Abstract
A thorough investigation of perovskite structures formed through doping is essential for advancing the efficiency and stability of perovskite solar cells. In this study, Bi-doped FAPbI3 perovskite films with varying Bi concentrations (0.5–2%) were fabricated using a spin-coating technique on ITO glass substrates. Then the films’ phase structure, local structure, and optical characteristics were analyzed. X-ray diffraction (XRD) analysis revealed that the pristine FAPbI3 film exhibited both hexagonal and cubic phases, indicating structural instability. In contrast, Bi-doped FAPbI3 films predominantly displayed a cubic perovskite structure, with a notable reduction in the XRD peak intensity corresponding to the hexagonal phase. UV–Vis spectroscopy showed that the undoped FAPbI3 film had an absorption edge in the visible-near infrared range, while Bi-doping caused a redshift, indicating a reduction in the optical band gap. The calculated results show that optical band gaps decrease with increasing Bi, from a value of 1.49 (pure) to 1.43 (2% Bi) eV. X-ray absorption near edge structure (XANES) analysis confirmed the oxidation states of Pb2+ and Bi3+ ions across all samples, with Bi ions replacing Pb in the local structure. Photoluminescence (PL) measurements revealed an increased PL intensity with 1% Bi doping (7 \(\times\) 105) compared with pristine FAPbI3 (4.7 \(\times\) 105), suggesting a reduction in carrier recombination. These findings demonstrate the potential of Bi-doping to stabilize perovskite structures with improved optoelectronic properties.
Similar content being viewed by others
Introduction
Currently, conventional non-renewable energy is extensively utilized in many industries, including fossil energy. This releases toxic gases and radioactive wastes during the production of electrical and thermal energies1. These energy sources present significant disadvantages, not only due to their finite nature but also due to their harmful environmental impacts2. As a result, there has been a shift towards renewable energy resources, such as solar energy, to address these concerns. Solar energy, a renewable and environmentally friendly source, is widely applied in various fields, including photocatalysis and photovoltaics3. The benefits of solar energy include its renewability, non-toxicity, and its role in green technology4. The current research aims to apply solar energy harvested using solar cells to convert sunlight into electrical energy and to conduct an in-depth study of materials to improve the stability and efficiency of solar cells.
Various types of solar cells, such as silicon-based and thin-film solar devices5, are widely used and have been continually improved. Thin-film solar cells are deployed as quantum dot6, dye-sensitized7, organic8, and perovskite forms9. They are fabricated by coating single or multiple layers onto appropriate substrates. Recent studies reported that perovskite solar cells demonstrate higher efficiency than other thin-film solar cells. The advantages of perovskite solar cells include their low cost, facile fabrication, and high solar cell efficiency10. Normally, a perovskite solar cell consists of multiple layers, including transparent conducting oxide, electron transport, perovskite compound, hole transport, and electrode layers11. A perovskite compound is a material with a specific structure corresponding to the chemical formula ABX₃, where A is a cation, typically an organic or inorganic group (Cs, MA, FA), B is also a cation, usually an inorganic element (Pb), and X is an anion, commonly a halide (I, Cl, Br). However, the limitations of perovskite solar cells include their sensitivity to moisture and unstable perovskite crystallinity at ambient temperatures, which hinders their long-term stability in solar cell applications12. Generally, Pb-based perovskite compounds have been widely examined in many research studies. The toxicity of Pb-based perovskite compounds has raised concerns regarding their application as perovskite solar cells. However, Pb-based perovskite compounds possess advantageous properties, including a high absorption coefficient and a narrow optical bandgap13. To mitigate toxicity, Pb-based perovskite solar cells can be sealed using an encapsulation method to prevent Pb ion leakage, ensuring environmental protection14.
Previous research has demonstrated various approaches to improve perovskite solar cell stability by modifying the perovskite layer through variation of precursor ratios15, surface engineering16, additive engineering17, and doping18. Recently, several studies highlighted the impact of elemental doping in the perovskite layer, such as Sb- and Bi-co-doped MAPbBr318, Cs-doped MAPbI319, and Cs- and Rb-doped FAPbI3 compounds20, in enhancing the phase stability of the perovskite-structure. Researchers confirmed that the phase stability of various perovskite-based compounds used in the perovskite layer of these solar cells can be significantly improved through elemental doping.
This research aims to apply formamidinium lead iodide (FAPbI3) as a perovskite layer due to its low cost and facile synthesis with concurrent thermal stability21. However, a key drawback of FAPbI3 is the unstable crystallinity of its perovskite structure22. Generally, FAPbI3 crystal structures have two forms. These are cubic (\(\alpha\)-FAPbI3), which exhibits a perovskite structure, and hexagonal (\(\delta\)-FAPbI3) with its non-perovskite form. The \(\alpha\)-FAPbI3 phase is stable above 150 °C, while the \(\delta\)-FAPbI3 phase is stable at room temperature23. Moreover, previous research reported the fabrication of a large-scale perovskite solar cell module (30 × 30 cm) using 1 mol of PbI2, 0.88 mol of FAI, 0.613 mol of MACI, and 0.12 mol of CsI, dissolved in a 5:1 ratio of DMF/NMP solvents for practical applications24.
The goal of this research is to enhance the phase stability of the FAPbI3 crystal structure by doping it with varying levels of Bi. Previous studies on Bi-doped perovskite compounds have shown that Bi-doping in the CsSnI₃ perovskite layer improves its stability by substituting Bi at the B site in the perovskite structure25. The influence of Bi-doping into the MAPbBr3 perovskite layer improves its optical properties and phase stability26. Previous research reported that the influence of Bi doping in MAPbI2Br improves the structural phase and solar cell efficiency of perovskite solar cells27. Therefore, doping a perovskite layer is an effective process for improving phase stability and solar cell efficiency28. However, Bi-doped FAPbI3 perovskite films have not been thoroughly studied. Previous research investigated the local structure of the FAPbI3 perovskite layer, demonstrating improved phase stability due to a shorter Pb-I bonding distance, as observed through (XAS)15. However, the local structure of Bi-doped FAPbI3 perovskite films has not been investigated using XAS. Therefore, this work focuses on a precise investigation of the local structure of Bi in the FAPbI3 perovskite structure using XAS, along with calculation of XANES spectra using the FEFF9 code in combination with density functional theory. This examination provides an approach to improving the crystallinity and stability of the cubic FAPbI3 structure.
