Abstract
The RUNX1/ETO fusion protein is a chimeric transcription factor in acute myeloid leukemia (AML) created by chromosomal translocation t(8;21)(q22;q22). t(8;21) abnormality is associated with 12% of de novo AML cases and up to 40% in the AML subtype M2. Previously, we identified the small-molecule inhibitor 7.44, which interferes with NHR2 domain tetramerization of RUNX1/ETO, restores gene expression down-regulated by RUNX1/ETO, inhibits proliferation, and reduces RUNX1/ETO-related tumor growth in a mouse model. However, despite favorable properties, 7.44 is negatively charged at physiological pH and was predicted to have low to medium membrane permeability. Here, we identified M23, M27, and M10 as non-charged analogs of 7.44 using ligand-based virtual screening, in vivo hit identification, biophysical and in vivo hit validation, and integrative modeling and ADMET predictions. All three compounds interact with the NHR2 domain, have KD, app values of 39–114 µM in Microscale Thermophoresis experiments, and IC50 values of 33–77 µM as to cell viability in RUNX1/ETO-positive KASUMI cells, i.e., are ~ 5 to 10-fold more potent than 7.44. M23 is ~ 10-fold more potent than 7.44 in inhibiting cell proliferation of RUNX1/ETO-positive cells. Biological characterization of M23 in relevant RUNX1/ETO-positive -and negative cell lines indicates that M23 induces apoptosis and promotes differentiation in RUNX1/ETO-positive AML cells. M23 and M27 are negligibly protonated or in a ~ 1:1 ratio at physiological pH, while M10 has no (de-)protonatable group. The non-protonated species are predicted to be highly membrane-permeable, along with other favorable pharmacokinetic and toxicological properties. These compounds might serve as lead structures for compounds inhibiting RUNX1/ETO oncogenic function in t(8;21) AML.
Similar content being viewed by others
Introduction
The RUNX1/ETO fusion protein (also called RUNX1/RUNX1T1 or AML1/ETO) is a common chimeric transcription factor in acute myeloid leukemia (AML) created by the chromosomal translocation t(8;21)(q22;q22)1. This RUNX1/ETO protein inhibits the function of non-modified RUNX1 by blocking myeloid cell differentiation and apoptosis, thereby causing leukemogenesis2. The t(8;21) abnormality is associated with 12% of de novo AML cases and up to 40% in the AML subtype M21,3. Without treatment, AML is a rapidly fatal disease with a median survival time between 10 and 15 months4. With treatment, AML can be cured in 70% of pediatric patients, 35–40% of adults younger than 60, and 5–15% of adults above 60 years4,5. However, the 5-year cumulative incidence of relapse is ~ 53%, and the overall survival rate is ~ 50%6. Moreover, high-dose chemotherapy is not suitable for elderly patients7. As a first step towards treating AML after four decades of research, the United States Food & Drug Administration (FDA) has approved ten new small molecule inhibitors that target specific mutations or pathways in the cell cycle (specific AML subsets)8. However, no targeted treatment of t(8;21)-dependent AML is available at the moment. Being able to target the RUNX1/ETO fusion protein, which is a major driver in t(8;21)-dependent AML, has the potential to enable targeted therapy schemes with superior efficacy and lesser toxicities than conventional chemotherapy9.
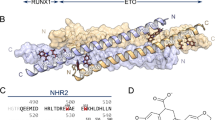
The t(8;21)-generated RUNX1/ETO fusion protein is composed of the DNA-binding Runt-domain encoded by the RUNX1 gene, and four nervy homology regions (NHR1-4) encoded by the ETO gene10,11. The NHR2 domain (residues 485–552 in RUNX1/ETO) is an oligomerization domain (Figure S1A) that plays a role in the dominant detrimental activity of RUNX1/ETO as well as its ability to repress basal transcription12. Moreover, co-expression of full-length and C-terminally truncated RUNX1/ETO fusion proteins (lacking NHR3-4 domains) causes a substantially earlier onset of leukemia and blocked myeloid differentiation at an earlier stage13. By contrast, spliced isoforms containing only the NHR1 domain did not exhibit clonogenic potential compared to isoforms containing NHR1 and NHR213,14. Thus, these studies indicated that the tetramerization conferred by the NHR2 domain plays a key role in promoting leukemogenesis15. Therefore, targeting the oligomerization function of NHR2 emerges as a promising therapeutic strategy for the treatment of AML.
