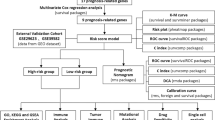
Abstract
Colon adenocarcinoma (COAD) is a leading cause of cancer-related mortality worldwide, with immune cells, particularly dendritic cells (DCs), playing an essential part in the advancement of tumors and immunotherapy response. However, the prognostic significance of genes associated with dendritic cells (DCRGs) in COAD remains underexplored. This study aims to identify DCRGs, construct a risk scoring system, and evaluate its prognostic and therapeutic implications. Data from single-cell RNA sequencing (scRNA-seq) of COAD tissues were examined for the detection of DCRGs. Transcriptomic and clinical data from TCGA and GEO were used to construct a DC Related Index (DCRI) via WGCNA, differential expression, univariate Cox regression, and LASSO-Cox analysis. The DCRI was validated in internal and external cohorts. Immune infiltration, MSI status, immune checkpoint expression, and drug sensitivity were analyzed to assess clinical relevance. Functional experiments were performed to investigate the role of PPP2CB in COAD progression. A five-gene signature (CTSD, DAPK1, TIMP1, TBXAS1, PPP2CB) was identified and used to construct a DCRI. The DCRI effectively stratified patients into high- and low-DCRI groups, with significant survival differences. High-DCRI patients exhibited distinct immune infiltration patterns, higher MSI scores, and increased sensitivity to immunotherapy. Functional experiments revealed PPP2CB as a protective factor, with its downregulation inhibits COAD cell proliferation, migration, and invasion. We developed a novel DCRI that accurately predicts COAD prognosis and immunotherapy response. PPP2CB was identified as a potential therapeutic target, offering new insights for personalized COAD treatment strategies.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) ranks among the prevalent malignant tumors globally concerning both occurrence and fatality rates, with colon adenocarcinoma (COAD) being the most common type1. Although the treatment of COAD currently includes surgery, radiotherapy, chemotherapy, targeted therapy, and immunotherapy2. Of concern, metastasis is one of the main causes of death in COAD patients3,and close to one-third of COAD patients are diagnosed at stage IV4. Fortunately, patients at stage I can be effectively cured4, making early detection particularly crucial. The initiation and progression of colorectal cancer is a complex biological process involving multiple steps and factors. Its pathological mechanisms encompass interactions at multiple levels, including the accumulation of genetic variations, aberrant epigenetic regulation, dynamic remodeling of the tumor microenvironment (TME), and dysregulation of host-microbiome interactions5,6. In recent years, the tumor microenvironment has attracted extensive attention as a critical hub regulating the malignant progression of CRC. Studies have shown that the TME is not merely a simple physical support structure but rather a dynamic interacting ecosystem composed of tumor cells, stromal cells, immune cells, vascular endothelial cells, and the extracellular matrix7,8.
While multiple immune cell types have been extensively studied in CRC, dendritic cells (DCs) hold unique clinical relevance due to their pivotal role in initiating adaptive immunity9. Dendritic cells (DCs) mainly include plasmacytoid dendritic cells (pDCs) and conventional dendritic cells (cDCs). They are a type of mononuclear phagocyte that bridges adaptive and innate immunity10. In the tumor immune microenvironment, DCs can transport tumor antigens to tumor-draining lymph nodes and interact with effector T cells, thereby mediating anti-tumor innate responses and blocking immune checkpoints11. One of the main functions of pDCs is to produce interferons, participating in antiviral and anti-tumor immune responses12. cDCs mainly consist of two subtypes: cDC1 and cDC213. cDC1 has strong antigen processing and presents capabilities, being able to degrade antigens into small fragments14. cDC1 primarily present antigens to CD8 + T cells via MHC-I molecules, while cDC2 present antigens to CD4 + T cells via MHC-II molecules14. Studies have indicated the substantial involvement of DCs in the advancement of colitis15. Notably, the prognostic value of DC subsets (cDC1, cDC2, pDCs) in COAD remains underexplored compared to other immune cells,
Through single-cell sequencing (scRNA-seq) analysis, a frontier high-throughput technology, we can delve deeply into the transcriptome makeup of individual cells, providing a novel vantage point for comprehending cellular diversity and functional distinctiveness. As a result, we utilized scRNA-seq data from COAD patient tissues to deeply analyze cell types, cell-to-cell communication, and extract DC-related genes (DCRGs). Subsequently, we systematically analyzed DC-related genes in conjunction with RNA-seq data from COAD patients.
In this study, we constructed a DCRI based on DCRGs that accurately predicts the prognosis of COAD patients. This DCRGs can effectively distinguish the tumor immune microenvironment and provide new insights for tailored clinical interventions for patients with COAD. Finally, we confirmed the tumor suppressor role of the key gene PPP2CB in COAD cells within the DCRG, offering new therapeutic targets for COAD.
Methods and materials
Dataset download
In this study, we systematically collected multi-source datasets to thoroughly examine the association between molecular characteristics and clinical prognosis of COAD.