In this work, we aim to comprehensively study the local structure of Bi-doped FAPbI3 films at various dopant levels (0.5, 1, 1.5, and 2%) using X-ray absorption spectroscopy. Pristine FAPbI3 and Bi-doped FAPbI3 films were spin-coated at 5000 rpm for 20 s. The structural phases of all films were examined using XRD. The light absorption properties of all films were analyzed with ultraviolet–visible spectroscopy (UV–Vis). Surface morphology was explored using scanning electron microscopy (SEM). PL was employed to evaluate light emissions due to carrier recombination of perovskite films. Local structures were investigated using (XAS), with calculated XANES spectra, performed with the FEFF9 code.
Experimental methodology
Materials
Indium tin oxide (ITO) coated glass slides (6 ~ 8 Ohm/sq, thin film: 185 nm, transmittance: > 83%) were purchased from Biotain Crystal Company. Lead iodide (PbI2) (99%), formamidinium iodide (FAI), methylammonium chloride (MACl), bismuth iodide (BiI3) (99%), diethyl ether, dimethyl sulfoxide (DMSO), and N–N dimethylformamide (DMF) were sourced from Sigma-Aldrich. Gamma-butyrolactone solvent was obtained from Acros Organics.
Synthesis
For preparation of FAPbI3 crystals, 1 molar PbI2 and FAI precursors were dissolved in 5 ml of gamma-butyrolactone and stirred for 2 h at 70 \(^\circ{\rm C}\). The solution was then heated at 150 \(^\circ{\rm C}\) to crystallize FAPbI3. After that, the crystals were cleaned multiple times using diethyl ether and subsequently dried at 100 \(^\circ{\rm C}\) to obtain pure FAPbI3. For preparation of a pristine FAPbI3 solution, 663 mg of the purified FAPbI3 crystals and 0.2 mol of an MACl commercial precursor were dissolved in 71 \(\mu\) l of DMSO and 568 \(\mu\) l of DMF. The mixture was stirred for 2 h at 80 \(^\circ{\rm C}\) until a homogeneous perovskite solution was achieved. This solution was passed through a syringe filter (13 mm diameter, 0.22 \(\mu\) m pore size) to obtain a pristine FAPbI3 solution. For preparation of Bi-doped FAPbI3 solutions, the BiI3 precursor at various dopant levels (0.5, 1, 1.5, and 2%), 663 mg of pure FAPbI3 crystals, and 0.2 mol of an MACl commercial precursor were dissolved in 71 \(\mu\) l of DMSO and 568 \(\mu\) l of DMF solvents. The solution was stirred for 2 h at 80 \(^\circ{\rm C}\) to form a homogeneous solution. Finally, the solution was filtered to obtain a Bi-doped FAPbI3 solution.
For preparation of pristine and Bi-doped FAPbI3 films, ITO glass slides were cleaned in an ultrasonic cleaner with alconox, acetone, and isopropyl alcohol for 90 min each, followed by drying. Before coating, the cleaned ITO glass slides were UV-ozone treated for 15 min. Perovskite solutions were spin-coated onto ITO glass slides at 5000 rpm for 20 s. Antisolvent diethyl ether was dropped onto the perovskite film 15 s after spinning. Finally, perovskite films were heated at 150 \(^\circ{\rm C}\) for 10 min to form pristine and Bi-doped FAPbI3 films.
Characterizations
The phase structures of all samples were analyzed using X-ray diffractometry (D8 Advance, Bruker). Their light absorption properties were evaluated under ultraviolet–visible spectrophotometry (Thermo Scientific Orion, AquaMate 8000). The surface morphology was examined with scanning electron microscopy (Jeol JSM-6010LV). Their local structure was further investigated using XAS. The XAS experiments were conducted at BL-1.1 W of the Synchrotron Light Research Institute, Thailand, with a beam current of 80–120 mA and a storage ring at 1.2 GeV. XAS spectra were measured in the fluorescence mode using a 19-element Ge detector for measuring the Pb L3-edge (13,035 eV) and Bi L3-edge (13,419 eV) XAS spectra. Beamline specifications include photon energy (4–18 keV), photon flux (1–8 \(\times\) 109 photon/s), and energy resolution of the BL-1.1 W (10–4). Normalization and background correction of the XAS spectra were done using ATHENA software.
Computational details
The structural model of the cubic FAPbI3 structure (a = 6.361 Å, b = 6.361 Å, c = 6.361 Å) was based on data from Weller et al., 1979. Density functional theory (DFT) calculations were done using the Vienna Ab initio Simulation Package (VASP) for structural relaxations29. To approximate the exchange–correlation energy, the generalized gradient approximation (GGA), proposed by Perdew, Burke, and Ernzerhof30, was used to approximate the exchange–correlation energy. Electron wave functions were described using the projector augmented wave (PAW) method31, with a plane wave cutoff energy of 520 eV. Brillouin zone integration was performed with k-points that were chosen using 11 × 11 × 11 Monkhorst–Pack grids32. Full relaxation was attained when the total energy and force difference between cycles were less than 0.1 meV/atom and 1 meV/Å, respectively, with self-consistent iterations. van der Waals (vdW) interactions were incorporated using the Grimme DFT-D3 approach33. Structural models of Bi substitution in each of the FA and Pb sites in the cubic FAPbI3 structure were used to simulate XANES spectra, which were calculated using the FEFF9 code.
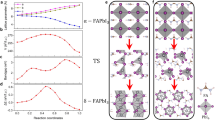
Figure 1(a) depicts the atomic structures of FAPbI3, consisting of 12 atoms: 1 C atom, 2 N atoms, 5 H atoms, 1 Pb atom, and 3 I atoms. The simulated Bi3+ substitution for the Pb atom of FAPbI3 is shown in Fig. 1(b). Bi3+ substitution at the FA site of FAPbI3 is depicted in Fig. 1(c). The fully relaxed atomic structures of hexagonal FAPbI3 within space group P63/m is shown in Fig. 1(d), which are made up of 84 atoms: 18 C atoms, 12 N atoms, 30 H atoms, 6 Pb atoms, and 18 I atoms. The calculated lattice parameters in comparison with previous works are shown in Table 1.
The optimized structural models, simulated by DFT calculation, of (a) the cubic FAPbI3 phase crystal structure within space group Pm-3 m. The predicted Bi substitution for the Pb atom of the cubic FAPbI3 phase is shown in (b). (c) Bi substitution at the cubic FAPbI3’s FA atom location. (d) The atomic structures of the hexagonal FAPbI3 phase within space group P63/m.
