Abstract
Quantification of soil carbon emissions in desert steppes is a key issue in determining the carbon budget in arid regions. However, the changes in and driving mechanisms of soil respiration and its components in response to drought in ecosystems under long-term water stress remain unclear. In this study, rain reduction by 30% and 50% experiments were conducted to simulate drought during the growing season in 2023 in the Stipa breviflora desert steppe. Total soil respiration and soil heterotrophic respiration were measured, and simultaneously, the surface soil temperature and moisture were measured at 0–10 cm. Surface soil microorganisms, microbial biomass carbon, and enzymatic activity were also tested. The results showed that drought significantly decreased soil microbial biomass carbon and enzymatic activity, but had no significant effects on soil microbial richness and diversity, as well as the dominant flora. The inhibitory effect of drought on soil autotrophic respiration only appeared at the beginning of the growing season and then disappeared with plant growth because of the drought-resistant ability of perennial plants in the desert steppe. Heterotrophic respiration is the primary soil carbon release process occurring in the desert steppe, approximately four times that of autotrophic respiration. Soil temperature and moisture jointly regulated heterotrophic respiration under extreme drought conditions (rainfall reduction of 50%); however, their influence on autotrophic respiration became insignificant. This study indicates that drought slowed the decomposition of soil organic carbon and had a weak effect on plant root respiration in the S. breviflora desert steppe. The response process of Rs and its components to drought stress provided theoretical basis for soil carbon feedback in desert steppe under extreme drought conditions.
Similar content being viewed by others
Introduction
Climate change has caused rising temperatures that accelerate soil evaporation, but water cannot be replenished in time, and the frequency and intensity of droughts are expected to increase in the future1. Drought, as an important factor regulating soil moisture, has a significant impact on ecosystem processes such as net primary productivity and carbon and nitrogen cycles2,3. Terrestrial ecosystems are the primary source of carbon emissions into the atmosphere, and their carbon cycle is strongly affected by changes in the global hydrological cycle, particularly soil respiration (Rs), which is considered the second-largest flux in the global terrestrial carbon cycle4. Aronson et al. (2019)5 speculated that if the grasslands in Southern California experience more frequent and extreme droughts in the future, Rs will decrease. Rs decreased by 8.2% and 6.1% in wet wetland ecosystems with 40% and 60% rainfall reductions, respectively6, whereas Rs decreased by 40.7% in arid desert steppe ecosystems with 50% rainfall reduction7. Therefore, the effects of drought on Rs show regional differences and are related to the hydrological conditions of the ecosystem. Previous studies have revealed that the influence of precipitation events on soil carbon emissions in the growing season of ecosystems is determined by antecedent soil moisture8, and the influence of geographical location on soil respiration was greater than that of average annual precipitation9. Desert steppe, located in the transitional zone between desert and grassland, is very sensitive and vulnerable to precipitation changes10. The process of drought stress’s impact on soil carbon emissions needs to be further discussed in desert steppe.
Litter microbial communities in semiarid regions may be metabolically adapted to maintain their functions under drought conditions11. However, due to Rs adaptability to moderate drought in arid ecosystems, drought stress may not significantly affect Rs12. By integrating the effects of global precipitation control experiments on Rs, Yang et al. (2017)13 found that soil moisture altered by precipitation was the primary driving factor for Rs, and the interpretation reached 98% when combined with soil microbial biomass carbon and vegetation biomass. Rs refers to the process of carbon dioxide (CO2) emission into the atmosphere by soil microorganisms, plant roots, and litter respiration13, which can be divided into heterotrophic respiration (Rh) and autotrophic respiration (Ra). Rh represents the CO2 released by microbial decomposition of organic carbon, which depends on microbial activity and substrate availability. Ra originates from the roots and rhizosphere and is regulated by the allocation of newly assimilated carbon14,15. Therefore, drought stress will affect Rs directly by altering soil microbial activity and root respiration or indirectly by altering matrix availability16. In order to better understand the mechanism of drought stress on soil respiration, it should be decomposed into different component.
The global average Ra/Rh ratio is indicative of underlying soil carbon dynamics, and the global variation in Ra/Rh is caused by the joint regulation of soil microbial and root activity by climate (temperature and humidity), soil (carbon/nitrogen and soil bulk density) and vegetation (biomass transfer)17. The responses of Ra and Rh to soil temperature and moisture differed, resulting in a change in the sensitivity of Rs to temperature and humidity in different sites18,19. The researchers have shown that soil moisture is the main driver of CO2 effusion from ecosystems, and Rh was the primary contributor to Rs during drought7,20,21. Moreover, Balogh et al. (2015)22 found that drought inhibited Ra to a greater extent than Rh, but Inglima et al. (2009)20 found that Ra did not play any significant role in CO2 exchange due to vegetation aging in the process of drying and rewetting. By observing the response of Rs and its components to drought and rewetting in different growth stages of desert steppe, Sun21 found that drought stress significantly inhibited Ra in the early growing season and had no significant effect in the growth peak period, but always had significant inhibitory effect on Rh. Most studies have looked at soil respiration as a whole, but there is still a lack of discussion on the effects of soil respiration and its components under extreme drought, especially in desert steppe. Thus, to better understand the feedback of soil carbon emission processes to climate change, and to more accurately predict soil CO2 flux and carbon sequestration potential, it is important to explore the response of Rs and its components to drought stress in arid ecosystems.
