 8, 299–326 (1927).
Connell, J. H. & Slatyer, R. O. Mechanisms of succession in natural communities and their role in community stability and organization. Am. Nat. 111, 1119–1144 (1977).
Chase, J. M. Community assembly: When should history matter? Oecologia 136, 489–498 (2003).
Prach, K. & Walker, L. R. Four opportunities for studies of ecological succession. Trends Ecol. Evol. 26, 119–123 (2011).
Gilmour, J. P., Smith, L. D., Heyward, A. J., Baird, A. H. & Pratchett, M. S. Recovery of an isolated Coral Reef system following severe disturbance. Science 340, 69–71 (2013).
Siegel, D. A. et al. The stochastic nature of larval connectivity among nearshore marine populations. Proc. Natl. Acad. Sci. USA 105, 8974–8979 (2008).
Kendall, M., Poti, M., Wynne, T., Kinlan, B. & Bauer, L. Consequences of the life history traits of pelagic larvae on interisland connectivity during a changing climate. Mar. Ecol. Prog. Ser. 489, 43–59 (2013).
Chang, C. & HilleRisLambers, J. Integrating succession and community assembly perspectives. F1000Res 5, 2294 (2016).
Li, S. et al. Convergence and divergence in a long-term old-field succession: the importance of spatial scale and species abundance. Ecol. Lett. 19, 1101–1109 (2016).
Knowlton, N. et al. Coral Reef Biodiversity (Wiley-Blackwell, 2010).
Plaisance, L., Caley, M. J., Brainard, R. E. & Knowlton, N. The diversity of coral reefs: What are we missing? PLoS ONE 6, e25026 (2011).
Fisher, R. et al. Species richness on coral reefs and the pursuit of convergent global estimates. Curr. Biol. 25, 500–505 (2015).
Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshwater Res. https://doi.org/10.1071/MF99078 (1999).
Hughes, T. P. et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 17, 360–365 (2007).
Done, T. J. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247, 121–132 (1992).
McClanahan, T. R. Primary succession of coral-reef algae: Differing patterns on fished versus unfished reefs. J. Exp. Mar. Biol. Ecol. 218, 77–102 (1997).
McClanahan, T., Polunin, N. & Done, T. Ecological states and the resilience of coral reefs. Conserv. Ecol. 6, 18 (2002).
Scopélitis, J. et al. The next step in shallow coral reef monitoring: Combining remote sensing and in situ approaches. Mar. Pollut. Bull. 60, 1956–1968 (2010).
Bellwood, D. R., Hoey, A. S., Ackerman, J. L. & Depczynski, M. Coral bleaching, reef fish community phase shifts and the resilience of coral reefs: Bleaching impacts on coral reefs. Global Change Biol. 12, 1587–1594 (2006).
Hoeksema, B. W. The hidden biodiversity of tropical coral reefs. Biodiversity 18, 8–12 (2017).
Brandl, S. J. et al. Demographic dynamics of the smallest marine vertebrates fuel coral reef ecosystem functioning. Science 364, 1189–1192 (2019).
Kornder, N. A. et al. Implications of 2D versus 3D surveys to measure the abundance and composition of benthic coral reef communities. Coral Reefs 40, 1137–1153 (2021).
de Goeij, J. M. et al. Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science 342, 108–110 (2013).
Carvalho, S. et al. Beyond the visual: Using metabarcoding to characterize the hidden reef cryptobiome. Proc. R. Soc. B 286, 20182697 (2019).
Leray, M. & Knowlton, N. DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proc. Natl. Acad. Sci. USA 112, 2076–2081 (2015).
Pearman, J. K. et al. Cross-shelf investigation of coral reef cryptic benthic organisms reveals diversity patterns of the hidden majority. Sci. Rep. 8, 8090 (2018).
Villalobos, R. et al. Biodiversity patterns of the coral reef cryptobiota around the Arabian Peninsula. Sci. Rep. 14, 9532 (2024).