The formation energy (Ef) measures the energy shift that occurs when a material forms a compound or introduces a defect from its constituent elements or pristine state. It offers insightful information about the viability and stability of different stages or flaws. The defect formation energy, or the energy needed to replace Pb or FA in FAPbI3 with Bi, was calculated as34.
Here, Et (pristine) is the total energy of a flawless FAPbI₃ structure, and Et (defect) is the total energy of the structure with Bi substitution. The chemical potentials of Pb or FA (removed from the pristine structure) and Bi (substituted) are denoted by μl and μs(Bi), respectively.
An energetically favorable compound or defect production is indicated by a negative formation energy. This implies that when the compound or defect arises, the system will release energy, resulting in a lower total energy state. A positive value of formation energy indicates that the process of generating the compound or defect is energetically unfavorable, meaning it is less likely to occur naturally and necessitates the input of energy as heat (thermal energy), pressure (mechanical energy), chemical reactions, electrical energy, or photon energy. Our calculated formation energy (Ef) for Bi substitution in both Pm-3 m and P6₃/m FAPbI3 shows that Bi substitution for Pb has an Ef of 1.01 eV and 1.45 eV for Pm-3 m and P6₃/m FAPbI3, respectively. In contrast, Bi substitution for FA has an Ef of 3.13 eV and 2.36 eV for Pm-3 m and P6₃/m FAPbI3, respectively. These results indicate that Bi substitution at the Pb site in both Pm-3 m and P6₃/m FAPbI3 is more energetically favorable than at the FA site, as a lower formation energy reflects a more favorable substitution process. The calculated formation energies are shown in Table 1.
Results and discussion
SEM analysis
The surface morphology of the perovskite films in the current study was investigated using SEM. These results are shown in Figs. 2(a-e). SEM imagery indicates orderly grain arrangements. For 0.5 and 1% Bi-FAPbI3 films, SEM shows larger grain sizes with smooth surfaces compared to the pristine FAPbI3 film. The SEM results for the 1.5% Bi-FAPbI3 film reveal irregular grain shapes. The 2% Bi-FAPbI3 film displays grains with various grain sizes, with pin-holes in its surface. This result suggests that Bi doping at 0.5 and 1% in pristine FAPbI3 film improves the morphology by increasing grain sizes on their surfaces. Bi doping beyond 1.5 to 2% negatively affects the formation of grains on their surfaces. The average thicknesses of films are approximately 500–800 nm (pristine), 669.47 ± 0.05 nm (0.5% Bi), 852.67 ± 0.06 nm (1% Bi), 953.93 ± 0.11 nm (1.5% Bi), and 991.8 ± 0.12 nm (2% Bi), as illustrated in the supporting information.
XRD analysis
Figure 3(a) shows the XRD patterns over the 2\(\theta\) range from 10° to 45° of all films. The XRD pattern of a pristine FAPbI3 film exhibits a mixture of hexagonal and cubic FAPbI3 structures, indicating non-perovskite and perovskite phases, respectively. A characteristic hexagonal FAPbI3 structure appears at 11.8° and 26.3°, while the cubic FAPbI3 structure shows peaks at 13.9°, 24.3°, 28.1°, 31.5°, 34.6°, 40.2°, and 42.7°. These results are confirmed by the calculated XRD spectrum of the cubic FAPbI3 structure using Vesta software. The XRD pattern of the pristine film further reveals that the peak intensity of the hexagonal phase (\(\delta\)-FAPbI3) is greater than that of the cubic phase (\(\alpha\)-FAPbI3), suggesting that the pristine FAPbI3 film predominantly exhibits a non-perovskite structure, indicating unstable crystallinity under ambient conditions39. XRD patterns of Bi-doped FAPbI3 films with varying Bi-dopant levels (0.5 to 2%) reveal that the main cubic crystal FAPbI3 structure is situated at 2\(\theta\)=13.9° and shows the smaller peak intensity of the hexagonal FAPbI3 structure. These results indicate that the influence of Bi-doping on the pristine FAPbI3 film suppresses the growth of the hexagonal FAPbI3 structure, shown by its decreased XRD peak intensity. The crystallinity of the cubic FAPbI3 structure is improved with increased XRD peak intensity, as shown by the arrows in Figs. 3. Secondary phases are detected as BiOCl at 12.1°, increasing with Bi levels from 1 to 2%, and PbI2 at 11.7° for only the 1.5% Bi-dopant level. However, Bi-doping reduces the intensity of hexagonal phase peaks and increases the cubic phase peak at 13.9°. This result suggests that the local site of the Bi ion replaces the Pb ion in the FAPbI3 structure, as indicated by the lowest calculated formation energy (Ef) in Table 1, inducing growth of the cubic FAPbI3 structure. This implies that the Bi ion preferentially bonds with FAPbI3 in the cubic perovskite structure rather than in the hexagonal structure. Moreover, time-dependent XRD patterns of all films are shown in Figures S5-S9, in the supporting information, to analyze phase stability at various times, including 1, 6, and 16 days. These results show that a decreased XRD peak intensity of a cubic structure is observed after 16 days for a pristine FAPbI3 film. For all conditions of the Bi-doped FAPbI3 films, the cubic FAPbI3 structure does not degrade under ambient conditions even after 6 and 16 days.
Figure 3(b) shows XRD patterns over the 2\(\theta\) range from 13° to 15°. The primary peaks of Bi-doped FAPbI3 films at 13.9°, corresponding to the cubic structure, are slightly shifted toward lower 2\(\theta\) angles due to Bi-doping. This shift suggests an increased d-spacing within the cubic FAPbI3 structure, as explained by Bragg’s law. Additionally, this significant shift toward a lower 2θ angle is caused by Bi doping in the perovskite structure, suggesting that Bi replaces Pb in the cubic FAPbI3 structure. This is confirmed by formation energy (Ef) calculations based on DFT, as shown in Table 1. These results induce increased lattice strain in the FAPbI3 structure, as presented in Table 2. This result improves the crystallinity and stabilization of the cubic FAPbI3 structure because the formation energy of Bi doping in the Pb site for a cubic perovskite structure (1.01 eV) is less than the hexagonal structure (1.45 eV). Moreover, the shift of the main XRD peaks confirms substitution of Bi atoms into Pb sites, validated by XAS analysis. The average crystallite sizes of pristine and Bi-doped FAPbI3 films were calculated using the Debye–Scherrer relation (Eq. 2) with these results shown in Table 2.