First, we meticulously selected and downloaded transcriptomic data related to COAD along with the corresponding clinical data from TCGA database. After rigorous quality control and selection processes, we ultimately included transcriptomic data from 364 colon adenocarcinoma samples, with the associated clinical data covering key survival indicators (including survival time and survival status), basic patient information (such as gender and age), and disease staging information (based on the TNM staging system). Additionally, to more comprehensively explore the genetic variation landscape of COAD, we also downloaded mutation data for COAD from the TCGA database. This data is crucial for identifying potential driver mutations, assessing mutation burden, and delving into the correlation between mutations and clinical phenotypes. Furthermore, we utilized resources from the GEO database to download the transcriptomic dataset GSE39582 and the single-cell sequencing dataset GSE161277 related to COAD. For the GSE39582 dataset, after a rigorous sample selection process, we successfully included transcriptomic data from 556 high-quality COAD samples. The GSE161277 dataset, on the other hand, contains single-cell sequencing data from 4 COAD samples.
scRNA-seq analysis
In this study, we initially screened the raw sequencing data according to preset quality standards, excluding poor-quality samples to ensure the reliability of subsequent analyses. Data filtering criteria: nFeature_RNA > 50 & percent_MT < 5. We systematically processed the single-cell dataset using the Seurat package, performing dimensionality reduction (PCA followed by t-SNE/UMAP) to visualize high-dimensional transcriptomic data and cluster cell populations into distinct subtypes16. Subsequently, cell type annotation was conducted via the SingleR package, leveraging reference transcriptomic datasets to classify cell identities and pinpoint dendritic cell (DC)-specific gene expression signatures17. Cell cycle analysis was integrated, stratifying immune cells into G1, S, and G2/M phases using phase-specific marker genes18. Finally, cell-cell communication networks were reconstructed by analyzing ligand-receptor interactions to map intercellular crosstalk mechanisms driving immune responses19.
WGCNA analysis
This study employed weighted gene co-expression network analysis (WGCNA) to identify co-expression gene modules that are highly associated with specific biological functions or disease phenotypes. Through gene screening within these modules, key biomarkers were mined. Furthermore, by correlating the modules with clinical characteristics, the intrinsic connections between gene expression patterns and disease progression were revealed. Combined with functional enrichment analysis, the biological significance of the modules was elucidated. Finally, a gene regulatory network was constructed to clarify the core regulatory mechanisms20. In this study, we used WGCNA to construct a gene co-expression network based on DCRGs expression data, identified gene modules significantly associated with COAD prognosis, and conducted further in-depth analysis of these modules.
Modeling construction and validation
We employed the Lasso-Cox algorithm to construct a prognostic model for COAD patients and calculated the DCRI. The DCRI integrates the expression levels of multiple key molecules and their corresponding coefficients, with the specific calculation formula as follows: DCRI = Expression of CTSD × coefficient + Expression of TBXAS1 × coefficient + Expression of DAPK1 × coefficient + Expression of TIMP1 × coefficient + Expression of PPP2CB × coefficient.
In order to guarantee the robustness and applicability of the DCRI, we randomly partitioned the TCGA dataset into a training set and a validation set. Moreover, we used a ratio of 8:2 for the split21. The training set was utilized for building and refining the model, whereas the validation set was employed for the initial assessment of the model’s predictive efficacy. Additionally, we introduced the GSE39582 independent dataset to further externally validate the model. For the visual representation of prognostic variations among patients in distinct DCRI categories, we employed the Kaplan-Meier survival curve analysis technique. The survival curve effectively assessed the survival outcomes and mortality rates of individuals in high-DCRI groups (HDG) versus low-DCRI groups (LDG).
Simultaneously, by plotting a heatmap, we visually displayed the expression differences of key DCRGs constructing the DCRI between HDG and LDG. The ROC curve functions as a quantitative indicator of the DCRI’s performance, reflecting its ability to distinguish between disease states (such as survival and death). In addition, we used Cox regression analysis to further explore the independence and correlation of the DCRI and clinical traits on prognosis.
Drug sensitivity analysis
Chemotherapy, as a method of using chemical agents to intervene in organisms for the treatment of cancer and other pathological conditions, has its core mechanism in interfering with the proliferation and division cycle of cancer cells through specific molecular pathways, thereby achieving the purpose of inhibiting cancer cell growth or inducing their apoptosis. This study systematically delved into the potential effectiveness of 198 chemotherapy medications. By comparing the IC50 scores of chemotherapy drugs between these two groups, we aimed to uncover the differences in response to specific chemotherapy drugs among COAD patients at different risk levels.
Immunoassay
To comprehensively analyze the immune cell infiltration in the tumor tissues, we utilized the ssGSEA algorithm to accurately quantify the abundance of 28 essential immune cell types. To further evaluate the immune infiltration status of tumor tissues, we employed the ESTIMATE algorithm. This algorithm integrates gene expression data to calculate each patient’s immune score, stromal score, and an overall score adjusted for tumor purity, thus achieving a quantitative assessment of tumor tissue infiltration in different patients. MSI, as a significant feature of tumor biology, has been widely recognized as a prognostic factor and a potential immunotherapy target in various cancers. In this study, we explored its potential in predicting immunotherapy efficacy by analyzing the variations in MSI scores between HDG and LDG. To validate the effectiveness of the constructed DCRI in predicting immunotherapy efficacy, we assessed the correlation between DCRI and mismatch repair status, and the expression of essential immune checkpoint molecules (ICMs). Additionally, we introduced the TIDE scoring system, which predicts patients’ response to immunotherapy by comprehensively analyzing the immune suppression and exclusion mechanisms in the tumor immune microenvironment. By comparing the TIDE scores between different groups, we further validated the precision and dependability of the DCRI in predicting immunotherapy efficacy. To integrate our research findings with clinical practice, we utilized the IMvigor210 dataset, which contains data from treatments using PD-L1 inhibitors. Through an in-depth analysis of this dataset, we predicted the immunotherapy efficacy in different COAD patients.
