Abstract
In studies evaluating the efficacy of anti-programmed cell death 1/ligand 1 immune checkpoint inhibitors (anti-PD-(L)1) among patients with non-small cell lung cancer (NSCLC), smokers tend to have better clinical outcomes than non-smokers. However, it is unclear whether NSCLC patients with co-existing chronic obstructive pulmonary disease (COPD) have better clinical outcomes than patients without COPD, regardless of smoking history. The potential correlation of COPD with an improved response to anti-PD-(L)1 was examined in a large cohort of patients with available pulmonary function test results. Patients with stage IV NSCLC who received a minimum of two doses of anti-PD-(L)1 across various treatment lines from 2015 to 2021 were enrolled. Among the 387 patients, pulmonary function test (PFT) data were available for 234 (61%), 139 (59%) of whom had spirometry diagnosed COPD. A retrospective analysis was conducted to evaluate overall survival (OS) and progression-free survival (PFS) based on the presence or absence of COPD. In the univariate analyses, both PFS and OS significantly improved among patients with COPD, compared with patients who did not have COPD (HR 0.71, 95% CI 0.56–0.89 for PFS; HR 0.69, 95% CI 0.52–0.92 for OS), regardless of smoking status. In the multivariate analyses, PFS and OS remained superior among patients with COPD (HR 0.66, 95% CI 0.51–0.85 for PFS; HR 0.63, 95% CI 0.47–0.85 for OS). Additionally, patients with milder COPD (GOLD 1/2 vs. 3/4) had better clinical outcomes than patients with more severe disease. However, neither lung distension (defined as a total lung capacity > 120%) nor pre-COPD status (defined as a diffusing capacity of lung for carbon monoxide < 70%) had a significant impact on PFS or OS. Our study, conducted in the largest cohort with available PFT data to date, showed that COPD was associated with improved survival outcomes among patients with stage IV NSCLC who received anti-PD-(L)1 treatment, regardless of smoking history. The differences were mainly driven by mild and moderate obstruction (GOLD 1 and 2). The dysregulated PD-1/PD-L1 expression that occurs in COPD may offer insights into the different outcomes and thus warrants further investigation.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) is a major public health problem that affects 251 million people worldwide1. According to the World Health Organization, it is the third leading cause of death. Many studies have highlighted the risk factors shared by COPD and lung cancer, especially cigarette smoking, and the common pathogenetic pathways (e.g., aberrant immune function, epigenetic changes, oxidative stress, and impaired DNA repair as a result of exposure to inhaled particles)2. However, COPD increases the risk of lung cancer by 4- to 6-fold, independent of tobacco use and age, both of which worsen the prognosis3.
Several studies regarding anti-programmed cell death 1/ligand 1 immune checkpoint inhibitor (anti-PD-(L)1) efficacy in patients with non-small cell lung cancer (NSCLC) have shown a higher response rate in smokers4,5,6. In contrast, only four observational retrospective studies, based on data from 19 to 65 COPD patients, have shown improvements in progression-free survival (PFS) among COPD patients with NSCLC receiving anti-PD-(L)1 treatment; there are conflicting data regarding overall survival (OS). A limitation of these studies was that they did not include data from comprehensive pulmonary function tests (PFTs). Moreover, they often used composite criteria, such as smoking status and/or respiratory symptoms and/or emphysema, to diagnose COPD7,8,9,10. Only one study, based on comprehensive spirometry evaluations but with a limited sample size of 59 COPD patients with NSCLC, revealed a negative correlation between airflow obstruction and PFS or OS; the hazard ratio (HR) for disease progression or death was 0.50 (95% CI, 0.31–0.79), and the HR for death was 0.45 (95% CI, 0.26–0.78)10. Explorations beyond simple spirometry parameters can provide insights concerning the role of COPD severity in NSCLC patients. For instance, as airflow limitation due to COPD worsens, gas trapping and thoracic hyperinflation occur. However, the impact of thoracic hyperinflation on the response to anti-PD-(L)1 therapy in lung cancer remains unexplored. An additional risk factor for the development of COPD, included in the definition of pre-COPD, is a reduced diffusing lung capacity for carbon monoxide (DLCO)11.
