Abstract
Venous thrombosis is a serious complication that adversely impacts the prognosis of patients with intracerebral hemorrhage (ICH) and even threatens their lives. Triglyceride glucose product (TyG) index is closely related to the pathophysiological process of cerebral hemorrhage and venous thrombosis. A total of 308 ICH patients were divided into training (n = 215) and validation groups (n = 93). Single factor logistic regression analysis and multiple factor logistic regression analysis were performed. Seven factors associated with lower extremity venous thrombosis were identified: emergency cranial surgery, pneumonia, stroke history, age, TyG index, capryini score ≥ 10, and glomerular filtration rate. The area under the curve was found to be 0.79 (95% CI: 0.72–0.86) in the training group and 0.70 (95% CI: 0.58–0.83) in the validation group. Calibration plots demonstrated strong concordance between predicted probabilities from our model and observed outcomes; Decision curve analysis further confirmed that our nomogram provides substantial clinical net benefit. By integrating the TyG index with recognized clinical risk factors for ICH patients, we have developed a predictive nomogram that aids in early identification of individuals at high risk for lower extremity venous thrombosis as well as supports personalized management strategies for these patients.
Similar content being viewed by others
Introduction
Intracerebral hemorrhage (ICH) is a prevalent condition in the cerebrovascular system, closely associated with risk factors such as hyperlipidemia, diabetes, hypertension, and smoking. The disease is characterized by its acute onset and poor prognosis in elderly patients, leading to high morbidity and mortality rates1. Individuals suffering from ICH face an elevated risk of venous thromboembolism (VTE) due to complications such as limb paralysis, prolonged bed rest, vascular wall damage, and abnormal coagulation function2. The incidence of pulmonary embolism among ICH patients is 1.1%, while the risk for deep vein thrombosis is 2.4% 3. Given the significant risk of rebleeding in ICH patients, national guidelines for VTE prevention offer only weak recommendations supported by low-quality evidence4. Compared with the general population, individuals with ICH not only face a heightened risk of recurrent ICH but also have an increased likelihood of experiencing arterial ischemic events5. Nevertheless, the advisability of employing antithrombotic agents and statins for secondary prevention against thrombotic events in these patients remains contentious—particularly within specific subgroups such as those with atrial fibrillation—due to concerns regarding potential recurrence of ICH5. In summary, determining appropriate secondary prevention strategies for survivors of ICH presents a pressing clinical challenge. At present, there are no effective clinical methods available to predict embolism due to deep vein thrombosis; thus, it is critical to identify high-risk groups early on and implement corresponding nursing interventions.
Insulin resistance (IR) is recognized as a significant risk factor for VTE and pulmonary embolism. It has been demonstrated that IR is closely associated with human coagulation function. Specifically, levels of coagulation factors VII, IX, X, and XII are markedly elevated in patients with IR6,7. The PREVEND cohort study has indicated that IR can increase the risk of VTE8. Furthermore, due to the body’s insensitivity to insulin, there is a reduction in the amount of insulin bound to its receptor in individuals with IR9. This phenomenon may lead to the upregulation of P2Y12 signaling and an increase in platelet activity, consequently enhancing platelet adhesion, aggregation, and coagulation processes—ultimately contributing to thrombosis induction.
IR can be assessed through various methods, but the hyperinsulinemic-euglycemic clamp test is recognized as the gold standard for evaluating IR10. However, as this method is invasive, complex, time-consuming, and costly, it is impractical for routine clinical use. Simental-Mendia introduced a novel index known as the serum triglyceride–glucose product (TyG), which serves as an indicator of IR. The TyG index is calculated by multiplying triglyceride (TG) levels by fasting blood glucose (FBG)11. Previous research has demonstrated that FBG primarily reflects liver-related IR, while TG predominantly indicates adipocyte-related IR; thus, the TyG index encompasses both aspects of IR. Furthermore, FBG and TG are widely used clinical markers and are straightforward to measure12,13. Recent studies have also indicated that the TyG index may function as a risk biomarker for identifying ST-elevation myocardial infarction patients who are at high risk of adverse outcomes following percutaneous coronary intervention. This could facilitate personalized management strategies for ST-elevation myocardial infarction patients and potentially reduce major adverse cardiac events11. The increase of TyG index can lead to the decrease of nitric oxide (NO) synthesis in endothelial cells, and promote vascular inflammation and oxidative stress14. These will increase the permeability of the blood-brain barrier and aggravate cerebral edema after cerebral hemorrhage.