The following three hypotheses were tested to provide a theoretical basis for further understanding the carbon cycle of desert steppe ecosystems: (1) Rs and its components decrease with the increase of drought stress during growing season; (2) Drought stress had different inhibitory effects on Ra and Rh; (3) The influence of soil moisture on Rs and its components exceeds soil temperature. Therefore, three gradients of natural precipitation, 30% rain reduction and 50% rain reduction were set up in the desert steppe of Inner Mongolia, China, and simultaneous monitoring of soil respiration and Rh to explore the potential response process of Rs and its components to increasing aridity in a desert steppe during the growing season, and to analysis the key driving factors of Rs and its components.
Materials and methods
Study area description
The study area is located in Adege, Siwangqi Banner, Ulanqab, Inner Mongolia Autonomous Region, China, with geographical coordinates of 42°02′17′′ North and 112°30′57′′ East. It is located at an elevation of 1328 m and has a temperate continental monsoon climate. The average annual precipitation over the past 60 years (1961–2020) was primarily concentrated from May to October, with an average of approximately 275.8 mm, accounting for more than 90% of the total annual precipitation. The annual evaporation was 2180 mm, and the average annual temperature was 3.8 °C. The soil type in the study area is light chestnut calcareous soil with the texture of sandy loam. The plant community is simple, with an average vegetation cover of approximately 9%, primarily comprising perennial or annual herbs, with Stipa breviflora Griseb, Cleistogenes songorica (Roshev.) Ohwi and Neopallasia pectinate (Pall.) Poljak as the dominant species.
Experimental design
In 2022, a 50 m × 50 m experimental plot was established as a flat area with typical vegetation distribution, and fencing was used to prevent human and livestock interference. The experimental subplots were arranged in the central area of the plot, and three treatments were established: natural rainfall (control), drought intensity increased by 30% (rain reduction by 30%), and drought intensity increased by 50% (rain reduction by 50%). The area of each subplot was 7 m × 4 m, and the rain reduction treatment was provided with a canopy. Based on the long-term historical meteorological records of desert steppe from 1959 to 2012, the inter-annual variation of precipitation ranges from − 42.3 to 34.9%. Therefore, rain reduction by 30% represents most of the variation observed in annual precipitation in the desert steppe, and rainfall reduction by 50% represents the prospective drought intensity. The rain shelter was constructed of transparent acrylic boards to minimize its impact on plant lighting. The rain shield areas were 30% and 50% of the plot areas. The inclination angle of the rain shield was 6°, its height was approximately 1.3 m, and a rainwater-collection device was installed at the bottom. Two homemade Polyvinyl chloride (PVC) rings with diameters of 20 cm were installed in each subplot at the end of March 2022. One was 6 cm high and was inserted 3 cm into the ground for Rs measurement, which ensured stability during observation and minimized the impact on root growth in the upper soil layer. The other was 40 cm high and was inserted 37 cm into the ground for Rh measurement, which could eliminate approximately 88–91% of the newly formed root systems, eliminating the associated root respiration. The Ra was obtained by subtracting Rh from Rs.
Data measurements
Rs and Rh, and soil temperature and water content
During the 2023 growing season (May–October), Rs and Rh were measured using a portable automatic soil carbon flux system (Li-6800; Li-Cor Inc., Lincoln, NE, USA) at the beginning, middle, and end of each month from 8:00 am to 11:00 am on a cloudless day. The new green vegetation within the PVC ring for measuring Rs was trimmed flush with the ground to eliminate plant respiration, 24 h before each measurement, and the new vegetation within the PVC ring for measuring Rh was uprooted. Simultaneously, the 0–10 cm soil temperature and volumetric water content were measured using a Li-6800 soil thermocouple probe.
Soil microorganisms, microbial biomass carbon and enzymatic activity
Soil samples were collected in the middle of the growing season (26 July). Soil samples were randomly selected from the soil surface with a sterile sampling shovel in each PVC ring, loaded into a sterile centrifuge tube, placed in a foam box with ice packs at below 0 °C and shipped to the laboratory, stored in a refrigerator at − 80 °C for the determination of soil microorganisms, microbial biomass carbon and enzymatic activity.