We conducted a large retrospective study to examine the association between the presence of COPD, as delineated by spirometric parameters, and the clinical outcomes of NSCLC patients undergoing treatment with anti-PD-(L)1. We hypothesised that a more complete assessment of lung function parameters in COPD, including total lung capacity (TLC) and DLCO, would offer valuable predictive insights concerning the response to anti-PD-(L)1 therapy in patients with NSCLC.
Methods
Patients
This monocentric retrospective study was conducted within the respiratory department of the University Hospital of Bordeaux. Patients with advanced NSCLC who received anti-PD-(L)1 therapy, either in combination with chemotherapy or as monotherapy, from January 1, 2015, to January 1, 2021, were consecutively enrolled. Patients enrolled in experimental protocols involving anti-PD-(L)1 and patients who received only a single dose of the medication were excluded from the analysis. Follow-up continued until death, loss to follow-up (defined as a lack of visits for at least 6 months), or the end of the follow-up period. Data regarding clinical and pathological characteristics, as well as treatment history, were extracted from a detailed review of the patients’ medical records.
COPD patients were considered as such when they met either of the following criteria: (1) a documented diagnosis of COPD based on available spirometry prior to inclusion, or (2) identification as COPD patients in their medical records. The presence of COPD was determined by spirometry utilising the post-bronchodilation forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio, with a threshold of < 0.70. The airway obstruction severity was assessed using the GOLD criteria for spirometry, based of post-bronchodilation FEV1: mild when FEV1 ≥ 80% predicted value, moderate when 50% ≤ FEV1 < 80% predicted value, severe when 30% ≤ FEV1 < 50% predicted value and very severe when 30% < FEV1 predicted value12. In addition to spirometry results, we gathered data regarding other relevant lung function parameters. Hyperinflation was defined as a TLC > 120%, and a reduced lung diffusing capacity was defined as a DLCO < 70%. These parameters were included to provide an assessment of pulmonary function that was more comprehensive than airflow obstruction alone11,12.
Endpoints
The two primary endpoints were PFS (from the first day of anti-PD-(L)1 until progression or death) and OS (from the first day of anti-PD-(L)1 until death or loss to follow-up) according to COPD status. The objective response rate (ORR; percentage of patients achieving a complete response [CR] or partial response [PR] at the first scan evaluation according to RECIST 1.1) was also investigated. The number of long-term responders to anti-PD-(L)1, defined as patients continuing treatment after 2 years (including patients with temporary interruptions or oligoprogression) was determined in each group. Additionally, PFS and OS were compared among COPD patients, non-COPD smokers, and non-COPD non-smokers. The relationships of smoking status with PFS and OS, and the associations of cigarette consumption (measured in pack-years) with PFS and OS outcomes, were investigated.
Statistical analysis
Statistical analyses were performed using R statistical software and GraphPad Prism V10. Continuous data are presented as the median and interquartile range. The chi-squared test was used to compare categorical variables across the following subgroups: age, sex, smoking habits, cell type, PD-L1 status, anti-PD-(L)1 type, treatment modality, and anterior oncologic treatment. Fisher’s exact test was used to compare the number of lines of treatment. Student’s t-test was used to compare the following continuous variables: number of pack-years, performance status, and number of anti-PD-(L)1 doses. Univariate and multivariate analyses were performed using Cox proportional hazards models to investigate risk factors associated with PFS and OS. HRs are reported with 95% confidence intervals (CIs). The multivariate analysis was made with an adjustment on tobacco consumption based on pack year quantity, age, performance status, number of lines of treatment, cell type. The relationships of smoking intensity with clinical outcomes (PFS and OS duration) were assessed by Pearson correlation analysis. Statistical significance was determined based on a two-sided P-value < 0.05. Subgroup analyses were conducted to explore the interactions of COPD status with various factors, including the number of pack-years, age, performance status, number of treatment lines, cell type, and PD-L1 status. The proportional hazards assumption was checked by using the Schoenfeld residual methods.