Mutation analysis
Mutation analysis, as a core process in deciphering genetic variations in biological samples, aims to systematically detect, thoroughly describe, accurately classify, and scientifically interpret various mutation events in an organism’s genetic information. Specifically, we emphasized certain types of single nucleotide variations, namely ATCG mutations. These mutations play a pivotal role in revealing genomic mutation patterns and elucidating the molecular mechanisms of tumor development and progression. To explore the potential molecular mechanism differences between different risk groups, we will first use high-throughput sequencing technology to obtain genomic data from HDG and LDG. Subsequently, these data will be deeply mined to identify and classify various mutations. Through a comparison of mutation spectra across both groups, we aim to reveal the potential associations between specific mutation types and tumor risk, progression, and prognosis.
Functional enrichment analysis
Functional enrichment analysis, as a powerful bioinformatics tool, aims to deeply investigate the biological functions, metabolic pathways, and cellular components enriched within a specific set of genes or proteins. In this research, we employed GO and KEGG analyses22,23,24 to ensure the comprehensiveness and reliability of the results.
Cell culture and transfection
COAD cell lines (HCT116, HT29, and SW480) were purchased from the Cell Bank of the Typical Culture Collection Committee of the Chinese Academy of Sciences. All cells were cultured in DMEM containing 10% fetal bovine serum and 1% penicillin/streptomycin, and maintained in a constant 5% CO2 ¼and 37 °C cell culture incubator. Three siRNA sequences targeting PPP2CB were designed according to previous studies25, and the transfection steps were conducted as per earlier research21,26. In simple terms, cells were seeded in 6-well plates at a confluence density of 30-50% and cultured for 24 h until they reached 70-80% confluence. The siRNA was mixed with the transfection reagent according to the instructions provided with the transfection reagent, incubated, and then added to the cell culture medium. After 6 h of transfection, the medium was replaced with complete medium, and the cells were continued to be cultured for 24–72 h. Subsequently, the cells were used for further experiments.
CCK8 experiment, colony formation assay, scratch assay, and transwell assay
The CCK8 experiment, colony formation assay, scratch test, and Transwell assay involved in this study were carried out following previous research protocols21,26,27.The CCK8 Experiment involves seeding cells into 96-well plates. At 0 h, 24 h, 48 h, 72 h, 96 h, and 120 h, 10uL of CCK8 reagent solution is added to each well, followed by incubation in the dark for 2 h. The absorbance is then measured at 450 nm using a microplate reader. For the Colony Formation Assay, cells are seeded into 6-well plates at a density of 1 × 10³ cells per well and cultured for 10 days. The clonal cells are fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet solution for another 30 min. Finally, the colonies are washed with distilled water, dried, and photographed. In the Scratch Assay, cells are allowed to grow to 80-90% confluence to form a monolayer. A sterile pipette tip is used to make a linear “scratch” perpendicular to the bottom of the culture dish on the surface of the monolayer cells. The scratch closure is photographed and recorded at fixed positions under a microscope at 0 and 24 h. For the Transwell Assay, cells are seeded into the upper chamber containing 250ul of serum-free medium. For the migration assay, no Matrigel is added to the upper chamber, while for the invasion assay, Matrigel is added. The lower chamber contains 800ul of complete medium. After incubation for 24 h, the cells are fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The cells on the upper surface of the upper chamber are wiped off with a cotton swab, and the cells that have migrated or invaded are photographed under a microscope and counted.
Data statistics
All statistical computations and analyses were executed using R software (version 4.3.3). Differential analysis between the HDG and LDG was carried out using the Wilcoxon test, while correlations were assessed through the Spearman test. Statistical significance was set at P < 0.05 for all tests, with P-values being two-sided.
Results
ScRNA-seq analysis
We downloaded the ScRNA-seq dataset for COAD patients, labeled GSE161277, from the Gene Expression Omnibus database. After meticulous organization, we selected four representative COAD samples for further in-depth analysis. At first, we assessed the quality of the data, comprising the computation of the detected genes’ quantity (nFeature), the total gene expression count per cell (nCount), and the proportion of mitochondrial gene expression in relation to the overall gene expression. This process aimed to eliminate any probable low-quality cells or contamination (Fig. 1A–C). Subsequently, to capture cellular heterogeneity and reduce data dimensionality for analysis, we selected genes with high variability between cells as feature genes. Using these feature genes, we performed PCA and tSNE for dimensionality reduction to visualize the complex relationships between cells. Through unsupervised clustering analysis, we finely categorized the cells into 25 subgroups and identified key marker genes for each subgroup, which are crucial for distinguishing different cell types (Fig. 1D,E). Next, based on these marker genes and known cell type characteristics, we performed detailed annotation and classification of the cells. The results indicated a distinct classification of the cells into seven primary cell types: T cells, epithelial cells, B cells, NK cells, monocytes, DCs, and endothelial cells (Fig. 1F–G). To uncover the biological differences between different cell types, we analyzed the major differentially expressed genes among these cells and presented the findings using heatmaps and other visual representations (Fig. 1H–N).
Single-cell sequencing analysis identifies DC-related genes. (A–C) Violin plots showing the distribution of nFeature_RNA, nCount_RNA, and percent_MT metrics across four COAD samples. (D) Principal component analysis and t-distributed stochastic neighbor embedding clustering reveal 25 distinct cell clusters. (E) Heatmap illustrating the top differentially expressed genes across the identified clusters. (F–G) Cell annotation using the SingleR package, categorizing cells into seven major cell groups. (H–N) Violin plots depicting the expression levels of key differentially expressed genes across various cell types.