Previously, it has been shown that residues W498, W502, D533, E536, and W540 of the t(8;21)-generated RUNX1/ETO fusion protein, located in the NHR2 domain, are essential for the tetramerization of NHR2 (Figure S1B); mutating these ‘hot spot’ residues to alanine resulted in the formation of NHR2-dimers that do not block myeloid differentiation and fail to induce AML in mice16,17. Thus, targeting these five hot spot residues (Figure S1C) by a small molecule inhibitor appears as a promising therapeutic approach to cure AML. An 18-mer peptide containing all hot spot residues interfered with NHR2-mediated oligomerization and could revert the differentiation block16. The reported inhibition constant (IC), IC50, for this 18-mer peptide was 250 µM (BS3 cross-linking assay) and 390 µM (ELISA)16.
In general, small-molecule inhibitors are considered to have inherent advantages over peptidic ones, including generally lower production costs as well as better oral availability and membrane permeability18. Earlier, we identified the small-molecule inhibitor 7.44 (molecular weight 341 Da) (Fig. 1A), which was shown to (i) specifically interfere with NHR2, (ii) restore gene expression down-regulated by RUNX1/ETO, (iii) inhibit the proliferation, and (iv) reduce the RUNX1/ETO-related tumor growth in a mouse model19. We subsequently characterized the interaction of 7.44 with NHR2 (affinity KD = 4 µM, Figure S2) by biophysical experiments and an integrative structural biology approach. In addition, our data showed generally favorable physicochemical, pharmacokinetic, and toxicological properties of 7.44. However, 7.44 is negatively charged at physiological pH and has been predicted to have a low to medium membrane permeability20.
To obtain a better understanding of the structure-activity relationship (SAR) of inhibiting NHR2 tetramerization, here, we used ligand-based virtual screening with 7.44 as the template scaffold to perform “SAR-by-catalog”. We initially characterized 30 candidates by a cell viability assay in the AML cell line SKNO-1. The three best compounds (M23, M10, and M27), out of the 10 positive hits found by the cell viability assay, interact with the NHR2 domain (STD NMR and nanoDSF measurements) showing KD, app values of 39–114 µM (Microscale Thermophoresis (MST)) and IC50 values of 33–77 µM (cell viability in RUNX1/ETO-positive KASUMI cells). The latter corresponds to a ~ 5 to 10-fold increase in potency over 7.44, likely due to better cell permeability. Likewise, M23 is ~ 10-fold more potent than 7.44 in inhibiting cell proliferation of RUNX1/ETO-positive cells. Integrative modeling further predicted the molecular interactions of M23, M10, and M27 with the NHR2 domain. Finally, the three compounds show favorable physicochemical as well as predicted pharmacokinetic and toxicological properties, including a high membrane permeability.
