Abstract
Iron deficiency (ID) is frequent in adult patients with cystic fibrosis (pwCF). The effect of elexacaftor-tezacaftor-ivacaftor (ETI) on iron metabolism has rarely been reported. We aimed to study the trends and variables associated with iron store modulation under ETI. We conducted a prospective adult cohort in two referral centres for pwCF. Iron supplementation during the follow-up was an exclusion criterion. Clinical, biological data and pulmonary function tests were collected prospectively at ETI initiation (V0) and after 1 year of ETI (V12). The presence of Pseudomonas aeruginosa in forced sputum was assessed at V0 and V12. 220 (87 women) pwCF among the 278 screened were included. At V0, ID prevalence was 58% and was significantly associated with female sex and lower forced expiratory volume (FEV1). At V12, ID prevalence decreased significantly from 58 to 31% (p = 0.001). A significant decrease of C reactive protein and total globulins was found at V12. 60% of patients with ID at V0 achieved normalization of iron status at V12 with a significant association with the increase of FEV1 (moderate size effect: 0.68). A lower decrease of C reactive protein was significantly associated with the onset of ID in a small sample of patients (p < 0.001). The disappearance of Pseudomonas aeruginosa in sputum at V12 was not correlated to the evolution of iron status under ETI. ETI was associated with a decrease of ID prevalence, and improvement of pulmonary function and a correction of systemic inflammation.
Similar content being viewed by others
Introduction
Cystic fibrosis (CF) is an autosomal recessive disease due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene1,2, impairing the expression and/or function of CFTR protein acting as a chloride and bicarbonate channel. Abnormal ion transport, airway surface liquid dehydration and inflammation result in tissue damage responsible for the CF multisystem severe phenotype.
Iron deficiency (ID) is common in patients with cystic fibrosis (pwCF)3,4 with a prevalence ranging from 19 to 83% in the adult population of pwCF according to the biological thresholds used to define ID5. ID is either absolute by the decrease of the total body iron stores, and/or functional in patients with inflammation withholding iron from the plasma leading to inadequate erythropoiesis. Systemic inflammation induces an increase of hepcidin level reducing ferroportin transcription, thus limiting iron supply to the plasma causing functional ID6,7. Systemic inflammation is assumed to be one of the main pathways leading to the onset of ID in pwCF, highlighted by the increase of hepcidin level typically occurring after pulmonary exacerbation8,9,10. Reciprocally, it has been demonstrated that the treatment of pulmonary exacerbation waning the systemic inflammation induces a decrease of hepcidin level in pwCF8. Several other mechanisms may contribute to the onset of absolute ID including the malabsorption of micronutrients in the setting of exocrine pancreatic insufficiency and increase of iron loss via sputum11.
Elexacaftor Tezacaftor Ivacaftor (ETI) is the first triplet of CFTR modulators, demonstrating significant benefits for pwCF including improved lung function and quality of life12. The decrease of pulmonary infections under ETI therapy13,14 could restore normal iron metabolism in pwCF in line with the improvement of nutritional status15. In our previous study before the wider use of ETI, we found a high prevalence of ID in adult pwCF with no influence of the use of a double CFTR modulator (mostly Ivacaftor - Lumacaftor)5.
The aim of the present study was to prospectively assess the prevalence of ID in adult pwCF before and after one year of ETI, and to study its relation with clinical and nutritional status, pulmonary function, and biological markers.
Patients and methods
We conducted a one-year prospective bi-centric study in two adult referral centres for CF between 1st January 2021 and 31st January 2023. We included adult patients (age ≥ 18 years) with genetically proven CF who underwent a full iron store blood assessment at their ETI initiation visit (V0) and after 1 year of treatment (V12). Exclusion criteria were: (i) Clinically apparent exacerbation of CF during the 7 days before each visit, (ii) the use of iron supplements during the study period, (iii) solid organ transplantation during the follow-up, (iv) discontinuation of ETI treatment before V12.