D is the average crystallite size, k is the Scherrer constant (0.94), λ is the wavelength (0.154 nm) of incident X-ray irradiation, β is the full width at half maximum (FWHM) of the XRD peak, and θ is the Bragg’s diffraction angle, considered for the XRD peak of interest. These calculations indicate that Bi-doping at various levels affects the average crystallite size of the pristine FAPbI3 film. For 1 and 2% Bi-doped FAPbI3 films, the crystallite sizes increase to 67.21 and 72.85 nm, respectively, compared with 66.73 nm for pristine FAPbI3. However, 0.5 and 1.5% Bi-doped FAPbI3 films show smaller crystallite sizes, 55.96 and 54.3 nm, respectively. However, the average crystallite size obtained from the Debye–Scherrer equation for all samples does not exhibit trend values affected by instrumental broadening. These effects arise from various sources of instrumental broadening, including detector resolution, sample transparency, wavelength dispersion, and non-ideal optics. Additionally, lattice strain (\(\varepsilon\)) and d-spacing (d) values were calculated using the Williamson-Hall and Bragg’s law equations. These results are shown in Table 2. The Williamson-Hall and Bragg’s law equations are presented as Eqs. 3 and 4, respectively.
These results indicate that Bi-doping of a pristine FAPbI3 film does not significantly affect the d-spacing when compared to a pristine film. Lattice strain values of the films decrease as the average crystallite size increases, based on the calculated results. Additionally, crystallite sizes and lattice strain values of samples were calculated using a Williamson-Hall plot, as shown in Fig. 4, for an exact evaluation of thin films.
The results show that the crystallite size of the FAPbI3 perovskite film increases with Bi-dopant levels from 0.5 to 2%. Additionally, the lattice strain increases with greater Bi-doping, influenced by the larger crystallite sizes. There is a discrepancy in crystallite size values between these two approaches, with no clear trend from the Debye–Scherrer equation. This is likely due to lattice stress in the perovskite films. The Williamson-Hall plot approach can be simplified to the Scherrer equation when internal stress is zero. However, for non-zero residual stress, the Scherrer equation yields smaller crystallite sizes than the Williamson-Hall plot. Therefore, the Williamson-Hall plot is considered the more accurate approach in this work, which aligns with an earlier report comparing methods for estimating average crystallite size and strain40. The fitted values, including intercept, slope, standard error, and R-square, of the W–H plot of all samples are shown in the supporting information. Lattice constants, obtained from XRD data, of all samples were calculated using Eq. 6 for the cubic structure.
In this calculation, the XRD peak position at 2θ = 13.98°, corresponding to the (001) plane of the cubic FAPbI3 structure, was used to calculate the lattice constants. The results show that the lattice constants for all samples are 0.63 nm. This finding suggests that the lattice constants obtained from the XRD data correspond to the calculation of the optimized structure, as calculated by DFT.
UV–Vis analysis
The light absorption properties of all films were analyzed using UV–Vis, with these results shown in Fig. 5. The UV–Vis spectrum of pristine FAPbI3 film indicates light absorption with an absorption edge at approximately 819 nm, confirming light response in the visible-near infrared range. For 0.5 and 1.5% Bi-doped FAPbI3 films, the UV–Vis results show light absorption from the visible to near infrared range with absorption edges at around 828 and 830 nm, respectively. These findings suggest a redshift in the optical properties of the 0.5 and 1.5% Bi-doped films, with absorption edges shifting toward longer wavelengths compared to a pristine film. In the case of the 1 and 2% Bi-doped FAPbI3 films, the UV–Vis results present absorption edges at approximately 836 nm, also indicating a light response in the visible-near infrared range. These results further demonstrate a redshift in the optical properties of the 1 and 2% Bi-doped films compared to a pristine material. Moreover, optical properties of the 1 and 2% Bi-doped FAPbI3 films indicate a maximum shift of absorption edges toward longer wavelengths, representing a redshift, compared with other conditions.
The shift toward longer wavelengths in all Bi-doped FAPbI3 films suggests a narrower optical band gap, indicating a decreased band gap from the pristine film. Additionally, Bi incorporation into the FAPbI3 structure, as confirmed by the XAS results, affects lattice distortion, suggesting a decreased optical band gap in the pristine FAPbI3 film41,42. This result implies that Bi-doping enhances the optical properties of a pristine film by improving light absorption at longer wavelengths. The optical band gaps displayed in Fig. 6 were calculated using the Tauc plot method. The optical band gap of pristine FAPbI3 film is 1.52 eV, while the optical band gaps of Bi-doped FAPbI3 films with varying Bi-dopant contents (0.5, 1, 1.5, and 2% Bi) are 1.52, 1.48, 1.50, and 1.48 eV, respectively. These findings demonstrate the influence of Bi-doping on a pristine FAPbI3 film.
XAS analysis
XAS is a powerful technique for investigating the electronic and local structures of materials43. In this study, XAS was used to investigate the oxidation states and local structures of Bi ions in perovskite films. Generally, an XAS spectrum is obtained by exciting the core electron of an absorbing atom using X-ray radiation. The excited core electron then transitions to an unoccupied state, generating an XANES. The distinct features of the XANES spectrum are utilized for analyzing the local structure of the absorbing atom, including its surrounding atoms. In this research, the local structures of Bi atoms in Bi-doped FAPbI3 films, with Bi-dopant levels ranging from 0.5 to 2%, were carefully examined by comparing the specific features of experimental XANES spectra with calculated XANES spectra. Structural models of Bi substitution in FA and Pb sites in cubic FAPbI3 crystals were developed based on density functional theory (DFT) for calculating XANES spectra, using the FEFF9 code.
The absorption edges of Pb and Bi L3-edge XANES spectra of all samples were determined in Athena software by considering the peak position of the first derivative, which provided the absorption edge value of each sample. The normalized Pb L3-edge XANES spectra of pristine and Bi-doped FAPbI3 films with varying Bi-dopant levels, compared with Pb-based oxide compounds such as Pb foil, PbO, and PbO2, are shown in Fig. 7.
of all conditions, compared with Pb foil, PbO, and PbO2 compounds.