Given that ICH involves multiple pathophysiological mechanisms, current research on the risk factors associated with posterior venous thrombosis in ICH has not yielded reliable assessments. Identifying predictors of ICH-related posterior venous thrombotic events could aid in stratifying risks and developing effective secondary prevention measures to mitigate these risks. Therefore, we developed a risk prediction model based on the TyG index to forecast lower-extremity venous thrombosis in ICH patients during their first week of hospitalization and investigated its predictive value regarding lower-extremity venous thrombosis in this patient population.
Methods
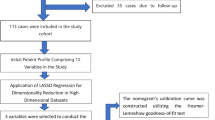
Study design and participants
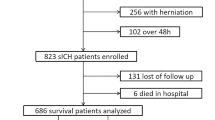
A total of 308 patients diagnosed with ICH at Changshu No. 1 People’s Hospital from 2022 to 2023 were enrolled in this retrospective study aimed at developing and validating a nomogram for predicting lower-extremity venous thrombosis in this patient population. All participants or their family members were informed about the details of the study and provided written informed consent. The research protocol received approval from the Ethics Committee of Changshu No. 1 People’s Hospital (Ethics Approval No. L-039), encompassing all pertinent details. All experiments were conducted in accordance with applicable guidelines and regulations. The prediction model underwent internal validation, with the participants randomly divided into the training and validation groups at a ratio of 7:3, yielding 215 individuals in the training group and 93 individuals in the validation group. Variables demonstrating P < 0.05 in univariate logistic regression analysis were included for subsequent multivariate regression analysis. The significant variables identified during this process contributed to the construction of the nomogram model, which was further validated using appropriate statistical methods, including decision curve analysis (DCA).
The inclusion criteria were as follows:
-
meeting diagnostic criteria for spontaneous ICH4;
-
confirmation of ICH via computed tomography or magnetic resonance imaging upon admission; and.
-
ICH patients who underwent screening for lower-extremity venous thrombosis via color Doppler ultrasonography.
The exclusion criteria were as follows:
-
age ≤ 18 years;
-
ICH resulting from brain trauma, tumors, or embolism;
-
hemorrhage confined to the ventricles or subarachnoid space;
-
history of deep vein thrombosis or thrombolysis;
-
ICH secondary to cerebral infarction;
-
ICH caused by hematological disorders;
-
hospital stay ≤ 72 h; and.
-
incomplete clinical data.
Baseline data collection
The pertinent clinical data of the participants were meticulously documented and encompassed the following: (1) basic information: body mass index (BMI), age, systolic blood pressure, and diastolic blood pressure; (2) medical history details: smoking history, alcohol consumption history, diabetes history, hypertension history, and cerebral infarction history; (3) laboratory indicators assessed on the first day after admission: FBG, estimated glomerular filtration rate (eGFR), high-density lipoprotein–cholesterol (HDL-C), TG, and low-density lipoprotein–cholesterol (LDL-C); and (4) conditions during hospitalization, including emergency cranial surgery, prophylactic heparin therapy with a Caprini score ≥ 10, as well as complications such as pneumonia or lower-limb venous thrombosis. Note: The product index of TG and FBG was calculated using the following formula: TyG = ln (fasting TG [mg/dL] × FBG [mg/dL])/2.
Observed outcome
The primary outcome of this study was the incidence of venous thrombosis in the lower extremities (including both superficial and deep vein thrombosis) within 1 week following hospitalization. Lower-extremity venous thrombosis was evaluated via color Doppler ultrasound. The patients underwent screening on admission day and on days 4 and 7 thereafter; additional examinations were conducted at any time during hospitalization if there were suspicions regarding lower-extremity venous thrombosis. Individuals who died or were discharged within 7 days were excluded from this analytical cohort. Two senior physicians evaluated all enrolled patients in the color ultrasound room. The diagnosis of lower-extremity venous thrombosis adhered to the guidelines outlined in the “Guidelines for Diagnosis and Treatment of Deep Vein Thrombosis” (third edition)15.