Clinical and biological data
We prospectively collected the following data at baseline (V0) and at V12:
-
Clinical data: age, sex, genotype; body mass index (BMI); the use of pump proton inhibitors (PPI); the use of CFTR modulators, Pseudomonas aeruginosa bronchial positivity with forced sputum samples after bacterial culture examination; Cystic Fibrosis Related Diabetes (CFRD) status.
-
Biological markers including: iron store markers (serum ferritin: SF, transferrin saturation: TSAT), C reactive protein, full blood count, total globulins on serum protein electrophoresis, vitamin D level, oral glucose tolerance test (OGTT) including insulin and glucose levels at 0, 60 and 120 min.
-
Pulmonary function tests (PFT) including forced expiratory volume in one second (FEV1) and forced vital capacity (FVC).
The primary outcome was the changes of iron status at V12 (after 1 year of ETI treatment). ID was defined by serum ferritin (SF) ≤ 20 (women) or 30 (men) µg/L or ≤ 100 µg/L in the case of systemic inflammation (CRP ≥ 10 mg/L) and/or transferrin saturation (TSAT) ≤ 16% similar to our previous cohort study5 which is also recommended for the assessment of iron status in chronic inflammation7,16,17. Normalization of iron stores was defined by the shift from ID at V0 to no ID at V12. Anaemia was defined according to the WHO thresholds for adult patients: haemoglobin < 120 g/L (women) or 130 g/L (men)18.
To further explore the potential underlying mechanism associated with iron status variations with ETI treatment, we included as secondary outcomes the study of PFT values variations, HOMA indices, prevalence of Pseudomonas aeruginosa bronchial positivity in forced sputum at V0 and V12, vitamin D levels, CRP level, and globulin level.
HOMA indices are validated methods for assessing insulin-resistance and β-cell function, requiring a single plasma assay19. β-cell function (HOMA-%β) and insulin resistance (HOMA-IR) were calculated from fasting plasma insulin and glucose plasma levels obtained from OGTT with the following formulas: HOMA-%β = [(fasting insulinemia (mUI/L) x 20) / (fasting plasma glucose (mmol/L) – 3.5)]; HOMA-IR = [(fasting insulinemia (mUI/L) x fasting plasma glucose (mmol/L)) / 22.5]20.
Statistical analysis
Categorical data were described with numbers (percentages). Continuous data were expressed as median and interquartile range, according to the statistical distribution. The assumption normality of the data was assessed using the Shapiro-Wilk test.
The comparisons between independent groups (such as (i) men vs. women, (ii) according to iron status course under ETI) were performed using analysis of variance (ANOVA) or the Kruskal-Wallis test when the assumptions to apply ANOVA were not met. The homoscedasticity (equality of variances) was assessed using the Bartlett test. The two by two post-hoc comparisons correcting type I error were conducted using Tukey-Kramer test after ANOVA and Dunn test after Kruskal-Wallis.
To assess changes between visits (i.e. baseline vs. after 1 year of ETI), statistical paired tests were performed using the paired Student t-test or Wilcoxon test if the assumptions of the t-test were not met. Pitman’s test was used to analyse the equality of variances. For categorical data, McNemar test was performed to analyse the evolution of ID prevalence between V0 and V12.
The relationships between continuous variables (i.e. between nutritional status and PFT at 12 months) were analysed using correlation coefficients (i.e. Pearson or Spearman according to the statistical distribution). Statistical analyses were performed using Stata software (version 15, StataCorp, College Station, US). All statistical tests were two-sided, with a type I error set at 5%. Emphasis was given to assessing the magnitude of differences using Hedge’s effect sizes (ES) and 95% confidence intervals (95% CI), and these were interpreted according to Cohen’s recommendations defining ES as small (≥ |0.2|), medium (≥ |0.5|), and large (≥ |0.8|)21.