The experimental Pb L3-edge XANES spectra displays an absorption edge at 13,036 eV, corresponding to the absorption edge of PbO (13,036 eV). This was used to analyze the oxidation states of Pb ions. These results suggest that the oxidation state of films under all conditions is due to Pb2+ ions. Additionally, normalized XANES spectra of Pb foil (Pb0) and PbO2 (Pb4+) compounds exhibit absorption edges at 13,032 and 13,039 eV, respectively. The normalized Bi L3-edge XANES spectra of Bi-doped FAPbI3 films, compared with Bi2O3, are displayed in Fig. 8. The experimental Bi L3-edge XANES spectra of films under all conditions demonstrate an absorption edge at 13,434 eV, corresponding to the absorption edge of Bi2O3. These results confirm that the oxidation states in all films arose from Bi3+ ions.
compared with Bi2O3.
Structural models of the Pb site in cubic and hexagonal FAPbI3 crystals were calculated using FEFF9 code to generate the specific features of the XANES spectrum, representing its local structure. The calculated XANES spectra (navy and purple color) and experimental Pb L3-edge XANES spectra of all conditions are shown in Fig. 9. The experimental Pb L3-edge XANES spectra present the characteristic features of XANES spectra at 13,044, 13,059, 13,089, and 13,121 eV. These features correspond to the characteristics of the calculated XANES spectrum of cubic FAPbI3 crystal, as indicated by arrows. This result suggests that the local structures of Pb atoms in all films are arranged in the form of a Pb-I octahedral bonding configuration, characteristic of the FAPbI3 perovskite structure.
To verify the local structure of Bi atoms in Bi-doped FAPbI3 films, models of Bi substitution at Pb and FA sites in cubic and hexagonal FAPbI3 crystals were calculated using the FEFF9 code. This was done to generate the specific features of calculated XANES spectra. The calculated XANES spectra, showing Bi substituted into Pb and FA sites in cubic and hexagonal FAPbI3 crystal, along with the experimental XANES spectra of Bi-doped FAPbI3 films, are displayed in Fig. 10. The experimental Bi L3-edge XANES spectra correspond to the calculated XANES spectrum for Bi substitution at the Pb site in a cubic FAPbI3 crystal, specifically at 13,439, 13,453, 13,483, and 13,520 eV, indicated by arrows. These experimental XANES spectra show good agreement with the calculated XANES spectrum of Bi substitution at Pb sites in cubic FAPbI3 crystals (green). This result suggests that the local structure of Bi atoms in these films corresponds to a local environment where Bi replaces Pb in cubic FAPbI3 crystals, calculated using the FEFF9 code. Conversely, the experimental XANES spectra of the films do not align with the calculated XANES spectra of other structural models.. This finding confirms that Bi atoms under all conditions exclusively replace Pb in a cubic FAPbI3 crystal, as validated by XANES calculations. Additionally, a comparison of the experimental Bi and Pb L3-edge XANES spectra is shown in Fig. 11 to confirm Bi substitution into the Pb sites of cubic FAPbI3 crystals. These results show that the specific features of experimental Bi L3-edge XANES spectra match the characteristic features of experimental Pb L3-edge XANES spectra, particularly at energies (E-E0) of 16, 27, 54, and 90 eV. This comparison indicates that the local structures of Bi atoms under all conditions corresponds to the local site of the Pb atom in cubic FAPbI3 crystals, indicating that Bi atoms are surrounded and bonded in the perovskite structure. DFT results suggest that the formation energy of the Bi-substituted Pb site (1.01 eV) in the cubic FAPbI3 model is three times lower than that of the Bi-substituted FA site (3.13 eV). This calculation suggests that a Bi atom replaces Pb in the cubic FAPbI3 structure. XAS result suggests that Bi substitution at Pb sites enhances the structural phase of pristine FAPbI3 for long-term phase stabilization, as shown in XRD results44. Formation energies of structural models calculated by substituting Bi at FA and Pb sites in cubic FAPbI3 crystals were calculated based on DFT. These calculations show that the formation energy of Bi-replacement at the Pb sites in cubic FAPbI3 crystals (1.01 eV) is lower than that of Bi-replacement at the FA sites in these crystals (3.13 eV), as shown in Table 1. This result confirms that a Bi atom preferentially replaces Pb in cubic FAPbI3 crystals, in agreement with experimental and calculated XAS results. However, some energy points of the calculated XANES spectra do not match those of the experimental spectra because the calculations, performed using the FEFF9 code, are based on structural models that only approximate the energies of XANES features. This approximation may contribute to the energy variation observed between the calculated and experimental spectra. Nevertheless, the specific features of the XANES spectra are still useful for analyzing the local structure of the samples. Therefore, the local structure of the Bi atom in the samples is investigated by analyzing these characteristic features. Notably, discrepancies between the experimental data and the simulations may indicate that the assumed local substitution sites in the models do not accurately reflect the actual substitution sites in the structure.
Photoluminescence analysis
Photoluminescence spectroscopy was used to evaluate carrier recombination in perovskite films by analyzing their photoluminescent properties. The photoluminescence (PL) spectra were obtained at a 405 nm excitation wavelength, as shown in Fig. 12. All samples exhibit light emission at a 771.5 nm wavelength. The PL intensity of 0.5% Bi-FAPbI3 film is comparable to that of a pristine FAPbI3 film. However, the 1% Bi-FAPbI3 film shows higher PL intensity than both the pristine and 0.5% Bi-FAPbI3 films, indicating an increase in photogenerated charge carriers and suppressed non-radiative recombination. In contrast, the PL intensity for 1.5 and 2%Bi-FAPbI3 films is lower than that of a pristine film, suggesting a reduction in photogenerated charge carriers and increased non-radiative recombination. Generally, decreased PL spectra in perovskite films are related to surface morphology, which affects photogenerated charge carriers and non-radiative recombination. Therefore, the PL intensities of 1.5 and 2% Bi-doped FAPbI3 films decrease due to the formation of irregularly shaped grains of various sizes on their surfaces, as shown in Fig. 2. This leads to an increased density of defect or trap states and a consequent reduction in PL intensity45.These observations demonstrate that a 1% Bi dopant concentration significantly enhances the photogenerated charge carrier properties of pristine FAPbI3, whereas higher dopant levels (1.5 and 2%) negatively impact the PL characteristics due to excessive Bi incorporation. Steady-state photoluminescence (PL) measurements provide valuable insights into the optoelectronic properties of materials and device efficiency46. For semiconductor materials, higher PL intensity generally corresponds to a greater concentration of photogenerated charge carriers, leading to increased radiative recombination47,48. This suggests that incorporating Bi at the 1% level enhances charge carrier dynamics by reducing defect-related recombination or suppressing non-radiative pathways49,50. Generally, the influence of Bi doping with various concentrations in perovskite films can significantly improve the electronic band structure by reducing the defect states that suppress the carrier recombination. However, increasing Bi doping beyond 1.5% leads to a decreased PL intensity in the FAPbI3 film due to irregularly shaped grains on its surface45, as shown in the SEM imagery. Consequently, the influence of Bi doping beyond 1.5% in Bi-doped FAPbI3 films improves the structural phase, according to XRD results, but does not enhance their PL properties. Therefore, a 1% Bi-doping concentration is optimal for pristine FAPbI3 films, enhancing their optical properties and making them suitable for optoelectronic applications.