Statistical analysis
R 4.2.2 and SPSS 26.0 version were used for statistical analysis. Count data were expressed as percentages (%) and analyzed using the χ2 test or Fisher’s exact test, as appropriate. Measurement data were presented as mean ± standard deviation (x̄ ± s). The normality of distribution was assessed using the Kolmogorov–Smirnov (K-S) test. Comparisons between two groups with normally distributed data were conducted using the independent-samples t test, whereas comparisons between two groups with non-normally distributed measurement data were performed using the Mann–Whitney U test.
Univariate and multivariate logistic regression analyses were employed to identify risk factors within the training cohort. Based on the results from multivariate logistic regression analysis, a nomogram was created to predict the risk of lower-limb venous thrombosis in ICH patients within 1 week of hospitalization. The receiver operating characteristic (ROC) curve was used to evaluate the discriminative ability of the nomogram. Additionally, performance assessment of the nomogram was conducted through a calibration curve, which illustrated agreement between observed outcomes and predicted probabilities. DCA was performed to assess clinical utility across all patients and quantify net benefits at varying threshold probabilities. A two-tailed P < 0.05 was considered statistically significant.
Results
Comparison of baseline data between the training and validation groups
This retrospective study involved 308 patients diagnosed with ICH. The cohort was divided into the training and validation groups at a 7:3 ratio, yielding 215 patients in the training group and 93 patients in the validation group. The mean age of all participants was 65.6 years. In the training group, the average age was 65.9 years, and 64 patients (29.77%) developed lower-extremity venous thrombosis. The average age in the validation group was 64.9 years, and 30 patients (32.26%) experienced lower-extremity venous thrombosis. The incidence rates of lower-extremity venous thrombosis were comparable between the two groups. Previous medical history and in-hospital conditions are detailed in Table 1. Statistical analysis of the baseline data indicated no significant differences between the training and validation groups (P > 0.05).
Predictors of lower-extremity venous thrombosis and construction of a nomogram
The occurrence of lower-limb venous thrombosis served as the dependent variable for subsequent analyses, and univariate and multivariate logistic regression analyses were conducted on the baseline characteristics and laboratory examination results from the included patients. Univariate logistic regression revealed that emergency craniocerebral surgery, Caprini score ≥ 10, pneumonia, stroke history, age, TyG index, and eGFR exhibited significant correlations with lower-extremity venous thrombosis (P < 0.05, Table 2). Multivariate logistic regression analysis identified emergency cranial surgery, complicated pneumonia, stroke history, age, and TyG index as the independent risk factors for lower-limb venous thrombosis among patients with ICH (P < 0.05, Table 2).
Several studies have shown that Caprini score ≥ 10 and eGFR are closely related to thrombosis16,17,18,19. Indeed, our univariate logistic regression analysis indicated that both Caprini score ≥ 10 and eGFR were associated with lower-limb venous thrombosis in ICH patients. However, multivariate logistic regression analysis revealed that these factors did not serve as independent risk factors for lower-extremity venous thrombosis, which may be attributed to the limited sample size in this investigation. Therefore, Caprini scores ≥ 10 and eGFR were retained in the development of the nomogram model. Based on the findings from both univariate and multivariate logistic regression analyses, a corresponding visual nomogram model was constructed, assigning specific scores to each predictor. This allowed for the prediction of the risk of lower-extremity venous thrombosis within 1 week after hospitalization for patients with ICH (Fig. 1).
Verification of the nomogram prediction model
To evaluate the discriminative ability of the nomogram model regarding the occurrence of lower-limb venous thrombosis among ICH patients within 1 week after hospitalization, we employed ROC curve analysis. The ROC curve yielded an area under the curve (AUC) of 0.79 (95% CI, 0.72–0.86) in the training group and 0.70 (95% CI, 0.58–0.83) in the validation group (Fig. 2A–B). The calibration plot was used to assess the concordance between predicted probabilities from the nomogram and observed outcomes concerning lower-extremity venous thrombosis among ICH patients during their first week after admission. The results indicated strong agreement between the predicted probabilities and the actual observations, as illustrated in Fig. 3A–B. Furthermore, we plotted DCA to evaluate the predictive performance of our nomogram assessment tool. The DCA results demonstrated that our nomogram model provided favorable net clinical benefits as shown in Fig. 4A–B.
