Abstract
Multidrug resistant Acinetobacter baumannii is declared as crucial level precedence pathogen by World Health Organization that needs new and upgraded antibiotics for better treatment. Against a vast extent of microbes, many silver nanoparticles have displayed anti-microbial activity because of their numerous methods of antimicrobial actions. This study was aimed to isolate and characterize the Acinetobacter baumannii by using standard microbiological technique and to analyze the anti-inflammatory and antagonistic effect of PB capped AgNPs in isolated A. baumannii. In antagonistic activity, PB capped AgNPs showed antagonistic effect in 8 out of 20 isolates tested and PB when combined with AgNPs showed antagonistic effect in all isolates tested. In in-vitro anti-inflammatory egg albumin assay, PB capped AgNPs gave inhibition rate of 96% at 12.5 µg, 93% at 25 µg, 82% at 50 µg and 62% at 100 µg whereas in Polymyxin B combined AgNPs it gave inhibition rate of 97% at the dose of 50 µg. In-silico analysis also showed that PB in combination with tri-sodium citrate gave good binding energy than tri-sodium citrate and Polymyxin used alone. Hence, this study represented that silver nanoparticles when used in combination proved to be a good alternative in the treatment of MDR Acinetobacter baumannii.
Similar content being viewed by others
Introduction
The antimicrobial resistance increasing extensiveness is a major risk to human health and it is enhancing mortality rates1. When studying about treatment of A. baumannii infections, awareness should be recompense to the definition of resistance used2. More research attempts have been made towards the evolution of substituted antimicrobials with multitargeting methods3. A. baumannii is a coco bacillus, Gram-negative bacteria. It is non-motile and aerobic bacterial pathogen. It is a major cause of nosocomial infections3. A. baumannii is the most frequent pathogen that causes bloodstream infections and ventilator associated pneumonia. It infects those with debilitating immune system. It is an antibiotic-resistant microbe. To all antimicrobial agents that are presently accessible, A. baumannii is found to be the most resistant4. To primary antimicrobial treatments, the rapidly increasing resistance of A. baumannii has made a lethal combination of AMR and pathogenicity that afflicts hospitals. At the time of admissions, Nosocomial infection is not present in patients. It arises within 48 h after infection. Because A. baumannii is common in humans, it is called the most common type of infection in nosocomial infections5. In critically ill patients, hospital acquired infections are seen and has risk of developing A. baumannii infections including burns, traumas, immunosuppression and use of previous antibiotics, while community-acquired infections found in hot and humid climate countries as a serious clinical disorder1. According to urgent need of new antibiotics, WHO has three categories, The most critical group includes MDR bacteria that pose a serious threat to patients such includes Acinetobacter, E.coli, Pseudomonas, Klebsiella, Proteus and Serratia. Resistance to multiple drugs defined as a great provocation as second line treatment options are less clarified in their potency and accompanies infectivity2. It is obligatory to evolve different drugs that can be used against A. baumannii4.
Although the pharmaceutical market offers a wide range of antibacterial medications, continued research into powerful antimicrobial agents—particularly those with novel mechanisms of action—is vital. This is key to curbing the development of resistance to current antibiotics and ensuring effective treatments for infections caused by antibiotic-resistant bacteria. Against a wide range of microbes many silver nanoparticles have displayed antimicrobial activity because of their numerous methods of antimicrobial action4. Silver nanoparticles are used in many fields involving food, health care, and medical purposes. The capability of AgNPs as antibiotics is linked to their numerous action modes which grant them the capability to kill numerous types of bacteria6,7. Silver has been used as an antimicrobial agent in all its forms when used alone as well as in combinations with other mechanisms6. Silver nanoparticles (AgNPs) generally pose no harm to human health at low concentrations due to their specific form, size, stability, and biocompatibility. However, prolonged exposure to silver can lead to serious conditions such as argyrosis. The chemical reduction method is employed to synthesize a colloidal solution of silver nanoparticles using silver nitrate as the precursor, with trisodium citrate acting as both the stabilizing and reducing agent in the process8. In case of A. baumannii high resistance is found in Amoxicillin, ampicillin, ciprofloxacin, imipenem, meropenem, cefuroxime, cefoxitin, cefepime, gentamicin, cefotaxime and nitrofurantoin9. Polymyxin B is an antibiotic that is used for the treatment of variety of infections. It is used for treatment of Gram-negative organism infections and all Gram-positive organisms remain resistant. It is used for treatment of blood sepsis and urinary tract infection. It has chemical formula C56H98N16O13. Polymyxin B damages the Gram-negative bacteria outer membrane. During bacterial lysis, Polymyxin B coheres and counteracts lipopolysaccharide. Polymyxin B and Colistin (Polymyxin E) are FDA approved drugs. They are considered as last line therapy for inflammation produced by Gram-negative bacteria. Polymyxin B is used as therapy for various infections including meningitis, urinary tract infections and blood stream infections caused by Gram negative bacteria10. Hence, the capping of Polymyxin B with silver nanoparticles must be studied to eliminate the resistance towards A. Baumannii. Capping of Polymyxin B with silver nanoparticles refers to the process in which Polymyxin B replaces the trisodium citrate on the surface of the silver nanoparticles, effectively attaching to it. Antagonistic efficacy and anti-inflammatory study will be done on A. baumannii by capping Polymyxin B and Citrate with silver nanoparticles and combining both CitAgNPs and PBAgNPs. The purpose of this study is to analyze the anti-inflammatory and antagonistic effect of PB capped AgNPs in isolated A. baumannii.