Ethics
The study protocol was registered on clinicaltrials.gov (NCT04584489). Written informed consent was obtained from each participant. The research was conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee (International Review Board, Hospices Civils de Lyon, IRB number: 69HCL20_0793).
Results
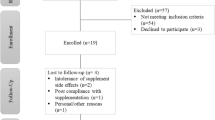
Among 278 patients who initiated ETI treatment between 1st January 2021 and 31st January 2023, 220 (87 women, 39.6%) were included. The main reasons for exclusion were the absence of interpretable iron data at V0 (n = 10) or V12 (n = 16), the use of iron treatment during follow-up (n = 9) and follow-up endpoint (V12) not reached when the study was closed to recruitment (n = 17). Five additional patients moved during the study (follow-up carried out in a non-participating centre) and one patient discontinued the ETI treatment due to side effects.
At V0, median age was 29.3 [IQR 23.6; 35.9]. 51 patients (23.5%) had CFRD, 84 (38.7%) used pump proton inhibitors, and 45 (20.5%) were treated by CFTR modulators before ETI, mostly Ivacaftor-Lumacaftor (96%).
Iron status at baseline (V0)
Table 1 show the main characteristics of the population at V0. ID prevalence was 58%, significantly associated with female sex (78% versus 44%, p < 0.001). ID was associated with lower FEV1% (FEV1p: 53 [41; 73] vs. 70 [52; 83], p < 0.001). In the gender subgroup analysis, a similar but non-significant trend was found. BMI and age were lower in ID patients (20.4 ± 4.1 kg.m− 2 vs. 21.1 ± 4.7 kg.m− 2 and 29.7 ± 9.2 kg.m− 2 vs. 31.9 ± 9.3 kg.m− 2 respectively, p = 0.003) but the difference did not reach statistical significance. Pseudomonas aeruginosa colonization, diabetes mellitus and PPI use were not associated with ID. CRP was higher in patients with ID (17.8 ± 29.1 mg/L vs. 5.1 ± 8.5 mg/L, p < 0.001). We found no association between HOMA indexes and iron deficiency.
Iron status after 1 year of ETI (V12)
At V12, the prevalence of ID decreased significantly from 58 to 31% (p = 0.001). At V12 after 1 year of ETI treatment, 60% of patients who had ID at V0 normalized their iron status. In the gender subgroup analysis, the decrease of ID prevalence was significant for females (78% to 44%, p = 0.03) while we observed a non-significant trend for males (44% to 23%). Serum ferritin (SF) increased significantly (46 µg/L [26.5; 85.5] to 53 µg/L [30.3; 97.8], p = 0.002, size effect 0.25) as well as TSAT (17% [12; 24] to 24% [17; 29.8], p = 0.001, size effect 0.57). Table 2 shows the evolution of the main characteristics of the population between V0 and V12.
Subgroup analysis by iron store trend after 1 year of ETI therapy
We conducted a subgroup analysis to describe covariates associated with the changes of iron status under ETI. The main results are showed in Table 3. Two patients were excluded from this subgroup analysis because of missing data on BMI and pulmonary function tests at V12.
We identified 4 subgroups: (i) patients without ID before and after ETI therapy (group 1, ID-/ID-); (ii) patients with ID before ETI and without ID after ETI (Group 2, ID+/ID-); (iii) patients without ID before ETI experiencing ID after ETI (group 3, ID-/ID+); and (iv) patients with ID before and after ETI (group 4, ID+/ID+).