Discussion
ICH accounts for approximately 30% of all cases of cerebral apoplexy, with a fatality rate exceeding 30% during the acute phase. The etiology of ICH is closely associated with cerebrovascular diseases. Conditions such as hyperlipidemia, diabetes, and hypertension are significant contributors to cerebrovascular disease and also heighten the risk of lower-limb VTE in patients with ICH. Due to complications such as limb paralysis, prolonged bed rest, vascular wall damage, and abnormal coagulation function, patients with cerebral hemorrhage face an elevated risk of VTE. Additionally, these patients are at a heightened risk for recurrent hemorrhage. The anticoagulation paradox of this unique dual risk of intracerebral hemorrhage remains unresolved, so early prediction of deep vein thrombosis (DVT) is critical to improving prognosis in patients with intracerebral hemorrhage.
The findings from this study indicate that patients with ICH face a high risk (30.52%) of developing lower-extremity venous thrombosis within 1 week after hospitalization. Factors such as emergency cranial surgery, Caprini score ≥ 10, presence of complicated pneumonia, history of stroke, age, TyG index, and eGFR significantly correlated with lower-extremity venous thrombosis in these patients during their first week in hospital. Multivariate logistic regression analysis identified emergency cranial surgery, complicated pneumonia, stroke history, age, and TyG index as the independent risk factors for lower-limb venous thrombosis among ICH patients within this timeframe. The Caprini score is an effective tool for risk stratification of venous thromboembolism in surgical populations. Although statistical significance was not achieved in our univariate multivariate logistic regression analysis (p = 0.25), its inclusion was consistent with clinical guidelines emphasizing cumulative risk assessment20. Our goal is to provide clinicians with a comprehensive predictive tool that integrates statistically significant variables and established clinical factors. Chronic neurologic deficits (e.g., limb weakness or immobility) post-stroke may reduce mobility, predisposing patients to venous stasis21. Stroke-induced prothrombotic tendencies (e.g., endothelial dysfunction or hypercoagulability) could further elevate DVT risk22. Based on both univariate and multivariate logistic regression analyses, a corresponding visual nomogram model was developed. ROC curve analysis along with calibration charts and DCA demonstrated that this model possesses substantial clinical utility in predicting the risk of lower-limb venous thrombosis in ICH patients within 1 week following hospitalization.
To quantify a patient’s risk of developing venous thrombosis, researchers have developed risk assessment tools tailored for various populations. Clinicians evaluate the likelihood of event occurrence through quantitative indicators, stratifying patients’ risk levels and selecting appropriate preventive measures. However, existing tools employ different threshold values to predict the risk degree of venous thrombosis in ICH patients, resulting in significant variability in predictive ability and insufficient accuracy23,24. Consequently, there is a need to identify new assessment methods for predicting the risk of lower-extremity venous thrombosis in ICH patients and to formulate precise prevention strategies. A multicenter clinical study that randomly assigned ICH patients to receive either platelet transfusion or standard care has revealed that those receiving platelet transfusions experienced a significantly higher incidence of serious adverse events during hospitalization than those receiving standard care (42% vs. 29%). Additionally, patients who received a platelet transfusion exhibited a markedly elevated 3-month mortality rate relative to standard care25. In the Multi-center Study of Cerebral Hemorrhage in Italy, follow-up data on ICH survivors over 30 days indicated that male gender, diabetes mellitus, hypercholesterolemia, atrial fibrillation, and personal history of coronary artery disease were associated with an increased long-term risk of thrombosis. In contrast, the use of statins and antithrombotic medications after acute cerebral hemorrhage was linked to a reduced risk1. Therefore, indiscriminate antiplatelet therapy may not provide optimal benefits for all patients suffering from ICH. There is a pressing need for improved indicators capable of accurately identifying ICH patients at high risk for lower-limb venous thrombosis and facilitating the development of individualized secondary prevention measures.
There is currently no consensus regarding the risk factors associated with lower-extremity venous thrombosis in ICH patients. Key risk factors for deep vein thrombosis in the lower limbs include trauma, surgical interventions, hypertensive disorders, vascular puncture procedures, and other elements that may lead to venous wall injury26. Additionally, factors contributing to reduced blood flow—such as a history of venous thrombosis and prolonged bed rest—as well as those promoting blood hypercoagulability—including advanced age, female gender, atrial fibrillation, and abnormal coagulation function—are significant contributors.