Soil microorganism 16 S rRNA and ITS amplicon sequencing were conducted by OE Biotech Co., Ltd. (Shanghai, China). Soil microbial community structure and abundance were determined to analyze the effects of drought on soil microbial community composition.
Chao index is used to characterize the richness of soil microbial community, and the greater the value, the greater the total species. The Chao index is defined as follows:
Shannon index was used to characterize soil microbial community diversity: the higher the species diversity, the more uniform the species distribution. The Shannon index is defined as follows:
Where SChao is the estimated number of Amplicon Sequence Variant (ASV); Sobs is the actual observed number of ASV; n1 is the number of ASV containing only one sequence; n2 is the number of ASV containing two sequences. N is total number of sample sequences; ni is the sequence number of the i-th ASV;
Soil microbial biomass carbon was determined using the chloroform-fumigation direct extraction process.
Samples were tested for lignin peroxidase, polyphenol oxidase and β-glucosidase enzymatic activity, using soil lignin peroxidase (S-Lip) activity and soil polyphenol oxidase (S-PPO) test kits and a soil β-glucosidase (S-β-GC) activity detection kit from Beijing BOXBIO Technology Co. LTD (Beijing, China), respectively. The specific analysis and calculation method of enzyme activity determination refer to sun’s research21.
Aboveground and belowground vegetation biomass
The vegetation biomass was estimated indirectly to ensure that the sample site was not damaged. Vegetation surveys were conducted at the end of each month of the growing season to record each plant species’ height, density, and coverage in the experimental plot. Thirty quadrats (1 m × 1 m) were selected near the experimental plots for vegetation investigation at the peak of the growing season (26 July), and clipped plants were used to determine the aboveground biomass of each plant. Ten quadrats were randomly selected to remove all plants with 0–20 cm roots, part of the soil attached to the roots was removed, and the plants were placed in bags. In the laboratory, the aboveground and belowground parts of the plants were separated based on vegetation species then washed, dried (65℃) and weighed. Finally, the aboveground and belowground biomass of each plant was obtained. Linear fitting equations for coverage and aboveground and belowground biomass was established according to the vegetation functional communities (both attained significance; Table 1)23. Perennial grasses included S. breviflora, Cleistogenes songorica, and Leymus chinensis; perennial forbs included Convolvulus ammannii, Allium mongolicum Regel, Allium tenuissimum, Aster altaicus, Lagochilus ilicifolius, Artemisia scoparia, and Astragalus scaberrimus; annual forbs included N. pectinate and Salsola collina; and semi-shrubs included Kochia prostrata and Artemisia frigida.
Data processing and analysis
The differences in soil temperature and moisture between the inner and outer rings of the PVC ring for measuring Rs and Rh were not significant (P > 0.05). The soil moisture at the experimental site was low; therefore, to reduce the measurement error of the soil thermocouple probe, the soil moisture was corrected by comparing the measured value with the soil moisture determined by the oven-drying method taken from a nearby soil sample at the same time as the instrument was measured. SPSS 21 (SPSS Inc., Chicago, IL USA) for windows was used for statistical analysis and analysis of variance for difference tests (P < 0.05), and charts were drawn using ORIGIN 2024 (OriginLab, Massachusetts, USA).
Results
Changes in Rs and its components, Rh/Rs and Ra/Rs, and soil temperature and moisture in the growing season under different drought stresses
The maximum Rs, Rh, and Ra values occurred on 3 May in the S. breviflora desert steppe (Fig. 1). Drought stress significantly decreased Rs and Rh; however, an inhibitory effect on Ra was only observed from May to June and the inhibitory effect was not significant from July to September. The average Rs values under the control, rain reduction by 30% and rain reduction by 50% were 0.85 µmol m–2 s–1, 0.67 µmol m–2 s–1, and 0.56 µmol m–2 s–1, respectively, the mean Rh values were 0.68 µmolm–2 s–1, 0.55 µmol m–2 s–1, and 0.45 µmol m–2 s–1, respectively, and the average Ra values were 0.16 µmol m–2 s–1, 0.12 µmol m–2 s–1, and 0.11 µmol m–2 s–1, respectively. Rainfall reductions by 30% and 50% significantly reduced Rs by 21.2% and 34.1%, respectively, with Rh contributing 16.2% and 27.5%, and Ra contributing 5.0% and 6.6%, respectively. This indicated that drought stress could inhibit Rs and its components, and the greater the drought stress, the stronger is the inhibitory effect; however, Ra was not inhibited by drought stress in the middle and end of the growing season.