We then systematically investigated the cell cycle distribution characteristics in different samples. Specifically, we used cell cycle density analysis to precisely quantify and categorize cells into distinct phases of the cell cycle (G1, S, G2/M, Fig. 2A). To further refine the analysis, we then focused on the cell type composition in samples of different stages. Using cell type proportion analysis techniques, we delved into the distribution of distinct cell types within each stage of the samples (Fig. 2B). We investigated the communication mechanisms among various cell types. Specifically, we observed that epithelial cells exhibited significant advantages in both the quantity and intensity of interactions (Fig. 2C,D), underscoring the pivotal role of epithelial cells in the cellular communication network. Furthermore, we focused on elucidating the molecular basis of these cell interactions. The study revealed that communication between epithelial cells and dendritic cells is primarily mediated by the Macrophage Migration Inhibitory Factor (MIF) signaling pathway, involving interactions between CD74 and CXCR4, as well as recognition between CD74 and CD44 (Fig. 2E). Subsequently, we employed a more refined analytical strategy to examine the specific effects of epithelial cells on other cell types (Fig. 2F–L). We then employed the Hallmark gene set to conduct an in-depth examination of the key signaling pathways associated with dendritic cells. Specifically, we focused on the COMPLEMENT, INTERFERON-GAMMA-RESPONSE, and INTERFERON-ALPHA-RESPONSE pathways, which are critical for immune regulation (P < 0.001) (Fig. 2M).
Cell communication and functional enrichment analyses in colorectal cancer. (A, B) Cell cycle analysis showing the proportion of immune cells in tumor samples across different cell cycle phases. (C, D) Interaction analysis using the “CellChat” package to evaluate the number and strength of interactions between distinct cell types. (E) Bubble plot illustrating the molecular mechanisms underlying cell-cell interactions across various cell types. (F–L) Cell communication analysis visualizing the intensity of interactions between a specific cell type and other cell types. (M) Heatmap displaying hallmark pathways enriched across different cell types.
Construction and validation of DCRI
Given the pivotal role of dendritic cells in the onset and progression of COAD, we focused on extracting key gene sets closely related to DCs function. Subsequently, we applied WGCNA to reveal the complex relationships between gene expression patterns and sample characteristics. Through detailed analysis, we successfully clustered the samples into four modules with significant internal consistency (Fig. 3A–C). To explore gene modules related to prognosis, we further filtered and analyzed the MEblue module, which showed potential importance in prognostic prediction (P < 0.05) (Fig. 3D). We then performed differential expression analysis on the genes within the MEblue module, using a volcano plot to visually display the significance and scale of gene expression changes, ultimately identifying 324 differentially expressed genes as candidates(P < 0.05) (Fig. 3E). To assess the independent impact of these candidate genes on prognosis, we conducted Cox univariate regression analysis. Notably, this analysis identified 33 genes from the 324 candidates as independent prognostic factors (Fig. 3F, Supplementary Fig. 1). We then expanded our analysis by evaluating the association of all candidate genes with prognosis through Kaplan-Meier survival analysis, finding a significant correlation for 240 genes (Fig. 3G). For the precise identification of genes with the most significant prognostic value, we intersected the outcomes of the variance analysis, Kaplan-Meier analysis, and Cox analysis, culminating in the identification of 27 genes that satisfied all three criteria (Fig. 3H). Finally, we employed the lasso-Cox regression analysis method, further refining the 27 candidate genes to five key genes: CTSD, TBXAS1, DAPK1, TIMP1, and PPP2CB (Fig. 3I–J, Supplementary Table 1).
Identification of key genes through WGCNA, differential expression, and prognostic analysis. (A, B) WGCNA determines the optimal soft threshold for network construction. (C) Gene dendrogram showing the clustering of genes into four distinct modules. (D) Genes in the MEblue module exhibit a significant association with survival time. (E) Heatmap illustrating the differentially expressed genes in the MEblue module between cancer and normal tissues. (F) Cox proportional hazards analysis identifies 33 genes with prognostic significance. (G) Kaplan-Meier analysis highlights 240 genes with prognostic value. (H) Venn diagram showing the overlap of genes identified through differential expression analysis, KM analysis, and Cox analysis, resulting in 27 genes. (I–J) LASSO-Cox regression analysis identifies genes suitable for constructing the optimal prognostic model.
We used three independent datasets to comprehensively construct and validate the prognostic prediction efficacy of the constructed DCRI. Specifically, we divided the TCGA dataset into training and internal validation sets, while the GSE39582 served as an external validation set. The analysis findings showed that in all three datasets, the prognosis of HDG was notably poorer compared to that of the LDG (Fig. 4A–C). For a more profound insight into the variations in survival status among distinct groups, we carried out a survival analysis and observed that the mortality rate within the HDG was notably higher than that of the LDG. Additionally, via heatmap visualizations, we noted that the expression levels of the candidate genes CTSD, DAPK1, and TIMP1 exhibited significant elevation in the HDG in contrast to the LDG. Conversely, TBXAS1 and PPP2CB displayed an inverse pattern, with notably lower expression levels in the HDG (Fig. 4D–F). To quantify the predictive performance of the DCRI, we calculated the AUC values for each dataset. The results indicated that the AUC values in the TCGA-Train, TCGA-Test and GSE41613 cohort were 0.707, 0.786, and 0.686, respectively (Fig. 4G–I).
Construction and validation of a risk prognosis model using the LASSO-Cox algorithm. (A–C) KM survival analysis demonstrates that patients in the high-risk group have a significantly worse prognosis compared to those in the low-risk group across different datasets. (D–F) Survival analysis indicates a higher mortality rate in the high-risk group. The heatmap shows elevated expression levels of CTSD, DAPK1, and TIMP1 in the high-risk group, while TBXAS1 and PPP2CB exhibit higher expression levels in the low-risk group. (G–I) ROC curves display AUC values for 1-year, 3-year, and 5-year survival predictions across different datasets.