Steyaert, M. et al. Remote reef cryptobenthic diversity: Integrating autonomous reef monitoring structures and in situ environmental parameters. Front Mar. Sci. 9, 932375 (2022).
Frattini, B. et al. Deterministic and stochastic processes drive sessile cryptobenthic community composition on coral reefs at different spatial scales. Mar. Ecol. Prog. Ser. https://doi.org/10.3354/meps14852 (2025).
Vicente, J. et al. Ecological succession of the sponge cryptofauna in Hawaiian reefs add new insights to detritus production by pioneering species. Sci. Rep. 12, 15093 (2022).
Villalobos, R. et al. Inter-annual variability patterns of reef cryptobiota in the central Red Sea across a shelf gradient. Sci. Rep. 12, 16944 (2022).
Sembiring, A. et al. Utilizing the Autonomous Reef Monitoring Structure (ARMS) to study the temporal variation of benthic community on coral reef ecosystems in Pemuteran, Bali, Indonesia. Reg. Stud. Mar. Sci. 62, 102925 (2023).
Couëdel, M. et al. Settlement patterns and temporal successions of coral reef cryptic communities affect diversity assessments using autonomous reef monitoring structures (ARMS). Sci. Rep. 14, 27061 (2024).
David, R. et al. Lessons from photo analyses of Autonomous Reef Monitoring Structures as tools to detect (bio-)geographical, spatial, and environmental effects. Mar. Pollut. Bull. 141, 420–429 (2019).
Pellerin, F. et al. Snapshots of coral reef biodiversity. In The Future of Coral Reefs. Evidence from Research. Coral Reefs of the World book series (ed. Planes, S.) (Springer) (in press).
Kohler, K. E. & Gill, S. M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269 (2006).
CoralNet. https://coralnet.ucsd.edu/source/.
Conand, F., Marsac, F., Tessier, E. & Conand, C. A ten-year period of daily sea surface temperature at a coastal station in Reunion Island, Indian Ocean (July 1993–April 2004): Patterns of variability and biological responses. West Ind. Oc. J Mar. Sci. 6, 1–16 (2009).
NASA Ocean Color. https://oceancolor.gsfc.nasa.gov/.
Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 43, 783 (1987).
Oksanen, J. et al. vegan: Community Ecology Package (2022).
Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests (2023).
Baselga, A. et al. betapart: Partitioning Beta Diversity into Turnover and Nestedness Components (2022).
Baselga, A. Partitioning the turnover and nestedness components of beta diversity: Partitioning beta diversity. Global Ecol Biogeogr 19, 134–143 (2010).
Gotelli, N. J., Hart, E. M. & Ellison, A. M. EcoSimR: Null Model Analysis for Ecological Data. https://doi.org/10.5281/zenodo.16522 (2015).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing (2022).
Odum, E. P. The Strategy of Ecosystem Development: An understanding of ecological succession provides a basis for resolving man’s conflict with nature. Science 164, 262–270 (1969).
Adey, W. H. & Vassar, J. M. Colonization, succession and growth rates of tropical crustose coralline algae (Rhodophyta, Cryptonemiales). Phycologia 14, 55–69 (1975).
Hughes, R. Differences in the growth, form and life history of Plumularia setacea (Ellis and Solander) (Hydrozoa: Plumulariidae) in two contrasting habitats. Proc. R. Soc. Lond. B 228, 113–125 (1986).
Zardus, J. D., Nedved, B. T., Huang, Y., Tran, C. & Hadfield, M. G. Microbial biofilms facilitate adhesion in biofouling invertebrates. Biol. Bull. 214, 91–98 (2008).
Choi, D. R. Ecological succession of reef cavity-dwellers (coelobites) in coral rubble. Bull. Mar. Sci. 35, 72–79 (1984).
Toledo, M.-I. et al. Ecological succession of benthic organisms on niche-type artificial reefs. Ecol. Process 9, 38 (2020).