The average soil temperature in PVC rings for measuring Rs and Rh were 33.2℃ and 33.7℃, respectively, the effect of rain reduction treatments on soil temperature was not significant. The average soil moisture in the PVC ring for measuring Rs was 8.0%, 6.2%, and 5.6% under the control and rain reduction by 30% and 50%, respectively, and the soil moisture (rain reduction by 30% and 50%) decreased significantly by 27.7% and 30.2%. The average soil moisture in the PVC ring for measuring Rh was 9.6%, 6.7%, and 5.6%, respectively, and the soil moisture (rain reduction by 30% and 50%) decreased significantly by 30.1% and 42.0%.
Variations of Rs, Rh, Ra, and soil temperature (Ts) and moisture (Ms) under different drought stresses in the growing season (Mean ± SE) (Note: W1: natural rainfall (control); W2: rain reduction by 30%; W3: rain reduction by 50%;Ts: the 0–10 cm soil temperature; MsW1: the 0–10 cm soil moisture under natural rainfall; MsW2: the 0–10 cm soil moisture under rain reduction by 30%; MsW3: the 0–10 cm soil moisture under rain reduction by 50%).
Under the control and rain reduction by 30% and 50% treatments, the Rh/Rs and Ra/Rs were 80.8% and 19.2%, 81.9% and 18.1%, and 80.7% and 19.3%, respectively. Rh/Rs was approximately four times that of Ra/Rs under different drought stress conditions; therefore, Rh was the primary form of soil carbon release in the desert steppe. Ra/Rs decreased with increasing drought stress in the early growing season (before July) and increased with increasing drought stress during the peak growth period (July-August) (Fig. 2).
Effects of drought stress on soil microorganisms and microbial biomass carbon
The soil microorganisms were primarily bacteria in the S. breviflora desert steppe. The abundance of bacteria and fungi spanned 900 to 1400 and 180 to 300, respectively, and the diversity of bacteria and fungi spanned 8 to 10 species and 3 to 6 species, respectively. The Chao abundance and Shannon diversity of soil bacteria and fungus had no significant difference between Rs and Rh under same treatment, moreover, they decreased under drought stress but the differences were not significant (Table 2). The average soil microbial biomass carbon under the control, rain reduction by 30% and 50% was 193.77 mg/kg, 183.21 mg/kg and 163.41 mg/kg, respectively, which decreased significantly with an increase in drought intensity.
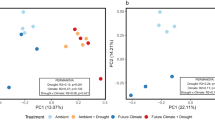
Among the bacterial community composition, the relative abundance of Bacteroidota and Actinobacteriota tended to stabilize, about 28% and 21%, respectively; the largest relative abundance of Proteobacteria, which was approximately 40%, and it showed a decreasing trend with increase of drought, but it suddenly increased in Rh under rain reduction by 50%. The relative abundance of Gemmatimonadota and Acidobacteriota were decreased by drought stress (Fig. 3). Among the fungal community composition, the largest relative abundance of Ascomycota, which was approximately 80%, and it showed stabilizing tend.
Effects of drought stress on soil enzymatic activity
Soil enzymatic activity observed for the test plot was low, and there was no significant difference between the PVC rings measuring for Rs and Rh (P > 0.05). The average lignin peroxidase activity under the control, rain reduction by 30% and 50% treatments were 11.01 U g–1, 8.03 U g–1, and 8.37 U g–1, respectively. The mean values of soil polyphenol oxidase and β-glucosidase activity under the control, rain reduction by 30% and 50% treatments were 85.70 U g–1, 64.76 U g–1, and 61.34 U g–1, and 0.69 U g–1, 0.60 U g–1, and 0.60 U g–1, respectively. The activity of soil lignin peroxidase, soil polyphenol oxidase and soil β-glucosidase significantly decreased under drought, however, the degree of inhibition did not increase with the increase of drought stress in the S. breviflora desert steppe (Fig. 4).
Effects of drought stress on aboveground and belowground biomass
By regulating the growth of perennial grasses and forbs, drought stress significantly increased the total underground biomass from June to September when the rainfall decreased by 30%, but had little effect on aboveground biomass in the S. breviflora desert steppe (Fig. 5 and Tables 1S and 2S). In terms of monthly vegetation growth (Tables 1S and 2S), drought stress significantly reduced the growth of total biomass in May and June, which primarily inhibited the aboveground and belowground growth of perennial grasses and forbs; however, no significant changes were observed in annual forbs and semi-shrubs. From July to September, no significant differences were observed in the growth of different vegetation functional communities and total biomass among the treatments (Tables 1S and 2S). This indicates that drought stress can significantly inhibit the growth of perennial grasses and forbs only at the beginning of the growing season.
Monthly variations of different plant functional communities aboveground and belowground biomass under different drought stresses during the growing season in the S. breviflora desert steppe (Note: W1: natural rainfall (control); W2: rain reduction by 30%; W3: rain reduction by 50%; Different lowercase letters indicate significant differences between the treatments in total plant aboveground or belowground biomass (P < 0.05)).