Materials and methods
Silver nanoparticles preparation (AgNPs)
0.084 g of silver nitrate was added in 50 ml deionized water in a beaker and heat it for 30 min with a stirrer in it. Then in 50 ml distilled water, 1 g tri sodium citrate was added. Then from prepared tri sodium citrate, 5 ml solution was added dropwise in prepared silver nitrate with continuous heating and stirring. Then it was heated for 15 min and cooled at room temperature. Silver nanoparticles was prepared11.
Polymyxin B capped silver nanoparticles preparation (PBAgNPs)
0.084 g of silver nitrate was added in 50 ml deionized water in a beaker and Polymyxin B with different concentrations (12.5 µg, 25 µg, 50 µg and 100 µg) was added in prepared silver nitrate solution and heat it for 30 min with a stirrer in it. Then in 50 ml distilled water, 1 g tri sodium citrate was added. Then from prepared tri sodium citrate, 5 ml solution was added dropwise in prepared silver nitrate with continuous heating and stirring. Then it was heated for 15 min and cool at room temperature. Polymyxin B capped silver nanoparticles was prepared12.
Characterization of AgNPs and PBAgNPs
The characterization of AgNPs and Polymyxin B capped AgNPs was done using techniques such as Dynamic light scattering (DLS) and Fourier transform infrared (FTIR) to determine their morphology.
Collection A. baumannii samples
A total 250 samples of Multidrug resistant A. baumannii was collected from tertiary care hospitals of Lahore. The sample was processed for isolation and identification of A. baumannii by using standard microbiological technique.
Isolation and identification of A. baumannii
The collected isolates of A. baumannii was cultured on Macconkey agar and was identified using biochemical tests as urease test, Simon citrate test, indole test, catalase test, Triple sugar iron test and gram staining.
Antagonistic activity
The Antagonistic activity of AgNPs, Polymyxin B and PBAgNPs at 100 µg, 50 µg, 25 µg and 12.5 µg were evaluated against multidrug A. baumannii using well diffusion method. Muller Hinton broth was made for determining the pure culture of bacteria. Then Muller Hinton agar plates were prepared and well has been made with in AgNPs, Polymyxin B and PBAgNPs at 100 µg, 50 µg, 25 µg and 12.5 µg were placed. Wells are created with diameter ranging from 6 to 8 mm using serile straw and plates are incubated at 37 C for 24 h. Zone of inhibition was measured in mm and antagonistic activity was evaluated against bacterial strains13.
Anti-inflammatory egg albumin
From fresh hen’s egg, 0.2 ml of 1–2% aqueous solution of egg albumin will be prepared. At different concentrations of 2 ml of PBAgNPs, AgNPs and Polymyxin B prepared egg albumin solution will be added and then phosphate buffer saline 2.8 ml (PH 7.4) were added and mixed to make volume up to 5 ml. Mixture will be incubated for 20 min and then heat for 20 min at 510C in water bath. After cooling, absorbance at 590 nm will be done by Elisa reader. A drug Diclofenac sodium (Standard) will be taken as positive control and distilled water as negative control. Then Anti-inflammatory % of inhibition was measured through the equation.
\(\% {\text{ INHIBITION}}={\text{1}}00{\text{ x }}\left( {\left[ {{\text{Vt}}/{\text{Vc}}} \right] -{\text{1}}} \right).\)
Vt is test sample absorbance and Vc is control absorbance14.