Results
Ligand-based virtual screening
To establish a SAR, we performed a ligand-based virtual screening to identify structural analogs of 7.44 using FastROCS (Fig. 1)21. FastROCS is an extremely fast shape comparison application, based on the idea that molecules have similar shapes if their volumes overlay well, and any volume mismatch is a measure of dissimilarity. Using 7.44 as a template, the similarity search was done on a subset of the eMolecules and ZINC15 databases containing 5.88 million compounds; the database subsets were chosen based on drug-likeliness. The Tanimoto-Combo score, which considers the molecular shape and pharmacophore according to the automatically assigned standard ROCS color features (donor, acceptor, anion, cation, hydrophobe, rings), was used for ranking the molecules (a higher score indicates more similar molecules, with the maximum being 2). The obtained Tanimoto-Combo scores for the screening results are: 10 molecules > 1.5, 33 molecules > 1.4, 360 molecules > 1.3, 3,800 molecules > 1.2, and 12,500 molecules > 1.0. These scores indicate to what extent substructures of the compounds overlap with 7.44. Among the top 13,000 molecules, the 650 most similar analogs showing the best Tanimoto-Combo scores were further inspected visually considering the main pharmacophore points. These are the key molecular recognition elements of 7.44 that target the interface of NHR2 according to Metz et al., i.e., the 1,3-benzodioxole moiety enters into a hydrophobic grove formed by W502, L505, L509, and L523, and the carboxyl group interacts with R527 and R528 of NHR216. Finally, we selected and purchased 30 candidates from commercial suppliers for the experimental testing (Table S1). All selected molecules have a molecular weight < 400 Da.
Screening of compounds by cell viability assay and STD NMR
The 30 molecules were initially screened in a cell viability assay after a 96 h treatment utilizing the CellTiter-Glo® assay in the AML cell line SKNO-1 carrying the t(8;21) translocation that results in RUNX1/ETO. Three compounds yielded cell viability with an IC50 value in the range of 60–150 µM, two compounds in the range of 150–250 µM, and five compounds in the range of 400–650 µM (Fig. 2A). Particularly, the compounds M23, M10, and M27 were found to have the strongest effects on cell viability with IC50 values of 62.14 µM, 108.67 µM, and 143.7 µM, respectively. The remaining compounds yielded IC50 values ≥ 1 mM indicating no effect on cell viability.
To test whether compounds identified in the cell viability assay interact with NHR2, saturation transfer difference (STD) NMR spectroscopy was used owing to its high sensitivity and low false-positive rate22. In the STD NMR experiments, resonances of NHR2 were selectively saturated for 2 s, and the magnetization was transferred to the interacting compound, which resulted in a decreased signal intensity of the bulk ligand. This spectrum was subtracted from a reference spectrum of the same sample recorded in the absence of saturation. Hence, signals in an STD spectrum indicate that a ligand interacts with NHR2 (Fig. 2B)23. All 10 compounds with IC50 values between 60 and 650 µM show STD signals. Compounds M3 and M6, which were inactive in the cell viability assay, also did not show STD signals. Hence, M6 was used as a negative control in the following cell-based assays. M18 showed STD signals but was not further considered because of the missing efficacy in the cell viability assay.
Cell viability assay and STD NMR experiments. (A) Anti-proliferative activity of the indicated compounds against the RUNX1/ETO-dependent leukemia cell line SKNO-1. Depicted are the mean ± STD IC50 values in µM calculated by using a sigmoid dose-response curve and nonlinear regression of the raw data normalized to the corresponding DMSO controls. Compounds with IC50 values over 1000 µM were classified as inactive (grey color). Data was collected from three independent experiments (n = 3). (B) Compounds from the cell-based assay were counter-screened by STD NMR experiments. For STD NMR experiments, 20–30 µM of 13C, 15N-labelled NHR2 protein were prepared in buffer containing 20 mM sodium phosphate, 50 mM sodium chloride, 0.5 mM tris(2-carboxyethyl)phosphine pH 6.5 (80% (v/v) H2O, 10% (v/v) DMSO-d6, 10% (v/v) D2O). The compound concentration was 1–3 mM in the complex. All compounds with IC50 values ~ 50–650 µM (denoted on the right side of the spectrum) showed STD signals in the NMR spectra, indicating their binding to NHR2. M3 and M6 showed no STD signals. DSS: Sodium 2,2-dimethyl-2-silapentane-5-sulfonate, used for chemical shift referencing (calibrated to zero ppm).