Linearity of Rs and its components with soil temperature and moisture under different drought stresses
The linear relationships between Rs and its components with soil temperature were not significant under the control and rain reduction by 30% treatment. However, with an increase in drought stress, the influence of soil temperature on Rs and Rh gradually increased, and was significantly negatively correlated under the 50% rain reduction treatment (P < 0.05). The linear relationships between Rs and Rh with soil moisture showed a significantly positive correlation under different drought stresses, and the influence of soil moisture on Rs and Rh gradually increased with increasing drought stress. However, the influence of soil moisture on Ra gradually decreased, and the relationship was not significantly correlated under the 50% rain reduction treatment (Fig. 6). This indicated that soil moisture was the dominant factor affecting Rs and its components in the S. breviflora desert steppe. When extreme drought occurs, the soil temperature and soil water content together regulate Rh, however, their influence on Ra becomes insignificant.
Linearity of Rs, Rh, Ra, with soil temperature (Ts) and moisture (Ms) under different drought stresses (Note: W1: natural rainfall (control); W2: rain reduction by 30%; W3: rain reduction by 50%;Ts: the 0–10 cm soil temperature; MsW1: the 0–10 cm soil moisture under natural rainfall; MsW2: the 0–10 cm soil moisture under rain reduction by 30%; MsW3: the 0–10 cm soil moisture under rain reduction by 50%; ns : P > 0.05; * : P < 0.05; ****: P < 0.0001).
Discussion
Effect of drought on soil Rh
Rh is the CO2 released by soil microorganisms that decompose organic matter. Proteobacteria, Bacteroidota, Actinobacteriota and Ascomycota were the dominant phyla in the S. breviflora desert steppe soils (Fig. 3), this was similar to the findings of Kang et al.24. Moreover, the study also found that the dominant phyla did not change with increasing drought, and the soil microbial diversity and abundance were not significantly affected by drought stress (Table 2). This may be that different bacterial communities responded to drought stress in the opposite ways, thus counterbalancing the effects of drought stress on the bacterial communities25,26. In addition, fungi can obtain sufficient water and nutrients from the pores of water-deficient soil through their mycelial structure to maintain their normal life activity27, so that drought stress has no significant impact on soil fungi.
Drought stress inhibited soil enzymatic activity (Fig. 4) and soil microbial biomass carbon (Table 2). The decomposition of organic matter by soil microorganisms depends on enzymatic catalysis, whereas drought stress reduces the leaching of soil water, and lowers the activity of soil microorganisms, which is unfavorable for the secretion of soil enzymes32. Soil microbial biomass carbon is the most active component in the turnover of soil organic carbon, and is positively correlated with soil water content28. Drought leads to the disconnection of soil pore water and the tight binding of soil colloids and aggregates, which limits the supply of soil microbial communities from the substrate29,30,31. Drought stress correspondingly increased the dependence of soil microorganisms and enzymatic activity on water32,33. Therefore, Rh decreases significantly with the increase of drought stress throughout the growing season. Moreover, the explanatory power of soil moisture for Rh increased (Fig. 6), surpassing that of soil temperature as a key factor affecting Rh. However, the influence of soil temperature on Rh becomes significant during extreme drought (rain reduction by 50%), which may be the decrease of precipitation has a threshold effect on the growth process of fungi by affecting the integrity of mycelial structure34, but fungi can actively explore nutrients and release CO2 across air-filled pores35.
Effect of drought on soil Ra
Drought stress only significantly reduced Ra in May and June, and had no significant difference from July to September (Fig. 1). The reason was that vegetation reduces transpiration by regulating stomata under drought stress, weakens plant photosynthesis, and reduces organic matter transportation to the roots, thereby limiting Ra36,37. The continuous weakening of photosynthesis during the growing season will inevitably reduce the accumulation of vegetation biomass. However, drought stress only significantly reduced the total aboveground biomass in June, and the total belowground biomass decreased significantly at the end of May (Fig. 5). By further analyzing found that drought stress significantly reduced the growth of total biomass in May and June, but there were no significant differences were observed in the growth of total biomass among the treatments from July to September (Tables 1S and 2S). This reason was that drought stress at the beginning of the growing season significantly inhibited the growth and development of plants and roots38,39. The decreased soil moisture in the S. breviflora desert steppe primarily inhibited the growth and development of perennial vegetation’s aboveground and subsurface parts (Tables 1S and 2S). However, the characteristics of S strategy were found in the perennial grasses (S. breviflora and C. songorica), and S. breviflora had strong adaptability to extreme drought and stressful environments40,41. So the vegetation growth rate under different drought stresses in the peak growth period was consistent, which further leaded to the insignificant difference of Ra under different drought treatments. Moreover, drought induced respiration rates by increasing specific root secretions, especially in legumes, which was also a strategy for plants to cope with drought42. And then it can be concluded that soil moisture is the key factor controlling Ra during general drought events, but when extreme drought occurs, the adaptability of perennial vegetation to extreme environments reduces its dependence on soil water and the relationship between Ra and soil moisture is no longer significant (Fig. 6). Moreover, Podzikowski et al.43 recently demonstrated that plant functional diversity determined the response of Rs to soil water availability, and highlighted the importance of plant community composition for predicting Rs.