In silico analysis
Selection of ligands and protein
As Acinetobacter baumannii causes hospital-acquired infections and is a multidrug-resistant bacteria. For the treatment of Acinetobacter baumannii, Polymyxin B an antibiotic is selected coated with silver nanoparticles. Hence ligands selected were tri-sodium citrate and Polymyxin B along with LPS protein (LptE) of A. baumannii for the treatment of MDR bacteria13.
Ligand preparations
SDS file of ligands (tri-sodium citrate and Polymyxin B) were selected from PUBCHEM and drawn on CHEMSKETCH. Then from PYMOL their 3D structure were obtained as PDB format13.
Protein preparations
3D structure of protein was downloaded from Protein data bank in PDB format with PBD ID 5TSE. The selected protein LptE was compressed by UCSF Chimera. All the ions, solvent and ligands were removed and single chain was saved13.
Prediction of active site
During a reaction, a site where an enzyme binds to protein is called active site. The active sites were predicted by CAST-p site. Amino acids were selected from chain C13.
Docking analysis
All Amino acids were selected in grid box. For LptE protein with Polymyxin B and Tri sodium citrate there were center x= − 0.0313, center y= − 43.38, center z = 1.194 and size x = 15.12, size y = 21.82 and size z = 25.57. Docking was done using PYRX and then protein ligand binding pattern will be obtained using Chimera. Same process was repeated with single Polymyxin B with Lpte protein as there was center x = − 0.0935, y= − 38.48, z= -11.63 and size x = 23.05 y = 12.02 and z = 26.86. The center for Tri-sodium citrate with Lpte protein was x = 1.057, y= − 36.94, z= -10.89, and size x = 25.0, y = 13.38, z = 27.3413.
Results
Collection of samples of Acinetobacter baumannii
A total of 250 samples were collected including pus, blood, wound and CSF from the Tertiary care hospital in Lahore. Out of 250 samples only 136 grew, other 114 samples were shown no prominent growth as shown in Fig. 1.
Isolation and identification of A. baumannii
The 136 samples were cultured and out of 136 only 14 were multi drug resistant Acinetobacter baumannii and 20 were A. baumannii which was sensitive against Polymyxin and AgNPs (Figs. 2 and 3). In this study, the 20 out of 136 samples which was sensitive to PB and Citrate capped AgNPs were considered for the antiinflammatory as well as antagonistic activity of Polymyxin B capped silver nanoparticles. Out of 20 MDR A. baumannii 6 samples were from wounds, 11 were from pus and 3 were from blood. Out of 20 A. baumannii 12 were MDR, 5 were XDR and 3 were PDR (Tables 1 and 2).
Acinetobacter baumannii was identified using biochemical tests including indole test, urease test, TSI test, Simon citrate test, Catalase test and gram staining. TSI results showed pink color that indicates positive test result for A. baumannii (Fig. 3). Indole showed no ring formation that indicates negative test results for A. baumannii. Urease were negative for A. baumannii, Simon citrate were positive as due to the appearance of blue color. Catalase test were positive for A. baumannii due to the formation of bubbles.
Synthesis of citrate capped and PB capped silver nanoparticles
A dark grey color obtained by addition of drop wise tri-sodium citrate on prepared silver nitrate solution indicated the formation of Citrate capped AgNPs. Polymyxin B with varying concentration (2 fold) 100 µg, 50 µg, 25 µg and 12.5 µg was added to silver nitrate solution and a dark grey color was obtained by addition of few drops of tri-sodium citrate (Fig. 4).
Characterization of AgNPs and PBAgNPs
Dynamic light scattering (DLS)
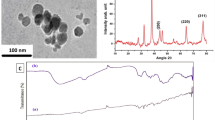
DLS is used to measure the size of a molecule or a particle. It includes Hydrodynamic diameter which indicates the size of a particle whereas zeta potential indicates the stability of a particle. In silver nanoparticles, the hydrodynamic diameter was 19,835 nm with the size of 1771 nm. Silver nanoparticles when capped with Polymyxin B at 12.5 µg shows hydrodynamic diameter of 296.6 nm with the size of 264.6 nm and at 25 µg hydrodynamic diameter was 18,824 nm with the size of 677 nm. Silver nanoparticles when capped with Polymyxin B at 50 µg shows hydrodynamic diameter of 45,882 nm and size shows 1031 nm and at 100 µg hydrodynamic diameter was 734.7 nm with two peaks, one is with size 159 nm and other is 964 nm. This shows that PB when capped with AgNPs at 50 µg showed increase in hydrodynamic diameter than citrate capped AgNPs with the decrease in size means PB capped AgNPs at 50 µg has a good stability than citrate capped AgNPs (Fig. 5)4.