Computational analysis
The calculated band structures and density of states (DOS) for cubic FAPbI3 are shown in Fig. 13(a). FAPbI3 exhibits a direct band gap at the E-point, measuring 1.43 eV, which aligns well with previous work reported using the PBE approximation (1.43 eV)35. This observed band gap confirms its semiconductor behavior. In the valence band maximum (VBM) region, the states are primarily composed of I p orbitals, spanning 0 eV to −3 eV, while the conduction band minimum (CBM) mainly consists of Pb p orbitals. N p states are primarily located between −2.6 and −3 eV in the valence band. The band structures and DOS of Bi substitution into the Pb site in cubic FAPbI3 are shown in Fig. 13(b). When Bi replaces Pb in cubic FAPbI3, the Fermi energy crosses the conduction band due to the donor defect introduced by the Bi p state. However, as reported in previous work on Eu-doped FAPbI351, electron excitation can still occur below the Fermi energy. Our results indicate that the band gap energy is 0.76 eV. Similarly, the band structures and DOS of Bi substitution into the FA site in FAPbI3 are shown in Fig. 13(c). When Bi replaces the FA in FAPbI3, the Fermi energy crosses the conduction band, but a band gap of approximately 1.72 eV remains, as shown in Fig. 13(d). In the case of the hexagonal structure of FAPbI3 within space group P6₃/m, the observed band gap confirms its semiconductor behavior, with a band gap energy of 1.91 eV. In the valence band maximum (VBM) region, the states are primarily composed of I p orbitals, spanning 0 to −3 eV, while the conduction band minimum (CBM) mainly consists of Pb p orbitals. The N p states are primarily located at −4 eV in the valence band. When Bi is substituted at the Pb site in hexagonal FAPbI3, the Fermi energy crosses the conduction band due to the donor defect introduced by the Bi p state. The band structures and DOS of Bi substituted at the Pb site in hexagonal FAPbI3 are shown in Fig. 13(e). In this case, the Fermi energy crosses the conduction band, but a band gap of approximately 1.94 eV remains. The band structures and DOS of Bi substitution at the FA site in hexagonal FAPbI3 are shown in Fig. 13(f). Similarly, when Bi replaces the FA site in hexagonal FAPbI3, the Fermi energy also crosses the conduction band, with a remaining band gap of approximately 2.03 eV. Interestingly, as we know, the PBE approximation tends to underestimate the band gap energy. However, PBE functional calculations can provide reasonable electronic properties for hybrid lead-iodine perovskite structures52,53. Our results show that in both cubic and hexagonal FAPbI3, when Bi substitutes for Pb, the band gap of FAPbI3 decreases. Conversely, when Bi replaces FA, the band gap also increases. Based on our results from formation energy, UV–Vis spectroscopy, and band gap analysis, we can confirm that in our experiment, Bi substitution occurs at the Pb site in FAPbI3.
The calculated band structures and density of state (DOS) of (a) cubic FAPbI3 within space group Pm-3 m FAPbI3. (b) Bi atom substitution at the Pb site in the cubic FAPbI3 structure. (c) Bi atom substitution at the FA site in cubic FAPbI3 structures. (d) Hexagonal structure of FAPbI3 within space group P63/m FAPbI3. (e) Bi atom substitution at the Pb site in the hexagonal FAPbI3 structure. (f) Bi atom substitution at the FA site in the hexagonal FAPbI3 structure.
Conclusions
Pristine and Bi-doped FAPbI3 perovskite films were prepared using a spin-coating method at 5000 rpm for 20 s. XRD results indicate that the pristine FAPbI3 film exhibits both non-perovskite (hexagonal phase, δ-FAPbI3) and perovskite structures (cubic phase, α-FAPbI3), suggesting unstable crystallinity. In contrast, Bi-doped FAPbI3 films consistently show a stable perovskite structure, with crystallite size increasing with Bi incorporation. The crystallite sizes of pristine and 2% Bi-doped FAPbI3 films, calculated using the Debye–Scherrer equation, are 66.73 nm and 72.85 nm, respectively. Those calculated using the Williamson-Hall plot are 72.19 nm and 135.88 nm. These calculations suggest that Bi-doping enhances the crystallinity of the structural phase of FAPbI3. UV–Vis results reveal that the pristine FAPbI3 film absorbs in the visible-near infrared range, while Bi-doped films show a redshift, indicating a narrower optical band gap. The calculated optical band gaps of pristine, 0.5, 1, 1.5, and 2% Bi-doped FAPbI3 films are 1.49, 1.49, 1.47, 1.44, and 1.43 eV, respectively. These results indicate that the effects of Bi-doping improve to decrease optical band gap of FAPbI3 films. Normalized Pb L3-edge XANES spectra of all films display an absorption edge at 13,036 eV corresponding to the oxidation state of Pb2+ ions, compared with the absorption edge of the PbO compound. Normalized Bi L3-edge XANES spectra of Bi-doped FAPbI3 under all conditions indicate an absorption edge at 13,434 eV corresponding to the oxidation state of Pb2+ ions, compared with the absorption edge of the PbO compound. XANES results confirm that Pb and Bi ions exist in the Pb2+ and Bi3+ oxidation states. The experimental Bi L3-edge XANES spectra show specific features at 13,442, 13,455, 13,485, and 13,520 eV, which resemble the characteristic features of the calculated XANES spectrum for Bi replacing Pb in the cubic FAPbI3 crystal. Calculated spectra suggest that Bi ions replace Pb in the perovskite structure. PL results demonstrate that Bi doping reduces PL intensity, especially at 1% Bi, indicating decreased carrier recombination.