Ethics
The study was conducted in accordance with French legislation and ethical codes; it complied with the protection of personal health data and the protection of privacy as specified by Article 65 − 2 of the amended Data Protection Act and general data protection regulations. Due to the retrospective nature of the study (number CHUBX2020RE0275, Ethics committee, CHU Bordeaux, France) waived the need of obtaining informed consent. The study design adhered to the STROBE guidelines.
Results
Patient characteristics
Of the 387 patients included in the study, 169 (43.6%) had physician diagnosed COPD (Fig. 1, Table S1). Among them, 234 patients had spirometry and 139 (59.0%) had spirometry diagnosed COPD (Table 1). Patients with COPD differed by having a higher cumulative tobacco exposure, receiving more lines of treatment, more frequently receiving ICI monotherapy (as opposed to combination with chemotherapy), and a higher number of ICI doses administered. The PFT results are detailed in Table 2. Briefly, 42 (30.2%) patients had mild COPD, 79 (56.8%) had moderate COPD, 15 (10.8%) had severe COPD, and 2 (1.4%) had very severe COPD. Only 11 (6.5%) patients had previously diagnosed COPD, with unavailable PFT data and no information regarding airway obstruction severity. Nineteen (4.9%) patients had hyperinflation, and 145 (37.5%) patients had a reduced DLCO. The median follow-up interval was 13 months. As expected, EGFR modifications were more frequent in non-COPD population whereas KRAS mutations were more frequent among COPD ones (Table S2).
Primary endpoints
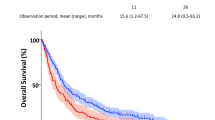
Significant improvements among COPD vs. non-COPD patients were identified based on differences in PFS and OS, with HRs of 0.71 (95% CI 0.56–0.89) for PFS and 0.69 (95% CI 0.52–0.92) for OS in univariate analysis (Fig. 2a, b). These differences were not due to smoking status; PFS and OS were better among non-smoking non-COPD than among smoking non-COPD (Suppl. Figure 1). Instead, subgroup analyses according to airway obstruction severity showed that the differences were mainly driven by mild and moderate obstruction: patients with GOLD 1 or GOLD 2 (FEV1/FVC < 70% or the lower limit of normal, and FEV1 > 50%) status had a better PFS compared with non-COPD patients (HR = 0.61; 95% CI = [0.39;0.96] and HR = 0.66; 95% CI [0.47;0.94]). Similar trends were observed for OS (HR = 0.66; 95% CI [0.47;0.94] and HR = 0.66; 95% CI [0.47;0.94]). In contrast, there were no differences for patients in the more severe COPD group (GOLD 3 and 4) with respect to either PFS (HR = 0.71; 95% CI [0.37;1.35]) or OS (HR = 0.71; 95% CI [0.37;1.36]) (Fig. 2c, d).
Survival curves for progression-free survival (PFS) and overall survival (OS) among chronic obstructive pulmonary disease (COPD) vs. non-COPD (A, B), and according to airflow obstruction severity (C, D). Dotted lines indicate median survival time. COPD chronic obstructive pulmonary disease, GOLD global obstructive lung disease, HR hazard ratio.
Secondary endpoints
Current and former smokers had a better PFS than never-smokers (HR = 0.39; 95% CI [0.25;0.60] and HR = 0.42; 95% CI [0.28;0.64]), whereas OS was not significantly improved among current smokers (HR = 0.64; 95% CI [0.37;1.11]) or former smokers (HR = 0.67; 95% CI [0.40;1.11]) (Fig. 3 and Suppl. Figure 2). There was a marginally significant correlation between cigarette consumption in pack-year and PFS and there was no correlation between cigarette consumption and OS (Suppl. Figure 3).
Neither a low DLCO (HR = 1.49; 95% CI [0.99;2.24] and HR = 1.29; 95% CI [0.76;2.17], respectively for PFS and OS), nor hyperinflation (HR = 0.89; 95% CI [0.51;1.57]; and HR = 1.04; 95% CI [0.53;2.04], respectively for PFS and OS)were associated with a worse prognosis) (Suppl. Figure 4).