Zeta potential value indicates the repulsion among Polymyxin B and AgNPs and increase stability of composition. Zeta potential of silver nanoparticles shows − 1.6 mV whereas Polymyxin B capped silver nanoparticles shows zeta potential of − 8.9 mV at 12.5 µg, − 0.7 mV at 25 µg, and − 1.8 mV at 50 µg and 100 µg. Silver nanoparticles are poly dispersed in nature. As because of negative zeta potential values repulsion of particles helps in aversion of accumulation of nanoparticles and helpful in long term stability (Fig. 6)4.
Fourier transform infrared (FTIR) results
FTIR is used for determining the biochemical differences between AgNPs and Polymyxin B capped AgNPs. The spectrum of AgNPs showed frequency within the band 1098 cm− 1–3408 cm− 1. The strongest peak was observed in 1577 cm− 1, and 3375 cm− 1 which indicates the functional group of C=C and O-H group. Silver nanoparticles when capped with Polymyxin B showed a slight conformational change and in band intensity. At 12.5 µg dose showed band frequency within 882 cm− 1– 3340 cm− 1. The strongest peak was observed in 1641 cm− 1 and 3340 cm− 1 which reflects the functional group of N–H and O–H group. At 25 µg, 50 µg and 100 µg dose, band frequency ranges within 1639 cm− 1–3330 cm− 1 which showed increase in intensity which contributes to symmetric and asymmetric stretching. Strongest peak was observed in 1639 cm− 1 which indicates presence of N–H bond. In AgNPs and Polymyxin B capped silver nanoparticles the results were same except the peak that was observed in 1639 cm− 1 indicating the amine group as also shown in the study of15 and a different peak was seen at 12.5 µg dose at 1524 cm− 1 indicating the functional group N–O bond which were both not present in AgNPs, indicating that Polymyxin B were functionalized with citrate capped AgNPs at N atom of amine group (Fig. 7)15.
Antagonistic activity
Antagonistic activity was performed on Muller Hinton agar, silver nanoparticles showed no effect, only the Polymyxin B showed the effect. Polymyxin sensitivity pattern were also varied some strains of A. baumannii that were resistant against the lowest dose of Polymyxin B and some were sensitive against the longer dose which reflects the pathetic condition of public health because Polymyxin B has many side effects, it cause neurotoxicity and nephrotoxicity. When Polymyxin B capped with AgNPs (Table 3), it showed similar results. Out of 20 samples no sample were sensitive in 12.5 µg dose, 2 samples were sensitive in 25 µg dose, 8 were sensitive in 50 µg dose and 4 were sensitive in 100 µg dose in our results. In combination dose with AgNPs we find that all 20 samples were found sensitive. As a result of antagonistic activity, it is considered from our result that citrate capped silver nanoparticles alone were not a sufficient choice of therapy for the control of MDR Acinetobacter baumannii as it is also considered in past studies3 but it can be beneficial when used in combination with Polymyxin B (Table 4; Fig. 8, and Fig. 9).
The antimicrobial susceptibility of Polymyxin B and silver nanoparticles was evaluated using well diffusion method. Zone of inhibition shows sensitivity of Polymyxin B capped silver nanoparticles with different concentrations, AgNPs, and Pure Polymyxin B as shown in previous research.
According to CLSI standards for Polymyxin B, it is resistant at < 11 mm and sensitive when > 12 mm.
Anti-inflammatory egg albumin assay
Anti-inflammatory activity was performed by egg albumin process by Elisa reader. PB Capped AgNPs were taken at various concentrations and it has been compared with standard (Diclofenac sodium). Results showed % inhibition of 96% at the dose of 12.5 µg, 93% at the dose of 25 µg, 82% at 50 µg and 62% at 100 µg whereas Polymyxin B when combined with AgNPs gave the inhibition rate of 97% at the dose of 50 µg. Hence, it is concluded that PB capped AgNPs and PB in combination with AgNPs showed good anti-inflammatory activity in egg albumin assay and it is found that PB capped AgNPs found comparative to the standard set even at all concentrations. PB capped AgNPs showed good anti-inflammatory activity in the method of egg albumin assay (Table 5, and Figs. 10 and 11).
Computational results
Evaluation of protein structure
LptE protein is a Lipopolysaccharide protein at the surface of outer membrane of Acinetobacter baumannii. It has three chains (A –C) and consists of 169 amino acids. Vadar analysis of LptE indicates 31% alpha helix, 46% beta helix, 28% coil and 9% turn. RAMACHANDRAN PLOT of LptE protein contains 95.12% amino acids in preferred region, 4.88% in allowed region and there are 0 outliers remaining (Fig. 12).