FEV1% variations were significantly correlated with the evolution of iron status among the subgroups (p < 0.001). The FEV1 increase was significantly higher in group 2 (ID+/ID-) versus group 1 (ID- (37% vs. 17% respectively, moderate size effect − 0.68 (CI95% -1.02; -0.34), p < 0.001), suggesting that ID correction under ETI was associated with a better improvement of pulmonary function. Sex ratio was statistically different among groups (p < 0.001) with a higher proportion of women in group 4 (ID+/ID-) compared to group 1 (66% versus 20% respectively, p = 0.001), compared to group 2 (66% versus 44% respectively, p = 0.02) and compared to group 3 (66% versus 23.5%, p = 0.004). This may suggest that ID persistency may be associated to female gender under ETI therapy. Age was statistically different between groups (ID-/ID+, 28.4 ± 9.8 years, p = 0.03), but no paired subgroup analysis was statistically significant.
The disappearance of Pseudomonas aeruginosa in sputum between V0 and V12 was not significantly correlated to the evolution of iron status. CRP decrease was significantly different between groups (p < 0.001) but no paired subgroup analysis was statistically significant. The increase of BMI was significantly different between groups (p = 0.018); no paired subgroup analysis was statistically significant.
Discussion
We present here the results of the largest prospective cohort study on iron metabolism in adult pwCF treated with ETI, finding a high prevalence of ID of 58%, in line with previous reports3,22,23,24,25. ID was associated with female sex and lower lung function, but not with PPI use or Pseudomonas aeruginosa colonization. The high prevalence of ID in men with pwCF (44%) compared to the prevalence of ID in the general male population26 highlights the specific underlying mechanisms of ID in CF. The robustness of our study lies in the use of stringent criteria for ID diagnosis based on the adjustment of ferritin threshold levels to systemic inflammation17, the exclusion of iron fortification use during the follow-up and the large sample size of our population.
Our results are in agreement with the retrospective cohort study by James et al.27. In this retrospective cohort characterized by a prolonged follow-up of 2 years, the authors studied the variations of biological iron markers under ETI treatment in 127 adult pwCF. While ID was not the main outcome, the authors reported a significant increase in ferritin and transferrin saturation even after adjustment for covariates (age, sex, BMI, diabetes, lung function and P.aeruginosa colonization). Interestingly, the authors included patients receiving iron therapy, which was an exclusion criterion in our cohort and reported that the increase in iron was not significantly different between patients taking iron supplementation and those without iron supplementation. Thus, the improvement of iron was mainly due to ETI treatment and not by iron supplementation, which is in line with our findings.
Ferritin is a well-recognized acute phase-reactant produced in response to the secretion of pro-inflammatory cytokines28. Among the 128 patients with ID, the use of adjusted ferritin threshold (≤ 100 µg/L) according to the level of systemic inflammation allowed us to diagnose 18 additional patients with ID. This diagnosis strategy is recommended in patients with chronic diseases characterized by recurrent or chronic inflammation, which is a hallmark of cystic fibrosis due to repeated infections29. In the most recent review focusing on ID diagnosis, the ferritin threshold for absolute ID in case of inflammation is 70–100 µg/L combined with a TSAT ≤ 20% 7. Jia et al. reported a retrospective cohort study of ID in adult pwCF treated with ET using the TSAT threshold ≤ 20% 30. Interestingly, among the 18 patients who were diagnosed with ID at baseline in our cohort using the inflammation corrected ferritin threshold ≤ 100 µg/L, only 7 wouldn’t have been classified as ID with the definitions used in the study by Jia et al. However, the mean ferritin level in this small subgroup was 60 µg/L (min 34, max 82), which was lower than the lowest threshold (≤ 70 µg/L) discussed by Pasricha et al.7.
In our previous cohort, we found no association between the use of Lumacaftor-Ivacaftor and the correction of iron status compared to patients receiving no CFTR modulators5. These results were confirmed in the present cohort as well as in the report from Jia et al. showing a mild improvement of iron biomarkers in patients receiving moderately effective modulator therapy30. ETI has demonstrated numerous benefits for pwCF, including the improvement of nutritional status, illustrated by the significant increase of BMI during the follow-up in our cohort. We found a clear improvement of lung function (increase of 16% of FEV1) consistent with previous randomized trials regardless of patient genotype31. These two parameters were significantly improved at V12 but we found significant correlations with the evolution of iron status only for FEV1p, with a moderate size effect for patients who normalized their iron status under ETI. These results are in line with Jia et al. study who also reported a higher increase of iron markers in patients with lower FEV1p.