Effect of drought on total Rs
Studies have found that the contribution ratio of Rh to Rs was 51% in the semiarid grasslands of Inner Mongolia44, and the relative contribution rate of Rh to Rs increased from 29% under non-drought state to 64% under drought stress2. However, this study showed that Rh contributed more than 80% to Rs in the S. breviflora desert steppe, and ratio of Rh to Rs did not change significantly with an increase in drought stress (Fig. 2). This was because the average soil moisture in the study area was only 6.9%, vegetation coverage of the desert steppe was low, root respiration was minimal, and the contribution rate of Ra to Rs was much lower than that of Rh. Therefore, Rh was the primary form of soil carbon released into the S. breviflora desert steppe ecosystem, and the seasonal dynamic changes in the Rs during growing season was highly consistent with that of Rh.
Seasonal dynamic changes in Rs and its components are driven by the direct or indirect effects of soil temperature and moisture on vegetation growth and soil microbial activity45,46. In this study, Although the air temperature was relatively low in the early growth period (May) and the vegetation had just turned green, the soil was in the freeze-thaw period at this stage, and then ice film on the surface of the soil particles and the ice in the soil pores melted into water and accumulated in the soil, so the soil moisture reached the maximum (Fig. 1). Adequate soil moisture increased the activity of soil microorganisms, and promoted the rate of production and decomposition processes of soil organic carbon47,48,49, thus Rs and Rh reached their maximum value at the beginning of the growing season. When the rainy season entered in July, the temperature increased and the vegetation grew rapidly, Rs and Rh increased slightly, but the evapotranspiration increased, and the soil moisture did not exceed the freeze-thaw period, and therefore Rs and Rh did not reach the maximum in the growth peak period. This further indicates that soil moisture is the main controlling factor of Rs and Rh in desert steppe, and its effect exceeds soil temperature.
Drought stress inhibited soil enzymatic activity (Fig. 3), vegetation growth (Tables 1S and 2S), and weakened plant photosynthesis, which reduced the amount of organic matter delivered to the roots, the amount of substrate for root respiration, and soil microbial respiration50,51, ultimately leading to a significant decrease in Rs and its components (Fig. 1). Lellei-Kovács52 found that extreme drought had a negative legacy effect on soil respiration that could extend well beyond the immediate effects, and speculated that this was due to changes in soil biota and reduced root activity.
Conclusions
Drought stress significantly decreased Rs in the growing season of S. breviflora desert steppe. Rh is the main process and form of soil carbon emission, and its contribution rate exceeds 80%. Drought stress had no significant effects on the abundance and diversity of soil bacteria and fungi, as well as the dominant flora (phylum). Drought inhibited Rh by reducing soil microbial biomass carbon and enzyme activities, and inhibited Ra by regulating monthly changes in perennial vegetation biomass. Due to the strong drought resistance of perennial vegetation in desert steppe, vegetation growth was not inhibited in the blooming period, and the inhibition of drought on Ra only appeared at the beginning of the growing season. Soil moisture is a key factor in Rs and its components, and its influence goes beyond soil temperature. This study elucidates the response process of Rs and its components to drought stress in the S. breviflora desert steppe, and provides a theoretical basis for soil carbon feedback in desert steppe under extreme drought conditions.
Data availability
The research data supporting the results of the manuscript are available from the corresponding author upon reasonablerequest.
References
Smith, M. D. An ecological perspective on extreme Climatic events: a synthetic definition and framework to guide future research. J. Ecol. 99, 656–663 (2011).
Balogh, J. et al. Autotrophic component of soil respiration is repressed by drought more than the heterotrophic one in dry grasslands. Biogeosciences 13, 5171–5182 (2016).
Reichstein, M. et al. Climate extremes and the carbon cycle. Nature 500, 287–295 (2013).
Bond-Lamberty, B. & Thomson, A. A global database of soil respiration data. Biogeosciences 7, 1915–1926 (2010).
Aronson, E. L., Goulden, M. L. & Allison, S. D. Greenhouse gas fluxes under drought and nitrogen addition in a Southern California grassland. Soil. Biol. Biochem. 131, 19–27 (2019).