Selectivity of hit compounds against RUNX1/ETO-dependent cells
To validate that the efficacy of the three most active compounds in the cell viability assay (M23, M10, and M27) originates from on-target effects at NHR2, the concentration-dependent cell viability in the presence of M23, M10, or M27 was measured in RUNX1/ETO-positive KASUMI cells, carrying the t(8;21) gene, and compared to RUNX1/ETO-negative K562 cells, where the NHR2 domain was replaced by the BCR tetramerization domain in the t(8;21) gene. The BCR domain is a coiled-coil tetramerization domain of the oncogenic tyrosine kinase BCR/ABL fusion gene24 and was chosen because of the high structural similarity, comparable biochemical properties, and because ETO- and BCR-interacting proteins do not show overlap. Yet, the BCR domain lacks the hot spot residues previously identified in the NHR2 domain17,25 and has been used previously to probe for the selectivity of NHR2-targeting compounds16.
Compounds M23, M10, and M27 reduced the viability of the KASUMI cells with IC50 values of 33 µM, 61 µM, and 77 µM, respectively (Fig. 3A; Table 1), indicative of an anti-leukemic effect. 7.44, measured for comparison, showed an IC50 value ~ 5 to 10-fold higher (Table 1). Compounds M23, M10, and M27 were approximately two-fold more potent in KASUMI (RUNX1/ETO+) than K562 (RUNX1/ETO−) cells, indicating selectivity of the compounds towards RUNX1/ETO-carrying leukemic entities with NHR2 domain. Compound M6 was used as a negative control and did not show activity in either cell type at a concentration of 1 mM.
Selectivity of hit compounds against RUNX1/ETO-dependent cells. (A) The efficacy of hit compounds M23, M27, and M10 identified in the initial screening, as well as the negative control M6, was further evaluated in the RUNX1/ETO-positive KASUMI cell line and compared against the RUNX1/ETO-negative K562 cell line. The mean ± STD of the IC50 values are depicted in µM, calculated by using a sigmoid dose-response curve and a nonlinear regression of the raw data normalized to the corresponding DMSO controls. Compounds with IC50 values over 1000 µM were classified as inactive (grey color). Data was collected from three independent experiments (n = 3). (B) Chemical structures of M23, M27, and M10.
Thermal stability, affinity determination, and ligand epitope mapping of hit compounds M23, M27, and M10. (A–B) Thermal unfolding of apo NHR2 and in complex with a 10-fold excess of the compounds were studied by nanoDSF ((A) Fluorescence intensity ratio at 350 nm / 330 nm; shown are representatives of unfolding events. (B) First derivative of the curves in (A)). Changes in the 350 nm / 330 nm fluorescence emission indicate blue- or redshifts. For apo NHR2 in 10% DMSO solvent, a Tm of 74.5 ± 0.16 °C was observed (the error was determined from fitting). Complexes showed reduced Tm (ΔTm of -1.6 ± 0.03 °C for M23, ΔTm of -1.0 ± 0.09 °C for M27, and ΔTm of -0.1 ± 0.04 °C for M10). (C–E) Hit compounds binding to NHR2 detected by MST assay. Titration of hit compounds to a constant concentration of Alexa488 dye-labeled NHR2 induces a change in thermophoresis. The data were fitted to a 1:1 binding model to obtain apparent KD values. The inflection point of the curve revealed a KD, app of 39 ± 21 µM for M23 (C), 114 ± 55 µM for M27 (D), and 89 ± 38 µM for M10 (E). (F–H) Binding of M23 (F), M27 (G), and M10 (H) to NHR2 studied by STD NMR (lower spectra) (see also Fig. 2B) and the corresponding reference 1D (STD-off) 1H-NMR (upper spectra). The assignment of the individual peaks is indicated in the chemical structures. The STD effects (Isat / I0) of each proton are indicated on top of the peaks and were mapped as a filled circle onto the structures. Darker red colors denote closer proximity to the protein. The STD effects reveal a particular ligand orientation at the protein.