The ORR was similar between the non-COPD and COPD groups (chi-squared = 0.03; p = 0.87). Among non-COPD patients, 64 (29.3%) had progressive disease (PD), 106 (48.6%) had stable disease (SD), and 28 (12.8%) had a partial response (PR). No patient had a complete response (CR), and data were unavailable for 20 (9.2%) patients. Among COPD patients, 41 (24.3%) had PD, 93 (55.0%) had SD, and 21 (12.4%) had a PR. No patient had a CR, and data were unavailable for 14 (8.3%) patients. Long-term responders tended to be more frequent among COPD patients than among non-COPD patients, although the difference was not statistically significant: (26 [15.4%] vs. 20 [9.2%]; p = 0.06). Finally, there were no difference in either PFS or OS according to KRAS G12C mutational status (not shown).
Multivariate analyses
Multivariate analysis, adjusted for smoking status, showed that coexisting COPD was independently associated with a better PFS (adjusted HR = 0.66; 95% CI [0.51–0.85]) and a better OS (adjusted HR = 0.63; 95% CI [0.47–0.85]). Multivariate Cox proportional hazards regression analyses showed that higher age and performans status tended to be associated with a lower PFS and a better OS (Tables 3 and 4).
Discussion
Our study of the association between COPD and improved outcomes in patients with stage IV NSCLC receiving anti-PD-(L)1, regardless of tobacco consumption, examined the largest cohort to date. The association was particularly strong among patients with milder COPD. Consistent with literature reports of a reduction in PFS of 44–50% in COPD patients vs. non-COPD patients, our results indicated a significant increase in OS among COPD patients9,10 and a better response among current smokers than among non-smokers6,13. Finally, multivariate analyses adjusted for tobacco consumption supported a longer PFS and longer OS among COPD patients. The chronic inflammation associated with COPD, not caused by smoking alone, may contribute to the early stages of carcinogenesis14,15. The immune system’s role in perpetuating the chronic inflammation in COPD and in controlling tumour burden in lung cancer is well-established7,8. However, the impact of COPD on the immune microenvironment in lung cancer and its implications for anti-PD-(L)1 efficacy are unclear. A previous study showed that in patients with GOLD1/2 COPD, an increased PD-L1 can prevent cigarette smoke-induced sustained lung injury, leading to an exhausted immune phenotype that is more permissive to lung cancer15. Among patients with more severe stages of COPD, an overactive immune system may act as a barrier to cancer development16. We showed that patients with anti-PD-(L)1-treated NSCLC and mild to moderate airflow obstruction had a better PFS and OS than either patients with normal lung function or patients with more severe COPD. The high cost of anti-PD-(L)1 treatment and the non-negligible toxicity of these drugs support targeting for a population most likely to benefit from such treatment. A reliable predictor of the anti-PD-(L)1 response has not been established (including PD-L1 expression and tumour mutation burden)17,18,19, although it would help physicians to identify patients with advanced lung cancer who are likely to be anti-PD-(L)1 responders.
Among our cohort of 387 patients, 139 had airflow obstruction; previous studies included only 60, 65, and 19 COPD patients, diagnosed based on composite criteria of spirometry, symptoms, or radiological characteristics7,8,9. Only a Korean study had systematic spirometry data for all 59 COPD patients. Nevertheless, 85% of those patients had a PD-L1 status > 50%, which could have favoured an anti-PD-(L)1 response. Furthermore, the COPD group had fewer previous lines of treatment than the non-COPD group10. Our study was able to clearly show that, independent of smoking, patients with anti-PD-(L)1-treated NSCLC and coexisting spirometry-defined COPD, especially mild to moderate COPD, had a longer PFS and longer OS. If patients lacking PFT data are regarded as non-COPD, this classification would reinforce our finding of better survival among COPD patients. Additionally, the large number of patients in our study allowed us to identify a better OS among COPD patients, whereas in previous studies the number of patients and the follow-up period were both insufficient to allow statistical analyses of significance7,8,9. Only one study found a better OS among COPD patients; however, PFTs were not available for each patient10. In the present study, we also evaluated whether the lower PFS and OS in non-COPD patients were explained by non-smokers, who are less responsive to anti-PD-(L)16,13, thus favouring a worse clinical outcome for the non-COPD group. Intriguingly, non-COPD smokers had a lower OS and PFS. Additionally, in the multivariate analysis adjusted for smoking status, COPD was independently associated with a better PFS and better OS.