Evaluation of ligand structure
Polymyxin B belongs to the category of drugs. It has broad spectrum of activity against gram-negatives10. Polymyxin B is produced by a gram positive microorganism Paenibacillus polymyxa. It is declared as a last line treatment against MDR gram negative organisms. Polymyxins are cyclic polypeptides16. Its chemical formula is C56H98N16O13 and molecular weight is about 1203.477 g/mol17. Tri-sodium citrate is a sodium salt of citric acid. It acts as a reducing agent in silver nanoparticles18. It is a white crystalline powder. It has chemical formula C6H5Na3O7 and molecular weight of 258.06 g/mol (Fig. 13).
LptE protein active site
The Binding center of LptE protein consists of 14 amino acids 52 ASP, 56 GLN, 59 VAL, 60 TYR, 124 ARG, 142 TYR, 145 ARG, 146 ILE, 148 ILE, 149 ASP, 150 ASP, 153 GLN, 154 GLN and 157 ARG. These amino acids were selected from chain C (Fig. 14).
Binding of protein and ligands
After the docking has been done, both the Ligands occupy in protein binding pocket. All the picked amino acids revealed that both the docked ligands were redundant to gain their binding interaction (Table 6; Figs. 15 and 16).
Docking results of LptE protein with Tri-sodium citrate
When docked in combination with Polymyxin B, after modifying the best possible side for ligand in binding center of LptE protein, the two hydrogen bonds represent binding between Tri-sodium citrate with LptE as ARG at position 157 shows 1.332 Å bond distance and GLN with position 153 shows 1.514 Å (Table 7; Fig. 17). When docked alone, no hydrogen bond formed between tri-sodium citrate and LptE protein of A. baumannii. While Table 8 shows opposite results, ARG 157 shows 1.49 Å bond distance when alone.
Docking complex of LptE protein with polymyxin B
When taken in combination, after modifying the best side for ligand in binding center of LptE protein, there is no hydrogen bond formed between Polymyxin B with LptE. When taken alone, Polymyxin B showed hydrogen bond shows binding between Polymyxin B and LptE protein of A. baumannii at 153 Å (Fig. 18).
Binding energies
Binding of tri-sodium citrate and Polymyxin B with LptE, Polymyxin B with LptE and Tri sodium citrate with LptE was done using PYRX. Tri-sodium citrate shows the highest binding energy of -4.0 whereas Polymyxin B shows highest binding energy of 6. When evaluated in combination Polymyxin B showed highest binding energy of -5 and tri sodium citrate showed highest binding energy of -4.8 (Table 9). Higher the negative value higher is the Bonding. Hence, Polymyxin B and Tri sodium citrate gave good binding energy with LptE protein when used in combination.
Discussion
Multi-drug resistance is a main issue now-a-day. (A) baumannii becoming more and more resistant to multi drugs except Polymyxin B and Tigecycline. Hence, new treatments are required to overcome this issue. Polymyxin B is found to cause side effects like neurotoxicity and nephrotoxicity but it is the last choice of drugs to treat bacterial infections. Hence, our study depends on combinational therapy of Polymyxin B with silver nanoparticles to reduce the toxicity of Polymyxin (B) In our study 8 out of 20 isolates showed antagonistic activity in Polymyxin B capped AgNPs whereas 20 out of 20 isolates showed antagonistic activity in Polymyxin B combined silver nanoparticles. As in Allend et al.., showed that bio-AgNPs when combined with Polymyxin B gave synergistic and additive effect and showed decrease in cells of A. baumannii with MIC ranges from 0.46 to 1.87 ml. Bio-AgNPs when combined with Polymyxin B gave synergy against 4–5 strains and additivity against one strain19. In Naser (2018) study showed that 45 A. baumannii were isolated. AgNPs when combined with Polymyxin B showed FIC index value of 0.24–0.51 which showed that interaction is synergistic20. PB when used alone was 84% sensitive and 15% resistant. This study also proved that AgNPs have better results when combined with Polymyxin B against Acinetobacter baumannii. In Wan et al.., combination of antibiotics with AgNPs and as well as alone is studied21. Study showed that AgNPs when combined with Polymyxin B shows good synergistic effect than used alone.