A major pathway of iron metabolism disorders in CF may be the systemic inflammation due to the recurrence of infectious events, inducing hepcidin secretion which in turn blocks iron absorption and bioavailability32. In a paediatric study focusing on iron metabolism in children with CF, it has been showed that hepcidin is decreased in case of ID, allowing to increase nutritional iron absorption and iron availability for erythropoiesis9. In adult pwCF, hepcidin level has been showed to be directly correlated to the number and severity of pulmonary exacerbation in a longitudinal study33. Similar findings were reported in children pwCF, with an increase of hepcidin in case of inflammation34. Even though we did not measure hepcidin in our study, the higher CRP level in baseline ID and the significant association between ID correction and the improvement of inflammatory parameters under ETI support the hypothesis that inflammation correction may be a significant contributing factor of the normalization of iron stores in pwCF. We did not collect the number of exacerbations requiring antibiotic treatment in our study, but numerous reports showed a drastic reduction of these events under ETI12. However, under ETI we observed a significant decrease of globulins whose high levels may indicate prolonged or repeated immune system stimulation due to bronchial infections35. These results are also in favour of the correction of inflammation as a cofactor of iron store normalization and were confirmed in a recent retrospective cohort study14. We found a decrease of Pseudomonas aeruginosa sputum positivity at V12 but did not find a significant link with the correction of ID. Increased iron excretion through sputum is presumed to play a pivotal role in ID genesis for pwCF25. Airway cells expressing the ΔF508-CFTR increase iron availability promoting Pseudomonas aeruginosa biofilm formation36. Our results cannot support this hypothesis but the study of bronchial positivity through sputum bacterial culture may not be a highly reliable criterion as the collection of sputum is more difficult owing to the reduced volume of bronchial secretion under ETI.
A small sample of patients developed ID under ETI. This subgroup (ID-/ID+) was characterized by a lower decrease of CRP at V12. Our study was observational with no controlled group, which cannot allow identifying causal relationship between ETI treatment and ID onset. We did not identify any correlation between covariables and ID onset under ETI, and further studies are required to fully understand the underlying mechanisms of iron metabolism disorders. This subgroup was characterized by a greater increase of BMI (7%). An increase of erythropoiesis consuming iron stores in response to the increase of body mass might be a hypothesis37, but further studies are required to fully understand the pathways involved in this specific evolution of iron under ETI.
The study by James27 highlighted that both patients with and without iron fortification improved the level of ferritin and TSAT under ETI treatment. The improvement of iron status under ETI may probably reduce the requirement for iron fortification, for which debate still exists with regard to the risk of infection and exacerbations of CF after iron treatment33,38.Some additional parameters would be of interest in further studies to investigate the mechanisms underlying iron deficiency correction under ETI, such as iron levels in sputum and nutritional iron absorption.
Conclusion
ETI therapy in a large prospective cohort study is associated with the improvement of iron status, and with the correction of systemic inflammation. Nutritional status was partly correlated to the improvement of FEV1p but was not directly linked to the improvement of iron status. Further studies are required to identify the driving processes leading to the onset of ID in CF, particularly in patients who become ID notwithstanding ETI treatment.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to local regulation constraints but are available from the corresponding author on reasonable request.
References
Lopez, A., Daly, C., Vega-Hernandez, G., MacGregor, G. & Rubin, J. L. Elexacaftor/tezacaftor/ivacaftor projected survival and long-term health outcomes in people with cystic fibrosis homozygous for F508del. J. Cyst. Fibros. 22, 607–614 (2023).