Data availability
All data generated or analyzed during this study are included in this published article. [and its supplementary information files].
References
Aslan, A. et al. Effect of economic policy uncertainty on CO2 with the discrimination of renewable and non renewable energy consumption. Energy 291, 130382 (2024).
Zhang, X. et al. Resource utilization of solid waste in the field of phase change thermal energy storage. J. Energy Storage 58, 106362 (2023).
Asghar, H., Riaz, T., Mannan, H. A., Khan, S. M. & Butt, O. M. Rheology and modeling insights into dye-sensitized solar cells (DSSCs) material: Bridging the gap to solar energy advancements. Renew. Sustain. Energy Rev. 193, 114298 (2024).
Lee, J. & Shepley, M. M. Benefits of solar photovoltaic systems for low-income families in social housing of Korea: Renewable energy applications as solutions to energy poverty. J. Buil. Eng. 28, 101016 (2020).
Khan, W. A. et al. Carbon nanomaterials in coatings: A review focusing thin film photovoltaic solar cells. Mater Sci Semicond Process 185, 108929 (2025).
Mahjoory, A., Karimi, K., Teimouri, R., Mohammadpour, R. & Kolahdouz, M. Thin film Cs2TiBr 6 perovskite solar cell incorporating functionalized PbS colloidal quantum dots as a hole transporting layer. J. Phys. Chem. Solids 196, 112306 (2025).
Bagyalakshmi, S. et al. Optimizing sensitizer concentration from senna tora leaves for enhanced interfacial interactions in dye-sensitized solar cells. Inorg. Chem. Commun. 170, 113224 (2024).
Semab, A. et al. A theoretical study on symmetrical non-fullerene electron acceptors molecules on BDTPT based derivatives to enhance photovoltaic properties of organic solar cells. Comput. Theor. Chem. 1241, 114891. https://doi.org/10.1016/j.comptc.2024.114891 (2024).
Khalifa, M., Dkhili, M., Aouida, S. & Ezzaouia, H. Optimizing electron transport layers for high-efficiency perovskite solar cells using impedance spectroscopy. Sol. Energy Mater. Sol. Cell. 278, 113196 (2024).
Madani, M. et al. A study of kapton as a flexible substrate for perovskite solar cells; advantages and disadvantages. Opt. Mater. (Amst) 154, 115697 (2024).
Karishma, et al. Efficiency assessment of perovskite solar cells: A focus on hole transporting layers. Sol. Energy 282, 112967 (2024).
Zhao, H. et al. High performance and stability of perovskite solar cells achieved through anti-solvent modulation of multi-orientation PbI2 and stress relief. Sol. Energy Mater. Sol. Cell. 277, 113115 (2024).
Green, M., Ho-Baillie, A. & Snaith, H. The emergence of perovskite solar cells. Nat. Photon. 8, 506 (2014).
Wang, R. et al. A review of perovskites solar cell stability. Adv. Funct. Mater. 29, 1808843 (2019).
Kang, D.-H., Park, Y.-J., Jeon, Y.-S. & Park, N.-G. Extended X-ray absorption fine structure (EXAFS) of FAPbI3 for understanding local structure-stability relation in perovskite solar cells. J. Energy Chem. 67, 549–554 (2022).
Chen, X. et al. Surface engineering for enhanced efficiency and stability of perovskite solar cells through Lewis acid-base modified tin oxide electron transport layers. Mater. Today Energy 41, 101531 (2024).
Zheng, L., Zhao, Y., Zhao, R., Xie, L. & Hua, Y. A. Additive engineering via multiple-anchoring enhances 2D perovskite solar cells’ performance††Electronic supplementary information (ESI) available. See. Chem. Commun. 60, 7487–7490. https://doi.org/10.1039/d4cc01590h (2024).
Mkawi, E. M. et al. Enhancement the performance of MAPbI3 perovskite solar cells via germanium sulfide doping. Opt. Mater (Amst) 157, 116089 (2024).
Batool, R., Mahmood, T., Mahmood, S. & Bhatti, A. A. A computational study of alkali (Na, K, Cs) doped methylammonium lead iodide perovskite. Physica B. Conden. Matter. 672, 415469 (2024).
Zhang, L. et al. Quantitative phase analysis on Cs- and Rb-doped FAPbI3 and corresponding solar cell efficiency simulations. Sol. Energy 188, 224–229 (2019).
Bechir, M. B., Almeshal, A. & Dhaou, M. H. Structural evolution, dielectric relaxation, and charge transport characteristics of formamidinium lead iodide (FAPbI3) perovskite. Mater. Res. Bull. 157, 112012 (2023).
Xie, Y. et al. Competing formation of α and δ-FAPbI3 in perovskite ink using 2-methoxyethanol as the solvent. J. Solid State Chem. 334, 124657 (2024).
Solis, O. E. et al. α-FAPbI3 powder presynthesized by microwave irradiation for photovoltaic applications. Electrochim. Acta 439, 141701 (2023).
Lan, A. et al. Toward commercial-scale perovskite solar cells: The role of ALD-SnO2 buffer layers in performance and stability. ACS Appl. Mater. Interface. 16, 64825–64833 (2024).
Wang, G., Cheng, L., Bi, J., Chang, J. & Meng, F. B-site doping with bismuth ion enhances the efficiency and stability of inorganic CsSnI3 perovskite solar cell. Mater Lett. 354, 135394 (2024).
Yu, J., Xu, H., Wu, L., Han, Q. & Wu, W. Highly sensitive photodetector of Zn/Bi doped MAPbBr 3 single crystals formed homojunction. Mater Sci. Semicond Process 149, 106824 (2022).
Khan, M. et al. Enhancing Photovoltaic Performance of Bi-Doped Perovskite Solar Cells with Novel Double Electron Transport Layer: Achieving High Open-Circuit Voltage and Efficiency. https://doi.org/10.21203/rs.3.rs-4498642/v1(2024).
Kumar, S. et al. Recent advances in perovskite materials: exploring multifaceted properties for energy harvesting applications. Ionics (Kiel) 30, 5159–5188 (2024).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
John, P. & K. B. and M. E.,. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 77, 3865 (1997).
Perdew, J. & Zunger, A. Self-interaction correction to density-functional approximations for many-body systems. Phys. Rev. B 23, 5048–5079 (1981).