As in Hetta et al.., A. baumannii has been inhibited by AgNPs at MIC ranging from 4 to 25 µg/ml by well diffusion method22. In presence of AgNPs, A. baumannii has showed very less growth and also showed good antibacterial activity against A. baumannii in in-vitro infection model. In study by3 showed that resistant to silver nanoparticles can also be found. This study revealed that silver nanoparticles showed resistance in multidrug resistant strains of A. baumannii. In study by Auda et al.., showed that citrate capped silver nanoparticles gave better results when given in combination with imipenem than used alone23. Citrate capped silver nanoparticles gave additive effect when combined with imipenem. MIC and MBC of citrate capped silver nanoparticles were very low against A. baumannii.
In our study, Polymyxin B were sensitive at the dose of 100 µg and 500 µg whereas at 50 µg it was found resistant against A. baumannii. In study by Thomas et al.., showed that Polymyxin B were susceptible to A. baumannii that were XDR within MIC ranges from (1–2 µg) whereas PB at > 4 µg were found to be resistant to A. Baumannii24. In study by Zhu et al.., it was found that all 11 isolates showed resistance to PB and amikacin at the dose of 8 mg against A. Baumannii25. In study by da Costa et al.., showed that among 38 isolates of A. baumannii 13.2% were positive for Polymyxin B and all of them were resistant to PB within MIC value of 32 µg26. In study by Shah et al.., showed that 100 isolates of A. baumannii were collected from tertiary care hospital and these isolates were recovered from tracheal secretion, CSF and urine27. In antibacterial activity it was found that AgNPs gave zone of 22 mm at 50 mg and 18 mm at 40 mg.
As in Zhang et al.., showed that in ten Carbapenem resistant A. baumannii isolates Polymyxin B and Tigecycline showed 0% resistance rate and imipenem, meropenem and SUL showed 100% resistance rate7. In study by Xi et al.., showed that isolates of A. baumannii collected were 4.2% and about 94% was found susceptible28. It is reported first time in3 that A. baumannii developed resistance to silver nanoparticles. In study by Lambadi et al.., showed that Polymyxin B when capped with AgNPs gave good anti-bacterial activity in Pseudomonas aeruginosa evaluated first time against A. Baumannii15. Liu et al.., showed that Polymyxin B is a good target against A. baumannii, it showed a good anti-bacterial activity but antibiotic combining therapies seem to give promising result than Polymyxin B used alone29.
This study Rather et al., also showed that Polymyxin B capped silver nanoparticles showed a good anti-bacterial activity against Pseudomonas aeruginosa as tested in a surgical blade30. This study also includes the inhibitory effect of both AgNPs as well as Polymyxin B and this study concludes that Polymyxin B capped AgNPs has superior anti-microbial effect than citrate capped AgNPs but our study was on Polymyxin B capped AgNPs against Acinetobacter baumannii that found sensitive in 8 out of 20 samples tested at the dose of 50 µg which was not studied before.
In our results of in vitro anti-inflammatory activity of egg albumin assay, Polymyxin B when capped with AgNPs gave inhibition rate of 96% at the dose of 12.5 µg, 93% at the dose of 25 µg, 82% at the dose of 50 µg and 62% at the dose of 100 µg. Whereas at 50 µg Polymyxin B gave inhibition rate of 65%, Citrate capped AgNPs gave inhibition rate of 82% and Polymyxin B when combined with silver nanoparticles gave inhibition rate of 97% at the dose of 50 µg. In comparison to our results, a study by Kedi et al.., showed that at 0.2 mg silver nanoparticles showed 99% inhibition rate and standard set acetyl salicylic acid gave inhibition rate of 65%31,32,33,34,35. In study by Revathi et al.., showed that silver nanoparticles gave inhibition rate of 78.65% and Diclofenac sodium gave inhibition rate of 81.23% at the dose of 500 µg36. In study by Ameena et al.., showed that diclofenac sodium gave inhibition rate of 84% at 50 µg and 47% at 10 µg14.
Conclusion
Consider from our result that in antagonistic activity citrate capped AgNPs alone is not a good choice of therapy against MDR A. baumannii but it can be beneficial when used in combination as also proved from in-silico study. In anti-inflammatory activity, PB combined and capped AgNPs found to be comparative than standard use (Diclofenac sodium). Hence Silver nanoparticles when combined with Polymyxin B found to be an excellent choice to the health issues and offered as a good alternative in future use against MDR Acinetobacter baumannii.
Data availability
All the data generated in this research work has been included in this manuscript.
References
Morris, F. C., Dexter, C., Kostoulias, X., Uddin, M. I. & Peleg, A. Y. The mechanisms of disease caused by Acinetobacter baumannii. Front. Microbiol. 10, 1601 (2019).