To explore the clinical application value of the DCRI, we performed univariate and multivariate Cox proportional hazards regression analyses. The analysis showed that only T stage and DCRI were independent risk factors affecting COAD patients (Fig. 5A,B). Additionally, we further analyzed the correlation between DCRI and clinical indicators. The results showed significant differences in T, N, and Stage between HDG and LDG (Fig. 5C). Lastly, in order to investigate the variations in sensitivity to different chemotherapeutic agents among COAD patients, we evaluated the IC50 values of 198 chemotherapeutic drugs in both the HDG and LDG. Interestingly, we discovered that the IC50 values of 10 drugs, including AZD1332, AZD8186, BMS-754,807, OTX015, Entospletinib, WIKI4, Dasatinib, WZ4003, AZ960, and XAV939, were higher in the LDG, suggesting that these drugs may be more effective for LDG. Conversely, 6 drugs, including OF-1, Dihydrorotenone, Daporinad, VX-11e, BI-2536, and Ulixertinib, had higher IC50 values in the HDG, indicating that these drugs may have higher therapeutic potential for HDG (Fig. 5D–S).
The efficacy validation of the riskscore model and the construction and validation of clinical predictive models. (A, B) Univariate and multivariate COX analyses revealed that the riskscore is a valuable independent prognostic factor. (C) A heatmap depicts the distribution of different clinical characteristics across patients in high- and low-risk groups. (D-S) The IC50 values of various chemotherapeutic drugs are compared between high- and low-risk groups.
Exploring the relationship between DCRI and the immune landscape
To delve into the complex relationship between DCRI and immune cell infiltration patterns, we employed the ssGSEA to systematically evaluate the distribution differences of 28 immune cells subpopulations between HDG and LDG. The results showed that multiple immune cell subpopulations (P < 0.001, Fig. 6A). Furthermore, we utilized the Estimate algorithm to comprehensively assess the tumor microenvironment, which comprises various components. The results showed that the immune scores, stromal scores, and total scores of the HDG were significantly lower than those of the LDG (Fig. 6B). Additionally, MSI analysis revealed that the MSI scores of the HDG were significantly higher than those of the LDG (Fig. 6C). Furthermore, the MMR gene EPCAM was positively correlated with the DCRI, while MLH1, MSH2, and PMS2 were negatively correlated with the DCRI (Fig. 6D). Meanwhile, immune checkpoint analysis confirmed a strong correlation between key ICMs, such as CTLA4, PD-1 and PD-L1 (Fig. 6E). To more intuitively present the potential differences in immunotherapy response between HDG and LDG, we conducted TIDE analysis and immunotherapy scoring analysis. TIDE results revealed that HDG patients had higher TIDE scores, which might reflect lower sensitivity to immunotherapy or more complex immune evasion mechanisms in their tumors (Fig. 6F). In contrast, immunotherapy scoring analysis directly suggested that LDG patients might achieve more significant therapeutic benefits from immunotherapy (Fig. 6G,H).
Analysis of Riskscore and Immune Landscape. (A) Differential analysis revealed that the degree of immune infiltration, calculated using the ssGSEA algorithm, varied between patients in different risk groups. (B) Differential analysis found that the high-risk group had a higher StromalScore, ImmuneScore and ESTIMATEScore. (C) Differential analysis found that the MSI score was higher in the high-risk group. (D) MMR genes were found to be closely associated with riskscore. (E) Radar plots showed the correlation between riskscore and multiple immune checkpoints. (F) Differential analysis found that the high-risk group had a higher tumor TIDE score. (G, H) Patients with higher riskscore were more likely to experience remission according to the IMvigor210 dataset.
Mutation and functional enrichment analysis of the DCRI
Gene mutations, representing elemental genetic variations, encompass alterations in the structure or sequence of base pairs within the genetic code. To systematically explore how these variations affect disease risk, we obtained detailed gene mutation data from TCGA database and conducted an in-depth analysis. Specifically, we focused on the differences in the mutation spectra between HDG and LDG, finding that the mutation frequencies of genes such as TP53, TTN and SYNE1 were significantly higher in the HDG, whereas the mutation rates of genes like APC, KRAS, and PIK3CA were relatively lower in the HDG (Fig. 7A,B). To further analyze the mutation characteristics of the key genes relied upon to construct the prognostic model (DCRI), we undertook a comprehensive analysis of the somatic mutation rates for genes such as DAPK1, TIMP1, PPP2CB, TBXAS1, and CTSD. We undertook a comprehensive review of the somatic mutation frequencies in genes like DAPK1, TIMP1, PPP2CB, TBXAS1, and CTSD. The results showed that DAPK1 exhibited a relatively high mutation rate (7.34%), while the mutation rates for TIMP1, PPP2CB, and TBXAS1 were lower, at 0.56%, 0.85%, and 1.69%, respectively. Notably, no significant mutations in the CTSD gene were observed in our study samples (Fig. 7C–F).
By conducting GO enrichment analysis, we explored the underlying biological basis of the significant differences between HDG and LDG. Specifically, in the BP analysis, significantly enriched terms included positive regulation of cell adhesion, regulation of cell-cell adhesion, and leukocyte cell-cell adhesion. These findings reveal the critical role of cell-cell interactions in distinguishing different risk groups. At the CC level, our analysis mainly pointed to the mitochondrial matrix, cell-substrate junction, and focal adhesion. The enrichment of these cellular components indicates potential impacts on cellular structural integrity, energy metabolism, and interactions with the extracellular matrix in disease risk stratification. In the MF analysis, we observed significant enrichment of functions such as extracellular matrix structural constituent (Fig. 7G). These molecular functions are closely related to the homeostasis of the cellular microenvironment and may directly influence cell migration, proliferation, and differentiation processes, thereby participating in risk stratification regulation. To further elucidate the potential mechanistic pathways underlying the differences between HDG and LDG, a KEGG analysis was carried out. The results showed significant enrichment in several key pathways such as cell adhesion molecules, allograft rejection, phagosome, and T cell receptor signaling pathway (Fig. 7H).