Our study also identified a link between the severity of airway obstruction and clinical outcome: a milder obstruction was associated with a better outcome, as previously reported. For instance, Shin et al. found that only patients with mild COPD had a significantly improved PFS and OS. This may be related to the immune system, which is an essential barrier to tumour development, and to the larger increase in PD-L1 in patients with milder vs. more severe COPD15, such that patients with mild COPD would derive the greatest benefit from anti-PD-(L)1 treatment. Furthermore, long-term responders tended to be more frequent among COPD patients.
An analysis of potential relationships between PFT parameters, such as TLC and DLCO, and pre-COPD status did not reveal any significant differences11. This may have been due to the limited availability of data: TLC and DLCO measurements were only available for half of the cohort. Alternatively, although individuals with pre-COPD are at risk of developing airflow obstruction over time, some of these patients will not undergo disease progression to that stage20. Using an emphysema quantification radiologic score, Noda et al. showed that the coexistence of emphysema with lung cancer predicted the efficacy of anti-PD-(L)121. Nevertheless, TLC is an indirect marker of emphysema. Further research is needed to better define the characteristics of emphysema that contribute to an anti-PD-(L)1 response. Finally, there was no significant difference in the ORR of the two groups in our study. This finding contrasts with the findings of a Korean study, in which the ORR was higher in the COPD group than in the non-COPD group. The disparity in these findings warrants further exploration.
Several limitations of our study should be noted. First, it was a retrospective cohort study conducted at a single centre, which limits the generalisability of the results. Second, 61% of the patients had available spirometry, but most such patients were in the COPD cohort. Thus, COPD patients without spirometry may have been misclassified in the non-COPD group, which might have reduced the magnitude of the difference in survival. In the COPD group, 11 patients had no spirometry but previously diagnosed COPD, had been examined by a pulmonologist and had received adequate inhaler treatment. However, even when considering only patients with spirometry data, the observed results are similar to those of the entire cohort. In addition, we believe that the significance of this work lies in its real-world data and, as such, the entire population (including those without available spirometry data) should be retained. Third, considering the possible interaction between COPD and PD-L1 expression, a prospective study is warranted to evaluate the effect of COPD on anti-PD-(L)1-treated NSCLC according to PD-L1 expression status. Furthermore, the presence of oncogenic addiction was less frequent than expected considering KRAS G12c mutation for instance, probably due to missing data; although neither KRAS mutation nor EGFR modified the difference of PFS and OS according to COPD status. Until 2015, successive thoracic oncology guidelines increasingly recommended anti-PD-(L)1 therapy as first-line treatment; nonetheless, most patients enrolled between 2015 and 2019 received it in subsequent lines. This difference may explain the more favourable response among patients treated with anti-PD-(L)1 as second-line therapy. Additionally, COPD patients were more frequently administered anti-PD-(L)1 monotherapy, perhaps because of their greater susceptibility to comorbidities22. Nevertheless, the performance statuses of the two patient groups were similar.
Conclusion
Our study found a robust association between COPD and improved survival outcomes among patients with stage IV NSCLC receiving anti-PD-(L)1 treatment, regardless of tobacco consumption. This finding underscores the importance of considering anti-PD-(L)1 therapy for a broader spectrum of COPD patients. Notably, patients with milder COPD cases had more favourable clinical outcomes than patients with severe COPD. Detection of the reason underlying this difference requires further investigation into the dysregulation of PD-1/PD-L1 expression in COPD patients.