Li, X. Effects of Change in Precipitation Amount on Soil Carbon Emissions in a Coastal Wetland in the Yellow River Delta, China (He Nan university, 2019).
Cui, Y. et al. Soil respiration and its determinants under simulated precipitation in a desert steppe. J. Soils Sediments 24, 552–562 (2024).
Han, Y., Wang, G., Xiong, L., Xu, Y. & Li, S. Rainfall effect on soil respiration depends on antecedent soil moisture. Sci.Total Env. 926, 742 (2024).
Liu, J., Hu, J., Liu, H. & Han, K. Global soil respiration Estimation based on ecological big data and machine learning model. Sci. Rep. 14, 412 (2024).
Hawinkel, P. et al. Vegetation response to precipitation variability in East Africa controlled by biogeographical factors. J. Geophys. Res. Biogeosci. 121, 2422–2444 (2016).
Nisson, D. M. & Allison, S. D. Litter microbial respiration and enzymatic resistance to drought stress. Elementa 8, 293–302 (2020).
Li, B., Zhu, W., Han, C., Yu, H. & Huang, J. Soil respiration and its influencing factors in a desert steppe in Northwestern China under changing precipitation regimes. Chin. J. Plant. Ecol. 47, 1310–1321 (2023).
Hanson, P. J., Edwards, N. T., Garrten, C. T. & Andrews, J. A. Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48, 115–146 (2000).
Baggs, E. M. Partitioning the components of soil respiration: a research challenge. Plant. Soil. 284, 1–5 (2006).
Chen, J. Differential responses of ecosystem respiration components to experimental warming in a meadow grassland on the Tibetan plateau. Agric. Meteorol. 220, 21–29 (2016).
Sowerby, A., Emmett, B. A., Tietema, A. & Beier, C. Contrasting effects of repeated summer drought on soil carbon efflux in hydric and mesic heathland soils. Glob. Chang. Biol. 14, 2388–2404 (2010).
Jin, C. et al. Soil autotrophic-to-heterotrophic-respiration ratio and its controlling factors across several terrestrial biomes: a global synthesis. Catena (Amst.) 242, 45 (2024).
Gomez-Casanovas, N., Matamala, R., Cook, D. R. & Gonzalez-Meler, M. A. Net ecosystem exchange modifies the relationship between the autotrophic and heterotrophic components of soil respiration with abiotic factors in prairie grasslands. Glob. Chang. Biol. 18, 2532–2545 (2012).
Rey, A. et al. Annual variation in soil respiration and its components in a coppice oak forest in central Italy. Glob. Chang. Biol. 8, 851–866 (2002).
Inglima, I. et al. Precipitation pulses enhance respiration of mediterranean ecosystems: the balance between organic and inorganic components of increased soil CO2 efflux. Glob. Chang. Biol. 15, 1289–1301 (2009).
Sun, Z. Response of Soil Respiration and its Components To Drought Stress in Desert Grasslands (Inner Mongolia Agricultural University, 2024).
Balogh, J. et al. Autotrophic component of soil respiration is repressed by drought more than the heterotrophic one in a dry grassland. Biogeosci. Discuss. 12, 16885–16911 (2015).
Gherardi, L. A., Sala, O. E. & Penuelas, J. Enhanced interannual precipitation variability increases plant functional diversity that in turn ameliorates negative impact on productivity. Ecol. Lett. 18, 1293–1300 (2015).
Kang, P. et al. Soil saprophytic fungi could be used as an important ecological indicator for land management in desert steppe. Ecol. Indic. 150, 745 (2023).
Maestre, F. T., Delgado-Baquerizo, M., Jeffries, T. C., Eldridge, D. J. & Singh, B. K. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natil. Acad. Sci. 112, 15684–15689 (2015).
Ochoa-Hueso, R. et al. Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Glob. Chang. Biol. 24, 2818–2827 (2018).
Barnard, R. L., Osborne, C. A. & Firestone, M. K. Responses of soil bacterial and fungal communities to extreme desiccation and rewetting. ISME J. 7, 2229–2241 (2013).
Ren, C. et al. Responses of soil total microbial biomass and community compositions to rainfall reductions. Soil. Biol. Biochem. 116, 4–10 (2018).
Schjnning, P., Thomsen, I. K., Moldrup, P. & Christensen, B. T. Linking soil microbial activity to Water- and Air-Phase contents and diffusivities. Soil Sci. Soc. Am. J. 67, 156–165 (2003).
Skopp, J., Jawson, M. D. & Doran, J. W. Steady-State aerobic microbial activity as a function of soil water content. Soil Sci. Soc. Am. J. 54, 1619–1625 (1990).
Monger, H. C. & Gallegos, R. A. Biotic and Abiotic Processes and Rates of Pedogenic Carbonate Accumulation in the Southwestern United States—Relationship to Atmospheric CO2 Sequestration (Global Climate Change and Pedogenic Carbonates, 2000).