Chadi, D. J. Special points for Brillouin-zone integrations. Phys. Rev. B. 16, 1746–1747 (1977).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Du, K. et al. Synthesis and characterization of ZnGeN2 grown from elemental Zn and Ge sources. J. Cryst. Growth. 310, 1057–1061 (2008).
Muhammad, Z. & Rashid, A. Exciton binding energies and polaron interplay in the optically excited state of organic–inorganic lead halide perovskites. Mater. Adv. 6, 13–38 (2024).
Weller, M. T., Weber, O. J., Frost, J. M. & Walsh, A. Cubic perovskite structure of black formamidinium lead Iodide, α-[HC(NH2)2]PbI3, at 298 K. J. Phys. Chem. Lett. 6, 3209–3212 (2015).
Slimi, B. et al. Synthesis and characterization of perovskite FAPbBr 3−xIxthin films for solar cells. Monatshefte für Chemie – Chem. Mon. 148, 835–844 (2017).
Chen, T. et al. Entropy-driven structural transition and kinetic trapping in formamidinium lead iodide perovskite. Sci. Adv. 2, e1601650–e1601650 (2016).
Hidalgo, J. et al. Bulky cation hinders undesired secondary phases in FAPbI3 perovskite solar cells. Mater. Today 68, 13–21 (2023).
Alam, Md. K., Hossain, Md. S., Bahadur, N. M. & Ahmed, S. A comparative study in estimating of crystallite sizes of synthesized and natural hydroxyapatites using Scherrer Method, Williamson-Hall model, Size-Strain Plot and Halder-Wagner Method. J. Mol. Struct. 1306, 137820 (2024).
Kumar, S., Thakur, S., Kaur, G. A., Shandilya, M. & Rai, R. Optical coalition in the electrical and magnetic induction of Dy and Tb-doped BFO-based multiferroic. Appl. Phys. A 129, 21 (2022).
Kumar, S. et al. Interface engineering of composite systems: Focusing on the compatibility of reduced graphene oxide and Bi0.8La0.1Ba0.1Fe0.9Ti0.1O3 hybrid systems. Mat. Today Sustain. 27, 100813 (2024).
Bootchanont, A. et al. Characterization of structural orientation and optical properties of Al and Cr in rubies. Radiat. Phys. Chem. 199, 110315 (2022).
Dehingia, A., Das, U. & Roy, A. Partial replacement of B-site cation to stabilize the optically active cubic phase of FAPbI3 for optoelectronic applications. Mater Today Proc. 65, 29–34 (2022).
McGovern, L., Koschany, I., Grimaldi, G., Muscarella, L. A. & Ehrler, B. Grain size influences activation energy and migration pathways in MAPbBr 3 perovskite solar cells. J. Phys. Chem. Lett. 12, 2423–2428 (2021).
Campanari, V. et al. Re-evaluation of Photoluminescence Intensity as an Indicator of Efficiency in Perovskite Solar Cells. Solar RRL https://doi.org/10.1002/solr.202200049 (2022).
Lacombe, A. et al. Photoluminescence intensity modulation by charge carrier injection in silicon nanocrystals at room temperature. Appl. Phys. Lett. – Appl. Phys. Lett. 94, 012112 (2009).
Wu, H. et al. Boosted charge carrier concentration via luminescent quenching of photoexcited electrons in CdS/FI S-scheme heterostructures for efficient photocatalytic hydrogen production. J. Clean Prod. 425, 138921 (2023).
Feldmann, S. et al. Photodoping through local charge carrier accumulation in alloyed hybrid perovskites for highly efficient luminescence. Nat. Photon. 14, 1–6 (2020).
Yao, J., Wang, H., Wu, J., Zou, B. & Zeng, R. Boosting photoluminescence of rare-earth-based double perovskites by isoelectronic doping of ns metal ions. Small https://doi.org/10.1002/smll.202405724 (2024).
Suzuki, A. & Oku, T. Effects of mixed-valence states of Eu-doped FAPbI3 perovskite crystals studied by first-principles calculation. Mater. Adv. 2, 2609–2616 (2021).
Kim, J., Lee, S.-C., Lee, S.-H. & Hong, K.-H. Importance of orbital interactions in determining electronic band structures of organo-lead Iodide. J. Phys. Chem. C 119, 4627–4634 (2015).
Tang, Z.-K. et al. Enhanced optical absorption via cation doping hybrid lead iodine perovskites. Sci. Rep. 7, 7843 (2017).
Acknowledgements
This work was supported by the Thailand Science Research and Innovation Fundamental Fund fiscal year 2024; the Thammasat University Research Unit in Energy Innovations and Modern Physics (EIMP); and Suranaree University of Technology. Thanks for computing resources provided by Suranaree university of Technology, Thailand. It was also supported by the Development of International Cooperation Network on Science Technology and Innovation, Program Management Unit for Human Resources & Institutional Development, Research and Innovation (PMU-B) of Thailand (which is under the Program 14 N39(S3P18) Grant No. B39G670015, National Higher Education Science Research and Innovation Policy Council. This research has received funding support from the NSRF via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation [Grant Number B37 F660011], National Research Council of Thailand [Grant No. 192042].
Funding
Thailand Science Research and Innovation Fundamental Fund fiscal year 2024; the Thammasat University Research Unit in Energy Innovations and Modern Physics (EIMP), Development of International Cooperation Network on Science Technology and Innovation, Program Management Unit for Human Resources & Institutional Development, Research and Innovation (PMU-B) of Thailand, Grant No. B39G670015, National Higher Education Science Research and Innovation Policy Council, NSRF via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation, Grant No. B37 F660011, National Research Council of Thailand.
Author information
Authors and Affiliations
Contributions
Tirapat Wechprasit and Wasan Maiaugree wrote the main manuscript text. Atipong Bootchanont revised and reviewed the manuscript. Wutthigrai Sailuam wrote and calculated computational details. Yingyot Infahsaeng, Poramed Wongjom, Suttipong Wannapaiboon, Anusit Kaewprajak, Pisist Kumnorkaew, Wittawat Saenrang, and Wisanu Pecharapa reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wechprasit, T., Bootchanont, A., Infahsaeng, Y. et al. Characterization of Bi-doped FAPbI3 perovskite films investigated by X-ray absorption spectroscopy. Sci Rep 15, 18351 (2025). https://doi.org/10.1038/s41598-025-02226-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02226-1