Grasemann, H. & Ratjen, F. Cystic fibrosis. N. Engl. J. Med. 389, 1693–1707 (2023).
von Drygalski, A. & Biller, J. Anemia in cystic fibrosis: incidence, mechanisms, and association with pulmonary function and vitamin deficiency. Nutr. Clin. Pract. 23, 557–563 (2008).
Smith, D. J. et al. Accurate assessment of systemic iron status in cystic fibrosis will avoid the hazards of inappropriate iron supplementation. J. Cyst. Fibros. 12, 303–304 (2013).
Lobbes, H. et al. Iron deficiency in cystic fibrosis: A Cross-Sectional Single-Centre study in a referral adult centre. Nutrients 14, 673 (2022).
Guida, C. et al. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood 125, 2265–2275 (2015).
Pasricha, S. R., Tye-Din, J., Muckenthaler, M. U. & Swinkels, D. W. Iron deficiency. Lancet 397, 233–248 (2021).
Gifford, A. H. et al. Iron homeostasis during cystic fibrosis pulmonary exacerbation. Clin. Transl. Sci. 5, 368–373 (2012).
Uijterschout, L. et al. The value of soluble transferrin receptor and Hepcidin in the assessment of iron status in children with cystic fibrosis. J. Cyst. Fibros. 13, 639–644 (2014).
Gifford, A. H. What is Hepcidin telling Us about the natural history of cystic fibrosis? J. Cyst. Fibros. 14, 155–157 (2015).
Fischer, R., Simmerlein, R., Huber, R. M., Schiffl, H. & Lang, S. M. Lung disease severity, chronic inflammation, iron deficiency, and erythropoietin response in adults with cystic fibrosis. Pediatr. Pulmonol. 42, 1193–1197 (2007).
Heijerman, H. G. M. et al. Efficacy and safety of the Elexacaftor plus Tezacaftor plus Ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 394, 1940–1948 (2019).
Miller, A. C. et al. The rapid reduction of Infection-Related visits and antibiotic use among people with cystic fibrosis after starting Elexacaftor-Tezacaftor-Ivacaftor. Clin. Infect. Dis. 75, 1115–1122 (2022).
Pepe, A. et al. Elexacaftor/tezacaftor/ivacaftor and inflammation in children and adolescents with cystic fibrosis: a retrospective dual-center cohort study. Ther. Adv. Respir Dis. 19, 17534666251314706 (2025).
Stastna, N. et al. Improved nutritional outcomes and Gastrointestinal symptoms in adult cystic fibrosis patients treated with Elexacaftor/Tezacaftor/Ivacaftor. Dig. Dis. 42, 361–368 (2024).
Lopez, A., Cacoub, P. & Macdougall, I. C. Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 387, 907–916 (2016).
Camaschella, C. Iron deficiency. Blood 133, 30–39 (2019).
World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. (2011). https://apps.who.int/iris/handle/10665/85839
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care. 27, 1487–1495 (2004).
Toin, T. et al. HOMA indices as screening tests for cystic fibrosis-related diabetes. J. Cyst. Fibros. 21, 123–128 (2022).
Cohen, J. A power primer. Psychol. Bull. 112, 155–159 (1992).
Gifford, A. H. et al. A pilot study of cystic fibrosis exacerbation response phenotypes reveals contrasting serum and sputum iron trends. Sci. Rep. 11, 4897 (2021).
Gettle, L. S., Harden, A., Bridges, M. & Albon, D. Prevalence and risk factors for Iron deficiency in adults with cystic fibrosis. Nutr. Clin. Pract. 35, 1101–1109 (2020).
Khalid, S., McGrowder, D., Kemp, M. & Johnson, P. The use of soluble transferin receptor to assess iron deficiency in adults with cystic fibrosis. Clin. Chim. Acta. 378, 194–200 (2007).