Šnajdr, J. et al. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil. Biol. Biochem. 40, 2068–2075 (2008).
Linn, D. M. & Doran, J. W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci. Soc. Am. J. 48, 1267–1272 (1984).
Hawkes, C. V. et al. Fungal community responses to precipitation. Glob Chang. Biol. 17, 1637–1645 (2015).
Griffin M. D. Soil water in the ecology of Fungi. Phytopathology 7, 289–310 (1969).
Chandregowda, M. H., Tjoelker, M. G., Power, S. A. & Pendall, E. Drought and warming alter gross primary production allocation and reduce productivity in a widespread pasture grass. Plant. Cell. Environ. 45, 2271–2291 (2022).
Sperlich, D., Barbeta, A., Ogaya, R., Sabaté, S. & Peuelas, J. Balance between carbon gain and loss under long-term drought: impacts on foliar respiration and photosynthesis in Quercus ilex L. J. Exp. Bot. 67, 821–833 (2016).
Chelli, S. et al. The response of sub-Mediterranean grasslands to rainfall variation is influenced by early season precipitation. Appl. Veg. Sci. 19, 611–619 (2016).
Suttle, K. B., Thomsen, M. A. & Power, M. E. Species interactions reverse grassland responses to changing climate. Sci. (1979) 315, 640–642 (2007).
Liu, Y., Gao, G., Li, Z., Wang, C. & Tian, L. Differences in plant water use characteristics and responses to environmental factors in the desert grassland of the inner Mongolia. Acta Ecol. Sin. 43, 1–12 (2023).
Zheng, J. et al. Soil deterioration due to long-term grazing of desert-steppe promotes stress-tolerant ecological strategies in plants. Sci. Total Env. 907, 745 (2024).
Hou, F. et al. Root exudates from drought-affected plants increase soil respiration across a range of grassland species. Soil Biol. Biochem. 203, 456 (2025).
Podzikowski, L. Y., Billings, S. A. & Bever, J. D. Plant functional diversity shapes soil respiration response to soil moisture availability. Ecosystems 28, 15 (2025).
Li, W. et al. Partitioning of soil respiration components and evaluating the mycorrhizal contribution to soil respiration in a semiarid grassland. Chin. J. Plant. Ecol. 42, 850–862 (2018).
Xu, J. et al. Influence of timber harvesting alternatives on forest soil respiration and its biophysical regulatory factors over a 5-year period in the Missouri Ozarks. Ecosystems 14, 1310–1327 (2011).
Martin, J. G., Bolstad, P. V., Ryu, S. R. & Chen, J. Modeling soil respiration based on carbon, nitrogen, and root mass across diverse great lake forests. Agric. Meteorol. 149, 1722–1729 (2009).
Rey, A., Oyonarte, C., Morán-López, T., Raimundo, J. & Pegoraro, E. Changes in soil moisture predict soil carbon losses upon rewetting in a perennial semiarid steppe in SE Spain. Geoderma 287, 135–146 (2017).
Van Gestel, M., Merckx, R. & Vlassak, K. Microbial biomass responses to soil drying and rewetting - the fate of fast-growing and slow-growing microorganisms in soils from different climates. Soil. Eiol Biochem. 25, 109–123 (1993).
Chu, X. et al. Seasonal not annual precipitation drives 8-year variability of interannual net CO2 exchange in a salt marsh. Agric. Meteorol. 2021, 308–309 (2021).
Wang, C. et al. Aridity threshold in controlling ecosystem nitrogen cycling in arid and semi-arid grasslands. Nat. Commun. 5, 4799 (2014).
Pflug, A. & Wolters, V. Influence of drought and litter age on Collembola communities. Eur. J. Soil. Biol. 37, 305–308 (2001).
Lellei-Kovács, E., Botta‐Dukát, Z., Ónodi, G., Mojzes, A. & Kröel‐Dulay, G. The negative legacy effect of extreme drought on soil respiration is unaffected by Post‐Drought precipitation regime in a temperate grassland. Glob Chang. Biol. 31, 145 (2025).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 5210921), Program for Natural Science Foundation of Inner Mongolia Autonomous Region (Grant 2021BS05021), and High-level Talents Introduction and Research Launch Project of Inner Mongolia Agricultural University (Grant NDYB2019-8).
Author information
Authors and Affiliations
Contributions
C.H. and Z.S. wrote the main manuscript text. H.L. provided ideas for the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, C., Sun, Z. & Li, H. Soil heterotrophic respiration repressed by drought stress more than soil autotrophic respiration in Stipa breviflora desert steppe, China. Sci Rep 15, 18235 (2025). https://doi.org/10.1038/s41598-025-01977-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41598-025-01977-1