Data availability
The datasets used and/or analyzed in the study are available from the corresponding author on reasonable request.
References
Young, R. P. et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur. Respir J. 34 (2), 380–386 (2009).
Adcock, I. M., Caramori, G. & Barnes, P. J. Chronic obstructive pulmonary disease and lung cancer: new molecular insights. Respir Int. Rev. Thorac. Dis. 81 (4), 265–284 (2011).
Mathers, C. D. & Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 3 (11), e442 (2006).
Reck, M. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl. J. Med. 375 (19), 1823–1833 (2016).
Kim, J. H., Kim, H. S. & Kim, B. J. Prognostic value of smoking status in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Oncotarget 8 (54), 93149–93155 (2017).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl. J. Med. 373 (17), 1627–1639 (2015).
Biton, J. et al. Impaired tumor-infiltrating T cells in patients with chronic obstructive pulmonary disease impact lung cancer response to PD-1 Blockade. Am. J. Respir Crit. Care Med. 198 (7), 928–940 (2018).
Mark, N. M. et al. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Am. J. Respir Crit. Care Med. 197 (3), 325–336 (2018).
Zhou, J. et al. Impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor efficacy in advanced lung cancer and the potential prognostic factors. Transl Lung Cancer Res. 10 (5), 2148–2162 (2021).
Shin, S. H. et al. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int. J. Cancer. 145 (9), 2433–2439 (2019).
Han, M. K. et al. From GOLD 0 to pre-COPD. Am. J. Respir Crit. Care Med. 203 (4), 414–423 (2021).
Agustí, A. et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur. Respir J. 61 (4), 2300239 (2023).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl. J. Med. 372 (21), 2018–2028 (2015).
Zhu, H-X. et al. Myocyte enhancer factor 2D provides a cross-talk between chronic inflammation and lung cancer. J. Transl Med. 15 (1), 65 (2017).
Polverino, F. et al. Similar programmed death ligand 1 (PD-L1) expression profile in patients with mild COPD and lung cancer. Sci. Rep. 12 (1), 22402 (2022).
Grumelli, S. et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 1 (1), e8 (2004).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl. J. Med. 378 (22), 2078–2092 (2018).
Rittmeyer, A. et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389 (10066), 255–265 (2017).
Forde, P. M. et al. Neoadjuvant PD-1 Blockade in resectable lung cancer. N Engl. J. Med. 378 (21), 1976–1986 (2018).
Martinez, F. J. et al. Treatment trials in young patients with chronic obstructive pulmonary disease and pre-chronic obstructive pulmonary disease patients: time to move forward. Am. J. Respir Crit. Care Med. 205 (3), 275–287 (2022).
Noda, Y. et al. Quantitative evaluation of emphysema for predicting immunotherapy response in patients with advanced non-small-cell lung cancer. Sci. Rep. 12 (1), 8881 (2022).
Corsonello, A. et al. Comorbidities of chronic obstructive pulmonary disease. Curr. Opin. Pulm Med. 17 (Supplement 1), S21–S28 (2011).
Author information
Authors and Affiliations
Contributions
AB: formal analysis; investigation; writing of the original draft. IL: Methodology, Software, Data Curation, Formal analysis, Writing - Original Draft, PH : Validation, Writing - Original Draft, Writing - Review & Editing, RV: Validation, Investigation, CB: Validation, Investigation, CC: Validation, Investigation, ChC: Validation, Investigation, PS: Validation, Investigation, MD: Validation, Formal analysis, Investigation, manuscript preparation and drafting, Writing - Review & Editing. MZ: study concept, data analysis and interpretation, Investigation, manuscript preparation and drafting, Writing - Review & Editing, Supervision, Project administration. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Boudoussier, A., Larrouture, I., Henrot, P. et al. COPD patients with non-small cell lung cancer respond better to anti-PD-(L)1 immune checkpoint inhibitors. Sci Rep 15, 17145 (2025). https://doi.org/10.1038/s41598-025-02251-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02251-0