Reid, D. W., Withers, N. J., Francis, L., Wilson, J. W. & Kotsimbos, T. C. Iron deficiency in cystic fibrosis: relationship to lung disease severity and chronic Pseudomonas aeruginosa infection. Chest 121, 48–54 (2002).
Centers for Disease Control and Prevention (CDC). Iron deficiency–United States, 1999–2000. MMWR Morb Mortal. Wkly. Rep. 51, 897–899 (2002).
James, A. et al. Analysis of iron status after initiation of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis. Pediatr. Pulmonol. 59(3), 669–678. https://doi.org/10.1002/ppul.26805 (2024).
Kernan, K. F. & Carcillo, J. A. Hyperferritinemia and inflammation. Int. Immunol. 29, 401–409 (2017).
Weiss, G. & Goodnough, L. T. Anemia of chronic disease. N Engl. J. Med. 352, 1011–1023 (2005).
Jia, S. et al. Association between CFTR modulators and changes in iron deficiency markers in cystic fibrosis. J Cyst Fibros S1569-1993(24)00030–4 (2024). https://doi.org/10.1016/j.jcf.2024.03.002
Barry, P. J. et al. Triple therapy for cystic fibrosis Phe508del–Gating and –Residual function genotypes. N. Engl. J. Med. 385, 815–825 (2021).
Nemeth, E. & Ganz, T. Hepcidin-Ferroportin interaction controls systemic Iron homeostasis. Int. J. Mol. Sci. 22, 6493 (2021).
Gifford, A. H. et al. Iron supplementation does not worsen respiratory health or alter the sputum Microbiome in cystic fibrosis. J. Cyst. Fibros. 13, 311–318 (2014).
Kałużna-Czyż, M., Grzybowska-Chlebowczyk, U., Woś, H. & Więcek, S. Serum Hepcidin Level as a Marker of Iron Status in Children with Cystic Fibrosis. Mediators Inflamm 3040346 (2018). (2018).
Zhao, E. J., Cheng, C. V., Mattman, A. & Chen, L. Y. C. Polyclonal hypergammaglobulinaemia: assessment, clinical interpretation, and management. Lancet Haematol. 8, e365–e375 (2021).
Moreau-Marquis, S. et al. The ∆F508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L25–L37 (2008).
Kautz, L. et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 46, 678–684 (2014).
Hoo, Z. H. & Wildman, M. J. Intravenous iron among cystic fibrosis patients. J. Cyst. Fibros. 11, 560–562 (2012).
Acknowledgements
We warmly thank Dr Isabelle Petit and Dr Montcouquiol (Centre Hospitalier Universitaire de Clermont-Ferrand) who helped to identify eligible patients and participated in the data collection.
Author information
Authors and Affiliations
Contributions
H.L. : Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing - Original Draft, Writing - Review & Editing.B.P. : Formal analysis, Writing - Review & EditingM.R. : Investigation, Resources, Writing - Review & Editing, Visualization, M.RP. : Investigation, Resources, Writing - Review & Editing, Visualization, I.D. : Conceptualization, Methodology, Resources, Writing - Review & Editing, Visualization, Supervision, Q.R. : Conceptualization, Methodology, Resources, Investigation, Writing - Review & Editing, Visualization, Project administration.
Corresponding author
Ethics declarations
Competing interests
Prof. Isabelle Durieu reports a relationship outside this work with the French Ministry of Health and with the Non Profit Organization “Vaincre la mucoviscidose” that includes: funding grants from Zambon outside this work that includes: travel reimbursement. Prof. Isabelle Durieu reports a relationship with Vertex that includes: board membership outside this work. The other authors reports no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lobbes, H., Pereira, B., Richard, M. et al. Improvement of iron status with elexacaftor tezacaftor ivacaftor therapy is associated with the correction of systemic inflammation and improvement of lung function: a one-year prospective study. Sci Rep 15, 17394 (2025). https://doi.org/10.1038/s41598-025-02296-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02296-1