Viehman, J. A., Nguyen, M. H. & Doi, Y. Treatment options for carbapenem-resistant and extensively drug-resistant Acinetobacter baumannii infections. Drugs 74, 1315–1333 (2014).
McNeilly, O. et al. Development of nanoparticle adaptation phenomena in Acinetobacter baumannii: physiological change and defense response. Microbiol. Spectr. 11 (1), e02857–e02822 (2023).
Tiwari, V., Roy, R. & Tiwari, M. Antimicrobial active herbal compounds against Acinetobacter baumannii and other pathogens. Front. Microbiol. 6, 141854 (2015).
Bouadma, L., Wolff, M. & Lucet, J. C. Ventilator-associated pneumonia and its prevention. Curr. Opin. Infect. Dis. 25 (4), 395–404 (2012).
Bruna, T., Maldonado-Bravo, F., Jara, P. & Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 22 (13), 7202 (2021).
Zhang, X. F., Liu, Z. G., Shen, W. & Gurunathan, S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 17 (9), 1534 (2016).
International, B. R. & Retracted Vancomycin as an antibacterial agent capped with silver nanoparticles: an experimental potential analysis. Biomed. Res. Int. 2024, 9784637 (2024).
Agyepong, N., Fordjour, F. & Owusu-Ofori, A. Multidrug-resistant Acinetobacter baumannii in healthcare settings in Africa. Front. Trop. Dis. 4, 1110125 (2023).
Hîncu, S. et al. Drug–drug interactions in nosocomial infections: an updated review for clinicians. Pharmaceutics 16 (9), 1137 (2024).
Arif, M., Ulfiya, R., Erwin, E. & Panggabean, A. S. Synthesis of silver nanoparticles using trisodium citrate and development in analysis method. In AIP Conference Proceedings 2360 (1). (AIP Publishing, 2021).
Liu, T. et al. A polymyxin B–silver nanoparticle colloidal system and the application of lipopolysaccharide analysis. Analyst 143 (5), 1053–1058 (2018).
Siddiqui, F. et al. Scrutinizing the effect of additive and synergistic antibiotics against carbapenem-resistant Pseudomonas aeruginosa. Open. Chem. 22 (1), 20240074 (2024).
Ameena, M., Arumugham, M., Ramalingam, K., Rajeshkumar, S. & Shanmugam, R. Evaluation of the anti-inflammatory, antimicrobial, antioxidant, and cytotoxic effects of Chitosan thiocolchicoside-lauric acid nanogel. Cureus 15 (9). (2023).
Lambadi, P. R. et al. Facile biofunctionalization of silver nanoparticles for enhanced antibacterial properties, endotoxin removal, and biofilm control. Int. J. Nanomed., 2155–2171. (2015).
Velkov, T., Roberts, K. D., Thompson, P. E. & Li, J. Polymyxins: a new hope in combating Gram-negative superbugs? Future Med. Chem. 8 (10), 1017–1025 (2016).
Zaidi, M. S., Bakar, R. A., Musa, N., Mustafa, S. & Yusuf, W. N. W. Optimization of quadrupole time-of-flight liquid chromatography mass spectrometry (QTOF-LC/MS) conditions for determination of colistin in human plasma. Malaysian J. Anal. Sci. 26 (6), 1288–1302 (2022).
Husanu, E., Chiappe, C., Bernardini, A., Cappello, V. & Gemmi, M. Synthesis of colloidal ag nanoparticles with citrate-based ionic liquids as reducing and capping agents. Colloids Surf. Physicochem Eng. Asp. 538, 506–512 (2018).
Allend, S. O. et al. Biogenic silver nanoparticle (Bio-AgNP) has an antibacterial effect against carbapenem-resistant Acinetobacter baumannii with synergism and additivity when combined with polymyxin B. J. Appl. Microbiol. 132 (2), 1036–1047 (2022).
Naser, I. J. Synergistic effect of silver nanoparticles and polymyxin B on multidrug-resistant Acinetobacter baumannii isolated from burn wound infections. Iraqi J. Comm. Med. 31 (2), 64–68 (2018).
Wan, G. et al. Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria acinetobacter baumannii. Int. J. Nanomed. :3789–3800. (2016).
Hetta, H. F. et al. Antibiofilm and antivirulence potential of silver nanoparticles against multidrug-resistant Acinetobacter baumannii. Sci. Rep. 11 (1), 10751 (2021).
Auda, I. G., Salman, I. M. A., Al-Sattar, D. A. & Oduha, J. G. In-vivo and in-vitro anti-Acinetobacter baumannii activity of citrate-capped silver nanoparticles. Nano Biomed. Eng. 13 (3), 229–239 (2021).