Analysis of mutation patterns and functional enrichment in patients with different risk scores. (A, B) The waterfall plot revealed different mutated genes and mutation rates between the high and low-risk groups. (C–F) Mutation rates and mutation analysis of the four key genes in the model. (G, H) GO and KEGG enrichment analyses reveal potential mechanistic pathways in patients stratified into high- and low-risk groups.
Functional role of PPP2CB in COAD cell proliferation, migration, and invasion
Considering that PPP2CB has the largest |coef| among the model genes and there is no relevant biological research in COAD, we focused our research on PPP2CB In investigating the biological significance of PPP2CB in COAD, our initial step involved evaluating its expression in three COAD cell lines (HCT116, HT29, and SW480). PPP2CB expression increases in HCT116, HT29, and SW480 (Fig. 8A). Subsequently, we overexpressed PPP2CB in COAD cells HCT116 and silenced PPP2CB expression in HT29 and SW480. Overexpression and knockdown efficiency were confirmed by qRT-PCR (Fig. 8B). Immunohistochemical staining of colon tissue also demonstrated moderate staining intensity of PPP2CB in tumor tissues compared to high staining in normal tissues (Fig. 8C). The results of CCK-8 detection and colony formation experiments showed that overexpression of PPP2CB significantly inhibited the proliferation ability of HCT116 cells (Fig. 8D), and inhibition of PPP2CB significantly promoted the proliferation ability of HT29 and SW480 cells (Fig. 8E,F), highlighting the pivotal role of PPP2CB in stimulating cell proliferation.
PPP2CB expression affects COAD cell proliferation. (A) Relative expression of PPP2CB in COAD cell lines (HCT116, HT29, and SW480). (B) qRT-PCR analysis of transfection overexpression or knockdown efficiency. (C) Immunohistochemical staining of PPP2CB in normal and tumor colon tissues. (D–F) CCK-8 and colony formation assays were used to explore the effect of PPP2CB expression on the proliferation capacity of HCT116, HT29, and SW480 cells. Data are shown as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
To investigate the role of PPP2CB in migration and invasion, we performed wound healing and Transwell assays. The results showed that overexpression of PPP2CB significantly reduced the migration and invasion abilities of COAD cells HCT116 (Fig. 9A), while PPP2CB knockdown significantly promoted the migration and invasion abilities of COAD cells HT29 and SW480 (Fig. 9B-C).
PPP2CB regulates COAD cell migration and invasion. (A–C) Wound healing assays (left) and Transwell migration/invasion assays (right) demonstrating the effects of PPP2CB expression on migration and invasion in HCT116 (A), HT29 (B), and SW480 (C) cells. Data are presented as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
In light of DCs’ pivotal function in bridging innate and adaptive immunity, there has been a systematic and in-depth investigation of DCs in COAD. Numerous studies indicate that DCs play crucial roles in tumor progression and targeted/immunotherapy in COAD28,29,30,31. In this research, we utilized scRNA-seq from samples of 4 COAD patients. Our findings revealed that DCs are an indispensable and important component in the tumor microenvironment of COAD tissues, suggesting their potential crucial involvement in the advancement of COAD. On the other hand, prognostic markers constructed based on specific gene sets can accurately predict the prognosis and are beneficial for guiding treatment plans26,32. However, reliable markers based on DCRG for predicting prognosis are still lacking in COAD.
To address this research gap, we extracted 1539 DCRGs using scRNA-seq data. Subsequently, we utilized RNA-seq data from COAD patients to obtain the gene expression levels of these DCRGs. Subsequently, we conducted a multi-dimensional screening and selection process for the DCRGs. Eventually, we identified 27 prognosis-related DCRGs. Following this, we applied LASSO and multivariate Cox analysis to filter the prognosis-related DCRGs, resulting in the identification of 5 key DCRGs (CTSD, DAPK1, TIMP1, TBXAS1, PPP2CB). Previous studies have indicated that CTSD can serve as a biomarker for early screening of CRC33, and can induce apoptosis in CRC cells34. Reduced expression of DAPK1 can effectively weaken the invasive ability of CRC cells35. TIMP1 is a reliable and excellent diagnostic and prognostic marker in colorectal cancer36, and it can promote the malignant phenotype of colorectal cancer cells through the AKT pathway37,38. However, there are no reports on TBXAS1 and PPP2CB in COAD. Overall, most of the model genes are close associations to the onset and progression of COAD, demonstrating the reliability of the DCRI. Based on multifactor Cox regression analysis, we found that PPP2CB is the most significant factor affecting the prognosis of COAD and acts as a protective factor. Consequently, we conducted a comprehensive exploration of PPP2CB in COAD cells and found that decreased expression of PPP2CB significantly promoted the proliferation, migration, and invasive capacity of COAD cell lines.
Furthermore, we constructed a novel biomarker (DCRI) based on the 5 key DCRGs. In both internal (TCGA-Train and TCGA-Test) and external datasets (GSE39582), the DCRI demonstrated excellent ability to differentiate the overall survival (OS) of COAD patients. Specifically, COAD patients in the HDG group had shorter OS compared to the LDG. Importantly, the DCRI emerged as an independent risk factor influencing the prognosis. These findings strongly suggest that the DCRI is a robust and stable prognostic marker in COAD.