Thermal stability of NHR2 in the presence of hit compounds studied by nano differential scanning fluorimetry (nanoDSF)
nanoDSF was used to assess the thermal stability of NHR2 in the presence and absence of the hit compounds. nanoDSF measures the thermal unfolding transition of a target protein under native conditions, and no extra dye is required26. Rather, the fluorescence intensity ratio at 350 nm / 330 nm is evaluated, corresponding to tryptophan in nonpolar and polar environments27. A lower melting temperature Tm indicates a lower thermal stability27, as seen previously when 7.44 interacts with NHR220 and confirmed here (Table 1).
20 µM of NHR2 was incubated with increasing concentrations of 20, 40, 80, and 200 µM of the respective compound (M23, M27, and M10), and the melting curves were measured (Figure S3). The ratio of fluorescence intensities plotted against the temperature for apo NHR2 and in complex with a 1:10 ratio (protein to compound) and the corresponding first-order derivative are shown in Fig. 4A, B. A melting point of apo NHR2 Tm = 84.1 ± 0.15 °C was observed (Figure S3A), which is virtually identical to the one reported earlier17,20. The addition of 10% DMSO in the buffer containing NHR2 resulted in a destabilization of the protein (Tm = 74.5 ± 0.16 °C) (Fig. 4A, B), similar to what has been reported for other proteins in the literature28. In the presence of 200 µM of the hit compounds, the Tm of NHR2 decreased by 1.6 ± 0.03 °C for M23, 1.0 ± 0.09 °C for M27, and 0.1 ± 0.04 °C for M10 (Table 1; Fig. 4A, B). The Tm values of the complexes become lower with increasing compound concentrations except for M10 (Figure S3). These results indicate that M23 and M27 reduce the thermal stability of NHR2 in a concentration-dependent manner, likely by interfering with the tetramer-dimer equilibrium. It is unclear why M10 does not show this effect.
Affinity determination by microscale thermophoresis (MST)
MST is a biophysical technique to quantify molecular interactions based on the difference in the movement of molecules along microscopic temperature gradients29. To determine the apparent dissociation constant (KD, app) of NHR2 with compounds M10, M23, and M27, 100 nM of dye-labeled NHR2 was mixed with increasing compound concentrations (15 nM to 1 mM). The MST signals were measured for each compound at 24 °C, and normalized fluorescence changes as a function of the compound concentration are shown in Fig. 4C-E. As the concentration of the respective compound is increased, changes in the thermophoretic curve were observed, implying the binding of the compound to the NHR2 (Figure S4A–C). This data was fitted to a 1:1 binding model to obtain KD, app: 39 ± 21 µM for M23, 114 ± 55 µM for M27, and 89 ± 38 µM for M10 (Table 1). Previous data of 7.4420, reanalyzed for comparison, yielded a KD, app of 2.3 ± 0.7 µM (Table 1, Figure S2), which is within the uncertainty of the dissociation constant of 7.44 binding to NHR2 determined previously20. The poor solubility of the compounds at higher concentrations gave rise to fluctuations in the data fit and limited reaching a plateau. The KD, app values obtained by the MST method are in the same range as the IC50 values obtained from the cell viability assay except for 7.44 (Fig. 3; Table 1).