The exact mechanism between TyG index and the development and prognosis of ICH is unclear, but evidence supports the critical role of IR in this process. Previous studies have demonstrated that IR can exacerbate vascular damage, thrombosis, and the rupture of atherosclerotic plaques and is closely linked to thrombosis in lower-limb veins27,28. Under physiological conditions, insulin functions to inhibit platelet aggregation and thrombosis by enhancing fibrinolysis and suppressing tissue factors. However, elevated blood glucose levels alongside IR can lead to increased platelet activity and atherothrombotic events. On the one hand, IR elevates levels of plasminogen activator inhibitor-1 and fibrinogen while concurrently reducing tissue plasminogen activator concentrations. On the other hand, hyperinsulinemia stimulates the expression of tissue factors as well as procoagulant activity and thrombin production29. Consequently, patients with IR or diabetes exhibit heightened plasma clotting factor levels along with disease-associated coagulation agents; conversely, endogenous anticoagulant levels are diminished. Moreover, disturbances in glucose metabolism among individuals with IR result in abnormal megakaryocyte function. This leads to the production of larger platelets enriched with proteins and enzymes that release more active substances—such as serotonin and thromboprotein—thereby inducing a hypercoagulable state in these patients30. IR contributes to vascular dysfunction characterized by reduced synthesis of prostacyclin and nitric oxide; it accelerates platelet activation while enhancing coagulation processes alongside inflammatory responses leading to thrombosis31. The TyG index serves as a novel metric reflecting IR. As it is derived from the product of TG and FBG, it is relatively straightforward for clinical application. This index effectively indicates underlying IR conditions that may not be readily detectable in patients along with blood hypercoagulability associated with TyG. Through both univariate and multivariate logistic regression analyses conducted in this study, we found that a history of emergency cranial surgery, presence of complicated pneumonia, prior stroke incidents, age, and TyG index were the independent risk factors influencing lower-limb venous thrombosis among ICH patients during their first week after hospitalization. Moreover, postoperative elevations in blood glucose levels, blood pressure, and lipid profiles among acute ICH patients can persist over time. This condition increases coagulation factors and disrupts the balance of the coagulation system. Furthermore, abnormalities in lipid metabolism can impair vascular endothelial dilation functions, consequently heightening the risk of deep VTE in the lower extremities.
Inflammation and oxidative stress, along with abnormal glucose metabolism and endothelial damage, create conditions conducive to atypical lipid deposition, thereby facilitating the development of atherosclerotic lesions. In patients with ICH, following the rupture of cerebral vessels, blood components—including red blood cells and their metabolites, thrombin, and fibrinogen—can infiltrate the brain parenchyma through the compromised blood-brain barrier. This infiltration triggers a cascade of oxidative stress and inflammation that heightens the risk of thrombosis and plaque rupture. Endothelial injury, IR, inflammation, as well as disturbances in glucose and lipid metabolism are interrelated factors that often perpetuate a vicious cycle leading to disease progression. Within this context, endothelial injury is regarded as a central link in this pathological process while IR serves as a critical mediator of endothelial damage. In this study, we introduced a novel index for assessing insulin resistance—TyG—to underscore its significant role in lower limb venous thrombosis among ICH patients. This approach offers an innovative method for indicating lower limb venous thrombosis in individuals suffering from ICH. TyG index is involved in the pathophysiological process of cerebral hemorrhage through insulin resistance, vascular injury, inflammation and other mechanisms, and may affect short-term and long-term prognosis. At present, most of the evidence is based on observational studies, and future prospective cohort or mechanism studies are needed to clarify the causal association and clinical intervention strategies.
While our model demonstrated commendable predictive capabilities—with AUCs of 0.79 and 0.70 in the training and validation cohorts, respectively—the calibration curve indicated robust performance alongside favorable clinical net benefits as shown by DCA. These results underscore our predictive model’s clinical validity across both the training and validation groups. However, it is essential to acknowledge that this study was constrained by its limited sample size and single-center design. Our research excluded patients who either died or were discharged within 72 h of admission in order to enhance the accuracy of outcome ascertainment. While this strategy improves the internal validity of our analysis, it may restrict generalizability by underrepresenting the most severe cases. Future studies with broader inclusion criteria or those focused on early mortality would complement our findings. Therefore, it is imperative to validate the model’s robustness through a larger multi-center study and extended observation periods. This expansion will facilitate a comparison of the accuracy between two prediction models developed before and after incorporating the TyG index for assessing the risk of lower limb venous thrombosis in patients with cerebral hemorrhage. It will further evaluate the contribution of the TyG index to enhancing predictive capability within this framework. Moreover, the substantial sample size derived from a multi-center approach allows us to assess our predictive model’s accuracy through external validation, thereby augmenting the universality of this study.