Thomas, V. M., Brown, R. M., Ashcraft, D. S. & Pankey, G. A. Synergistic effect between Nisin and polymyxin B against pandrug-resistant and extensively drug-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents. 53 (5), 663–668 (2019).
Zhu, S. et al. Effects of Amikacin, polymyxin-B, and sulbactam combination on the pharmacodynamic indices of mutant selection against multi-drug resistant Acinetobacter baumannii. Front. Microbiol. 13, 1013939 (2022).
da Costa Júnior, S. D., da Silva, W. R. C., da Silva, A. M. C., Maciel, M. A. V. & Cavalcanti, I. M. F. Synergistic effect between Usnic acid and polymyxin B against resistant clinical isolates of Pseudomonas aeruginosa. Evid. Based Complement. Alternat Med. (2020).
Shah, A. A. et al. Antibacterial activity of silver nanoparticles against carbapenem-resistant Acinetobacter baumannii clinical isolates. Pak J. Pharm. Sci. 35 (2022).
Xi, J. et al. Antimicrobial susceptibility to polymyxin B and other comparators against Gram-negative bacteria isolated from bloodstream infections in China: results from CARVIS-NET program. Front. Microbiol. 13, 1017488 (2022).
Liu, H. et al. In vitro analysis of synergistic combination of polymyxin B with 12 other antibiotics against MDR Acinetobacter baumannii isolated from a Chinese tertiary hospital. J. Antibiot. 76 (1), 20–26 (2023).
Rather, G. A. et al. Antimicrobial efficacy of biogenic silver and zinc nanocrystals/nanoparticles to combat the drug resistance in human pathogens. In Materials at the Nanoscale. (IntechOpen, 2021).
Kedi, P. B. E. et al. Eco-friendly synthesis, characterization, in vitro and in vivo anti-inflammatory activity of silver nanoparticle-mediated Selaginella myosurus aqueous extract. Int. J. Nanomed., 8537–8548 (2018).
Ibraheem, R. D., Hussein, N. N. & Suliman, G. M. Antibacterial activity of silver nanoparticles against pathogenic bacterial isolates from diabetic foot patients. Iraqi J. Sci. 64 (5), 2223–2239 (2023).
Ibraheem, D. R. et al. Ciprofloxacin-Loaded silver nanoparticles as potent Nano-Antibiotics against resistant pathogenic Bacteria. Nanomaterials 12, 2808 (2022).
Esnaashari, F. & Zahmatkesh, H. Antivirulence activities of Rutin-loaded Chitosan nanoparticles against pathogenic Staphylococcus aureus. BMC Microbiol. 24 (1), 328 (2024).
Sigarchian, M., Langarudy, M. & Esnaashari, S. H. Samarium oxide coated by Rutin: synthesis, antibiofilm, antivirulence and bactericidal activity evaluation. Chem. Pap. https://doi.org/10.1007/s11696-025-03997-7 (2025).
Revathi, N. & Dhanaraj, T. S. A study on in vitro anti-inflammatory activity of silver nanoparticles synthesized from Dodonaea angustifolia leaf extract. J. Pharm. Pharmacognosy Res. 8 (4), 1878–1881 (2019).
Funding
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R228), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors also thank the the Deanship of Scientific Research (DSR) at King Faisal University under project no. [KFU250830].
Author information
Authors and Affiliations
Contributions
Conceptualization, Nureen Zahra; methodology, Fatima Siddiqui; software, Basit Zeeshan; validation, Fatma Alshehri and Fahad Al-Asmari; formal analysis, Muhammad Faisal Amjad.; investigation, Hafiza Madiha Jaffar; resources, Tariq Aziz.; data curation, Bandar K. Baothman.; writing—original draft preparation, Fatima Siddiqui.; writing—review and editing, Tariq Aziz; visualization, Fakhria A. Al-Joufi and Maher S. Alwethaynani; supervision, Tariq Aziz and Nureen Zahra.; project administration: Rabail Alam.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Ethical approval for this study was granted by Ethics approval committee Institute of Molecular Biology and Biotechnology under ref no, Ref-IMBB/BBBC/23/2409-C.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Siddiqui, F., Zahra, N., Amjad, M.F. et al. In-vitro anti-inflammatory and antagonistic efficacy of polymyxin B capped silver nanoparticles in multi drug resistant Acinetobacter baumannii. Sci Rep 15, 17465 (2025). https://doi.org/10.1038/s41598-025-01639-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01639-2