Epitope mapping of the hit compounds by STD-NMR
In addition to identifying molecules that bind to a protein (Fig. 2B), STD NMR also provides information about the binding epitope of the ligand22,23. The STD-NMR spectra obtained for NHR2 in the presence of M23, M27, or M10 allow quantifying the STD effect (the ratio of the intensity when the saturation time is 2 s (on-resonance) / the intensity in the reference spectrum (off-resonance), Isat / I0) for each proton (Fig. 4C). A higher STD effect denotes a closer proximity of the proton to the protein. The STD effects of all three compounds displayed pronounced variations (> 10%), suggesting that the compounds bind to NHR2 through a preferred orientation and not by unspecific binding30. As an example, the varying STD effects of the isopropyl moiety of M23 (41%, 66%, 91%) and the cyclopropyl moieties of M27 (91%, 100%/137%, 120%/199%; values are relative to the peak at 8 ppm) and M10 (45%, 25%, 22%) are given. The differences in the cyclopropyl moieties might arise from the one of M27 being more exposed than the one from M10, such that the former can interact better. Similar variations were also observed for the methyl groups and aromatic moieties of these compounds. Furthermore, the STD effects of the methyl groups of M23, M27, and M10 (74%, 77%, 89%) indicate that they are buried upon binding. STD effects of the aromatic protons of M10 are weaker (~ 50–60%) compared to those in M23 and M27 (~ 75–95%). Overall, the STD data suggests that M23, M27, and M10 do not bind unspecifically to NHR2 and that different structural moieties contribute differentially to the binding. Interestingly, although the KD, app values are in the same order of magnitude, the lower STD effects of M10 parallel the weak effect in nanoDSF of this compound.
Detection of binding epitopes on NHR2 by molecular dynamics simulations
To study the interactions of M23, M27, and M10 with NHR2 on a molecular level, we performed molecular dynamics (MD) simulations of free ligand diffusion31,32 to simulate the association and dissociation of the compounds to and from NHR2. For M23 and M27, the neutral bases were used as the physiologically most relevant form (see also section “pKa value determination by NMR” below). We observed that the compounds interact with NHR2 at multiple sites (Fig. 5 and Figures S5–S7). A clustering of the ligand poses based on fingerprints of interactions with NHR2 residues revealed two primary binding epitopes on NHR2. For M23, the most populated site (epitope I, 13.2%) matches the previously identified binding epitope of 7.44 where interactions with W498 and W502 are formed20. The second site (epitope II, 10.1%) is in the protein-protein interface of the interaction with transcription factor-1211. For M27 and M10, the same two epitopes were identified as most populated, although here epitope II (15.7 & 13.3%) is more populated than epitope I (13.5 & 6.0%). As the occupancies are very similar in the case of M23 and M27, neither one of the binding sites can be identified as preferred.
The STD NMR experiments revealed the isopropyl group of M23 and the structurally homologous cyclopropyl group of M27 as the major interacting groups. In the binding mode obtained from epitope I, the isopropyl and cyclopropyl groups are buried between the helices of NHR2, which is more predominant for M23 than M27. In contrast, in epitope II, both groups are solvent-exposed and only interact transiently with the protein, which is again more predominant for M23 than M27. This might support that epitope I is the binding site of M23 and M27. Moreover, the ratio of epitope I occupancy over epitope II occupancy is largest for M23 followed by M27, which correlates with the relative affinities of these compounds.
Molecular interactions of compound M23 with NHR2 from MD simulations of free ligand diffusion. On the left side, the heavy-atom particle density of M23 around the NHR2 tetramer is depicted in a color-coded manner (see color scale). When accounting for the internal symmetry of NHR2, two primary binding epitopes are identified (black rectangles). The left blowup shows M23 binding to epitope I, where the isopropyl group of M23 is buried between the NHR2 helices, allowing the compound to interact with the hotspot residues W498 and W502. The right blowup shows M23 bound to the protein-protein interface of NHR2 with transcription factor-1211 (epitope II). Here, the benzodioxepane ring is partially buried between the helices, while the thiazolopyrimidine ring is bound to a hydrophobic methionine-rich patch.