In order to enhance our comprehension of the relationship between the DCRI and tumor immunity, we carried out a comprehensive and multifaceted analysis. Initially, notable variances in the immune cell abundance were noted between the HDG and LDG (24/28), with the majority showing higher levels in the HDG. These observations were further confirmed through ESTIMATE analysis. Considering the pivotal role of MSI in guiding immunotherapy for COAD patients, it is also worth mentioning the notably elevated MSI ratings observed within the HDG, indicating that patients falling within this category could potentially derive advantages from immunotherapeutic interventions.
In the analysis of the correlation with ICMs, the DCRI exhibited positive correlations with most ICMs, including PDCD1 (PDL1), CD274 (PD1), and CTLA4, among others. These results support this perspective. Importantly, we observed this phenomenon in real-world datasets. Specifically, in the IMvigor210 dataset, the probability of CR/PR in the HDG was significantly higher than in the LDG (29% vs. 13%).
Repurposing existing drugs has significantly saved on economic and time costs, providing great convenience for drug development in cancer treatment. Discrepancies in drug responsiveness among patients categorized into the HDG and LDG were noted in this research, particularly in relation to 16 frequently prescribed medications. Pharmacological profiling revealed distinct drug sensitivity patterns between molecular subgroups. AZD1332, AZD8186, BMS-754,807, OTX015, Entospletinib, WIKI4, Dasatinib, WZ4003, AZ960, and XAV939 demonstrated heightened sensitivity in the LDG subgroup, suggesting potential efficacy for LDG-targeted therapies. Conversely, OF-1, Dihydrorotenone, Daporinad, VX-11e, BI-2536, and Ulixertinib exhibited elevated IC50 values in the HDG subgroup, indicating preferential therapeutic potential for HDG patients. These findings highlight subgroup-specific vulnerabilities and propose novel therapeutic avenues for personalized COAD treatment.
Studies have shown that mutant TP53 fails to activate cell cycle arrest genes such as CDKN1A (p21), leading to uncontrolled cell proliferation39. KRAS, on the other hand, is a commonly mutated oncogene in colorectal cancer (CRC), with mutations occurring in approximately 40% of CRC cases40. Loss of APC function results in abnormal accumulation of β-catenin, activating the Wnt pathway. TTN, as a large molecular scaffold protein, may cause mitotic errors and chromosomal breakage when mutated, exacerbating genomic heterogeneity41. The above studies indicate that TP53, TTN, APC, and KRAS are the most commonly mutated genes in colorectal adenocarcinoma (COAD), and their functional inactivation leads to genomic instability and apoptosis evasion. Our finding of differences in these mutations between the HDG and LDG groups also demonstrates the effectiveness of our prognostic score.
This study has the following limitations that need to be further addressed in subsequent work. Firstly, some cases in public databases lack detailed treatment histories, which may introduce unmeasured confounding factors and affect the generalization ability of the prognostic model. Secondly, the sample size of single-cell RNA sequencing included in this study is limited, which may not be sufficient to capture all heterogeneous subpopulations of dendritic cells in the tumor microenvironment of colorectal adenocarcinoma. Finally, our research on downstream mechanisms may be insufficient, which requires further investigation in the future.
In summary, our research harnessed the potential of DCs in COAD, culminating in the creation of an innovative risk assessment framework reliant on DCRGs, amalgamating scRNA-seq and RNA-seq data. The DCRI demonstrates a high level of precision in forecasting the prognosis of COAD patients and their reactivity to immunotherapeutic approaches.
Furthermore, foundational research indicates that decreased expression of PPP2CB significantly promotes the proliferation, migration, and invasive capacity of COAD cells, offering novel biomarkers for targeted prevention and management of COAD.
Data availability
The TCGA database (https://portal.gdc.cancer.gov/) and the GEO database (https://www.ncbi.nlm.nih.gov/geo/, dataset GSE39582) provide RNAseq data and clinical information of COAD; the GEO database (dataset GSE161277) provides single-cell RNAseq data of COAD patients. These data are all publicly available.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71(3), 209–249 (2021).
Wang, D. et al. CircSEC24B activates autophagy and induces chemoresistance of colorectal cancer via OTUB1-mediated deubiquitination of SRPX2. Cell Death Dis. 15(9), 693 (2024).
Dong, C. et al. FUT2 promotes colorectal cancer metastasis by reprogramming fatty acid metabolism via YAP/TAZ signaling and SREBP-1. Commun. Biol. 7(1), 1297 (2024).
Hultcrantz, R. Aspects of colorectal cancer screening, methods, age and gender. J. Intern. Med. 289(4), 493–507 (2021).
Eng, C. et al. A comprehensive framework for early-onset colorectal cancer research. Lancet Oncol. 23(3), e116–e28 (2022).
Patel, S. G., Karlitz, J. J., Yen, T., Lieu, C. H. & Boland, C. R. The rising tide of early-onset colorectal cancer: A comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. Hepatol. 7(3), 262–274 (2022).
AlMusawi, S., Ahmed, M. & Nateri, A. S. Understanding cell-cell communication and signaling in the colorectal cancer microenvironment. Clin. Transl. Med. 11(2), e308 (2021).
Zhong, X. et al. Warburg effect in colorectal cancer: The emerging roles in tumor microenvironment and therapeutic implications. J. Hematol. Oncol. 15(1), 160 (2022).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20(1), 7–24 (2020).
Lubin, R. et al. The lifespan and kinetics of human dendritic cell subsets and their precursors in health and inflammation. J. Exp. Med. 221(11) (2024).
Chudnovskiy, A. et al. Proximity-dependent labeling identifies dendritic cells that drive the tumor-specific CD4(+) T cell response. Sci. Immunol. 9(100), eadq8843 (2024).
Reizis, B. Plasmacytoid dendritic cells: Development, regulation, and function. Immunity 50(1), 37–50 (2019).