Maire, O., Duchêne, J. C., Amouroux, J. M. & Grémare, A. Activity patterns in the terebellid polychaete Eupolymnia nebulosa assessed using a new image analysis system. Mar. Biol. 151, 737–749 (2007).
Scheffers, S. R., Van Soest, R. W. M., Nieuwland, G. & Bak, R. P. M. Coral reef framework cavities: is functional similarity reflected in composition of the cryptic macrofaunal community? Atoll Res. Bull. 583, 1–24 (2010).
Nelson, H. R. & Altieri, A. H. Oxygen: The universal currency on coral reefs. Coral Reefs 38, 177–198 (2019).
Chadwick, N. E. & Morrow, K. M. Competition among sessile organisms on coral reefs. In Coral Reefs: An Ecosystem in Transition (eds Dubinsky, Z. & Stambler, N.) 347–371 (Springer, 2011). https://doi.org/10.1007/978-94-007-0114-4_20.
Littler, M. M. & Littler, D. S. The nature of crustose coralline algae and their interactions on reefs. Research and discoveries: the revolution of science through SCUBA (2013).
Hughes, T. P. et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018).
Sandin, S. A. & Sala, E. Using successional theory to measure marine ecosystem health. Evol. Ecol. 26, 435–448 (2012).
Bak, R. P. M., Sybesma, J. & van Duyl, F. C. The ecology of the tropical compound ascidian Trididemnum solidum. II. Abundance, growth and survival. Mar. Ecol. Prog. Ser. 6, 43–52 (1981).
Weersing, K. & Toonen, R. Population genetics, larval dispersal, and connectivity in marine systems. Mar. Ecol. Prog. Ser. 393, 1–12 (2009).
Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 85, 183–206 (2010).
McGill, B. J. et al. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 10, 995–1015 (2007).
Pearman, J. K., Anlauf, H., Irigoien, X. & Carvalho, S. Please mind the gap—Visual census and cryptic biodiversity assessment at central Red Sea coral reefs. Mar. Environ. Res. 118, 20–30 (2016).
Acknowledgements
This study was supported by the research program Fonds Européen de Développement Régional (FEDER) 20171591-0002633 CALIBIOME 2017-2022. ARMS deployments were conducted under permit n°2020-09-DEAL/SEB/UBIO and 2018-61 DEAL/SED/UBIO of the Direction de l’eEnvironnement, de l’aAménagement et du logement de La Réunion (Part 4) granted to J.H.B. and M.M.M.G., and permit nos 2019-083 and 2020-054 of the Direction de la Mer Sud Océan Indien. Baptiste Frattini benefited a PhD fellowship provided by ED 227 MNHN-SU. We thank Master students Gwennaïs Fustemberg and Auriane Serval for assistance in processing the ARMS, and Sophie Bureau and Lionel Bigot for their support during ARMS deployment and retrieval. We are indebted to Françoise Monniot† (ascidiaceans), Jean-Loup d’Hondt (bryozoans), Annachiara Bartolini (foraminiferans), David Ory (hydrozoans) for their identifications, and to François Guilhaumon for statistical advice. We are grateful to the AAP BOrEA Sud (2023/2024) and to Nathalie Niquil for her constant support of our research. We are also thankful to Christine Carrau, librarian at the Bibliothèque Théodore Monod at MNHN, for her assistance in accessing historical literature. Finally, we thank two anonymous reviewers for constructive comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Contributions
JHB, MMMG and MC conceptualised the project, and JHB and MMMG acquired the funding. JHB, BF, MC, FB and MMMG performed the fieldwork, participated in sample processing and data curation. BF performed the analyses, prepared the figures and wrote the original draft. JHB, MMMG and EG participated in investigation, supervision and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Frattini, B., Bruggemann, J.H., Goberville, E. et al. Seasonal colonisation and ecological succession shape coral reef sessile cryptobenthic communities in Autonomous Reef Monitoring Structures. Sci Rep 15, 23232 (2025). https://doi.org/10.1038/s41598-025-01624-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01624-9