Conclusion
We developed and internally validated a predictive nomogram for predicting the risk of developing lower-limb venous thrombosis within 1 week of hospitalization in ICH patients. The predictive nomogram combines the TyG index with recognized clinical risk factors for ICH patients. The validation group data confirmed that the TyG index can be used as a risk biomarker for lower-limb venous thrombosis in ICH patients within 1 week of hospitalization, which is helpful for early identification of ICH patients at high risk for lower-limb venous thrombosis and their individualized management.
Data availability
Datasets used during this study may be obtained from the corresponding author (fdcivilization@163.com) upon reasonable request.
References
Pezzini, A. et al. Long-Term risk of arterial thrombosis after intracerebral hemorrhage: MUCH-Italy. Stroke 55, 634–642. https://doi.org/10.1161/STROKEAHA.123.044626 (2024).
Han, Z., Du, Y. & Qi, H. Intraventricular Hemorrhage-Don’t miss the deep cerebral venous thrombosis. J. Emerg. Med. 63, 678–680. https://doi.org/10.1016/j.jemermed.2022.10.004 (2022).
Li, H., Wu, Z., Zhang, H., Qiu, B. & Wang, Y. Low-molecular-weight heparin in the prevention of venous thromboembolism among patients with acute intracerebral hemorrhage: A meta-analysis. PLoS One. 19, e0311858. https://doi.org/10.1371/journal.pone.0311858 (2024).
Greenberg, S. M. et al. Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 53, e282-e361 https://doi.org/10.1161/STR.0000000000000407 (2022).
Li, L. & Murthy, S. B. Cardiovascular events after intracerebral hemorrhage. Stroke 53, 2131–2141. https://doi.org/10.1161/STROKEAHA.122.036884 (2022).
Haffner, S. et al. Intensive lifestyle intervention or Metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 54, 1566–1572. https://doi.org/10.2337/diabetes.54.5.1566 (2005).
Samad, F., Pandey, M. & Loskutoff, D. J. Regulation of tissue factor gene expression in obesity. Blood 98, 3353–3358. https://doi.org/10.1182/blood.v98.12.3353 (2001).
Van Schouwenburg, I. M. et al. Insulin resistance and risk of venous thromboembolism: results of a population-based cohort study. J. Thromb. Haemost. 10, 1012–1018. https://doi.org/10.1111/j.1538-7836.2012.04707.x (2012).
Wang, Z. et al. Non-insulin-based insulin resistance indexes in predicting atrial fibrillation recurrence following ablation: a retrospective study. Cardiovasc. Diabetol. 23, 87. https://doi.org/10.1186/s12933-024-02158-6 (2024).
Minh, H. V. et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J. Clin. Hypertens. (Greenwich). 23, 529–537. https://doi.org/10.1111/jch.14155 (2021).
Ye, Z. et al. Predicting long-term prognosis after percutaneous coronary intervention in patients with new onset ST-elevation myocardial infarction: development and external validation of a nomogram model. Cardiovasc. Diabetol. 22, 87. https://doi.org/10.1186/s12933-023-01820-9 (2023).
Du, T. et al. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc. Diabetol. 13, 146. https://doi.org/10.1186/s12933-014-0146-3 (2014).
Tian, X. et al. Distinct triglyceride-glucose trajectories are associated with different risks of incident cardiovascular disease in normal-weight adults. Am. Heart J. 248, 63–71. https://doi.org/10.1016/j.ahj.2022.02.014 (2022).
Alarcon, G. et al. High fat diet-induced metabolically obese and normal weight rabbit model shows early vascular dysfunction: mechanisms involved. Int. J. Obes. (Lond). 42, 1535–1543. https://doi.org/10.1038/s41366-018-0020-6 (2018).
Rosemann, A. et al. Diagnosis and treatment of venous thrombosis. Praxis (Bern 1994). 113, 148–159. https://doi.org/10.23785/PRAXIS.2024.06.002 (2024).
Wang, J. et al. Clinical analysis of bleeding and thrombotic events in haematological-oncology patients with severe thrombocytopenia and a high risk of thrombosis. Sci. Rep. 14, 24272. https://doi.org/10.1038/s41598-024-75895-z (2024).