Guilliams, M. et al. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 14(8), 571–578 (2014).
Li, W. et al. Research progress on dendritic cells in hepatocellular carcinoma immune microenvironments. Biomolecules 14(9) (2024).
Cai, Y. et al. Intestinal epithelial damage-derived MtDNA activates STING-IL12 axis in dendritic cells to promote colitis. Theranostics 14(11), 4393–4410 (2024).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184(13), 3573–87e29 (2021).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20(2), 163–172 (2019).
Zheng, S. C. et al. Universal prediction of cell-cycle position using transfer learning. Genome Biol. 23(1), 41 (2022).
Jin, S. et al. Inference and analysis of cell-cell communication using cellchat. Nat. Commun. 12(1), 1088 (2021).
Langfelder, P. & Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 9, 559 (2008).
Sun, W. et al. Construction and validation of a novel senescence-related risk score can help predict the prognosis and tumor microenvironment of gastric cancer patients and determine that STK40 can affect the ROS accumulation and proliferation ability of gastric cancer cells. Front. Immunol. 14, 1259231 (2023).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53(D1), D672–d7 (2025).
Kanehisa, M. Toward Understanding the origin and evolution of cellular organisms. Protein Sci. Publ. Protein Soc. 28(11), 1947–1951 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28(1), 27–30 (2000).
Li, G. et al. Phosphatase type 2A-dependent and -independent pathways for ATR phosphorylation of Chk1. J. Biol. Chem. 282(10), 7287–7298 (2007).
Sun, W. et al. Combining WGCNA and machine learning to construct basement membrane-related gene index helps to predict the prognosis and tumor microenvironment of HCC patients and verifies the carcinogenesis of key gene CTSA. Front. Immunol. 14, 1185916 (2023).
Dai, W. et al. Silencing of OGDHL promotes liver cancer metastasis by enhancing hypoxia inducible factor 1 α protein stability. Cancer Sci. 114(4), 1309–1323 (2023).
Suzuki, S. et al. Type I-conventional dendritic cells support the progression of multiple myeloma in the bone marrow. Front. Immunol. 15, 1444821 (2024).
Reste, M. et al. The role of dendritic cells in tertiary lymphoid structures: lmplications in cancer and autoimmune diseases. Front. Immunol. 15, 1439413 (2024).
Liu, H. et al. Engineering dendritic cell biomimetic membrane as a delivery system for tumor targeted therapy. J. Nanobiotechnol. 22(1), 663 (2024).
Ribeiro, A. R. S., Neuper, T. & Horejs-Hoeck, J. The role of STING-Mediated activation of dendritic cells in cancer immunotherapy. Int. J. Nanomed. 19, 10685–10697 (2024).
Chen, C. et al. T cell-related ubiquitination genes as prognostic indicators in hepatocellular carcinoma. Front. Immunol. 15, 1424752 (2024).
Xie, L. Q. et al. Novel proteomic strategy reveal combined alpha1 antitrypsin and cathepsin D as biomarkers for colorectal cancer early screening. J. Proteome Res. 9(9), 4701–4709 (2010).
Alves, S. et al. Enhancement of acetate-induced apoptosis of colorectal cancer cells by cathepsin D inhibition depends on oligomycin A-sensitive respiration. Biomolecules 14(4) (2024).
Steinmann, S. et al. DAPK1 loss triggers tumor invasion in colorectal tumor cells. Cell Death Dis. 10(12), 895 (2019).
Qiu, X. et al. Unraveling TIMP1: A multifaceted biomarker in colorectal cancer. Front. Genet. 14, 1265137 (2023).
Ma, B. et al. TIMP1 promotes cell proliferation and invasion capability of right-sided colon cancers via the FAK/Akt signaling pathway. Cancer Sci. 113(12), 4244–4257 (2022).
Wang, L., Wang, J. & Chen, L. TIMP1 represses sorafenib-triggered ferroptosis in colorectal cancer cells by activating the PI3K/Akt signaling pathway. Immunopharmacol. Immunotoxicol. 45(4), 419–425 (2023).
Funk, J. S. et al. Deep CRISPR mutagenesis characterizes the functional diversity of TP53 mutations. Nat. Genet. 57(1), 140–153 (2025).
Zhu, G., Pei, L., Xia, H., Tang, Q. & Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 20(1), 143 (2021).
Heiser, C. N. et al. Molecular cartography uncovers evolutionary and microenvironmental dynamics in sporadic colorectal tumors. Cell 186(25), 5620–37e16 (2023).
Funding
This study was funded by Top Talent Support Program for Young and Middle-Aged People of Wuxi Health Committee (HB2023116).
Author information
Authors and Affiliations
Contributions
Chaobo Chen: Writing—original draft, Conceptualization, Data curation, Funding acquisition, Validation; Zhengqiu Wang: Writing—original draft, Validation, Visualization; Bo Xi: Writing—original draft, Data curation, Visualization; Zipeng Xu: Writing—original draft, Visualization; Chunlong Zhao: Writing—original draft, Visualization; Weidong Hu: Writing—original draft, Visualization; Chen Ge: Writing—original draft, Methodology; Genxi Tong: Writing—original draft, Methodology; Fengjun Cai: Writing—original draft, Methodology; Mingli Zhou: Writing—review and editing, Conceptualization; Yihang Yuan: Writing—original draft, Writing—review and editing, Conceptualization, Data curation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, C., Wang, Z., Xi, B. et al. Focusing on DC cells to optimize the prediction of prognosis and innovative treatment strategies for colon cancer. Sci Rep 15, 17298 (2025). https://doi.org/10.1038/s41598-025-01792-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-01792-8