Zhao, Q. et al. Predictive utility of a combination of the modified Caprini risk assessment score and D-dimer for the evaluation and management of lower extremity venous thrombosis after lung cancer surgery. J. Cardiothorac. Surg. 19, 562. https://doi.org/10.1186/s13019-024-03104-z (2024).
Fotiou, D. et al. Thrombotic and bleeding complications in patients with AL amyloidosis. Br. J. Haematol. 204, 1816–1824. https://doi.org/10.1111/bjh.19331 (2024).
Wang, T. F. et al. Risk of venous thromboembolism or hemorrhage among individuals with chronic kidney disease on prophylactic anticoagulant after hip or knee arthroplasty. Am. J. Hematol. 98, 1374–1382. https://doi.org/10.1002/ajh.26994 (2023).
Gu, Z. C. et al. A new simplified risk assessment model enhances postoperative prophylaxis of venous thromboembolism in Chinese adult patients with inguinal hernia (CHAT-3): a prospective, multicenter, randomized controlled trial. Int. J. Surg. 110, 5538–5544. https://doi.org/10.1097/JS9.0000000000001758 (2024).
Rothstein, A. et al. Construction of thermomaze. Bio Protoc. 14, e5044. https://doi.org/10.21769/BioProtoc.5044 (2024).
Sarkar, M., Madabhavi, I. V., Quy, P. N. & Govindagoudar, M. B. COVID-19 and coagulopathy. Clin. Respir J. 15, 1259–1274. https://doi.org/10.1111/crj.13438 (2021).
Aamodt, A. H. & Skattor, T. H. Cerebral venous thrombosis. Semin Thromb. Hemost. 48, 309–317. https://doi.org/10.1055/s-0042-1742738 (2022).
Galeano-Valle, F. et al. Cerebral venous thrombosis in adults: a case series of 35 patients from a tertiary hospital. Rev. Clin. Esp. (Barc). 223, 423–432. https://doi.org/10.1016/j.rceng.2023.06.005 (2023).
Baharoglu, M. I. et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 387, 2605–2613. https://doi.org/10.1016/S0140-6736(16)30392-0 (2016).
Ali, I. M., Reddy, M. V. & Shetty, V. Analysis of deep vein thrombosis: A prospective observational study. Cureus 16, e68014. https://doi.org/10.7759/cureus.68014 (2024).
Wittwer, J. & Bradley, D. Clusterin and its role in insulin resistance and the cardiometabolic syndrome. Front. Immunol. 12, 612496. https://doi.org/10.3389/fimmu.2021.612496 (2021).
Hill, M. A. et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 119, 154766. https://doi.org/10.1016/j.metabol.2021.154766 (2021).
Schneider, D. J. & Sobel, B. E. PAI-1 and diabetes: a journey from the bench to the bedside. Diabetes Care. 35, 1961–1967. https://doi.org/10.2337/dc12-0638 (2012).
Abd El-Kader, S. M. & Al-Jiffri, O. H. Impact of weight reduction on insulin resistance, adhesive molecules and adipokines dysregulation among obese type 2 diabetic patients. Afr. Health Sci. 18, 873–883. https://doi.org/10.4314/ahs.v18i4.5 (2018).
Wieczor, R., Wieczor, A. M., Kulwas, A. & Rosc, D. Type 2 diabetes and cardiovascular factors contrasted with fibrinolysis disorders in the blood of patients with peripheral arterial disease. Med. (Kaunas). 55. https://doi.org/10.3390/medicina55070395 (2019).
Acknowledgements
We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Funding
The paper is sponsored by Changshu City Science and Technology Development Program (CSWSQ202406).
Author information
Authors and Affiliations
Contributions
H.Z. developed the concept; H.Z. and F.L. performed data analysis and wrote manuscripts; L.H is responsible for research ethics and project management. All the authors reviewed the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, H., Huang, L. & Li, F. A nomogram based on the TyG index for the prediction of lower-limb venous thrombosis in patients with intracerebral hemorrhage. Sci Rep 15, 17406 (2025). https://doi.org/10.1038/s41598-025-01923-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01923-1
Keywords
This article is cited by
-
Triglyceride-glucose body mass index and risk of incident venous thromboembolism: a prospective cohort study from the UK Biobank
European Journal of Medical Research (2026)
-
The triglyceride-glucose index: updating evidence from clinical settings to molecular mechanisms in ageing-related cerebrovascular diseases
Cardiovascular Diabetology (2025)