M23 induces apoptosis and promotes differentiation in RUNX1/ETO-translocated AML cells
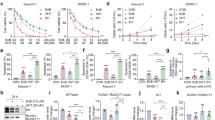
A colony formation assay is an in vitro cell survival assay based on the ability of a single cell to grow into a colony. We investigated the potency of M23, having the lowest IC50 and KD, app values (Table 1) of the three hit compounds, to inhibit colony formation, which is an important hallmark of myeloid cells33. For this, a colony-forming unit (CFU) assay was performed in RUNX1/ETO-positive SKNO-1 and KASUMI cells, and colonies were counted after 14 days (Fig. 6A). M23 demonstrated a dose-dependent effect on colony formation capabilities and is approximately 10-fold more potent than the previously published NHR2 tetramerization inhibitor 7.4419. M6 was used as a negative control and did not show activity in this assay. We next expanded the AML cell line panel and measured the viability using the ATP-based CellTiter-Glo luminescent assay. Of note, SKNO-1 cells showed the highest sensitivity to compound M23, whereas RUNX1/ETO-negative THP-1, K562, and HL-60 cells were considerably less affected (Fig. 6B). To exclude non-specific toxicity, we also included the non-malignant human B-lymphoblastoid cells NC-NC as a control. Consistent with selective activity, NC-NC cells remained unaffected by M23 treatment (Fig. 6B). Additionally, we analyzed the proliferation capacity of t(8;21)-positive and RUNX1/ETO-dependent SKNO-1 and Kasumi-1 cells upon treatment with compound M23, which triggered a significant reduction in the proliferation, while K562 cells were less affected (Fig. 6C). In addition, we analyzed cell cycle phase distribution after treatment with compound M23 in a FACS-based Nicoletti assay. The results showed that M23 treatment affected RUNX1/ETO-positive SKNO-1 cells, but not RUNX1/ETO-negative THP-1 cells. In SKNO-1 cells, M23 induced an accumulation in the subG1 and G1 phases, along with a reduction in the S and G2/M phases, indicating cell cycle arrest and potential induction of apoptosis (Fig. 6D). Apoptosis as the underlying cell death mechanism was further validated by co-treatment with the pan-caspase inhibitor Q-VD-OPh (QVD), which fully abrogated the effect of M23 (Fig. 6E). Additionally, a Caspase-3/7 Glo assay was performed to measure the apoptosis induction, which also showed that M23 treatment significantly increased caspase-3 and − 7 activity within SKNO-1 cells as compared to THP-1 cells (Fig. 6F). Importantly, healthy NC-NC control cells showed no increase in caspase-3/7 activity upon M23 treatment. We assessed the effect of M23 on granulocytic differentiation by measuring CD11b surface expression in both RUNX1/ETO-positive and -negative AML cell lines. Treatment with M23 at concentrations of 25 µM and 50 µM led to a significant increase in CD11b expression in SKNO-1 cells at 50 µM, indicating enhanced myeloid differentiation in SKNO-1 cells but not in K562 and THP-1 cells (Fig. 6G). These results suggest that M23 induces apoptosis and promotes differentiation in RUNX1/ETO-positive AML cells.
Biological characterization of compound M23 in relevant RUNX1/ETO-positive -and negative cell lines. (A) Colony-forming unit (CFU) assay. Representative bar graphs of counted colonies formed by the indicated cell lines 18 days post treatment with M23, M6, 7.44, or the control DMSO at indicated concentrations. Statistics was performed using a one-way ANOVA with Dunnett’s multiple comparisons test (*p < 0.05; ****p < 0.0001 vs. DMSO-treated group). (B) Cell Titer Glo assay after 72 h was used to measure cell viability in RUNX1/ETO-positive and -negative cell lines. NC-NC cells were used as non-cancerous, human B-cell control. Curve fitting was done using a sigmoid dose-response curve and a nonlinear regression. Data was collected from three independent experiments (n = 3). (C) Cell proliferation was measured over 10 days after treatment with M23. The proliferation rates of SKNO-1 and Kasumi cells were significantly more affected than those of K562 cells. (D) Nicoletti cell cycle analysis after 24 h of treatment revealed no effect on THP-1 cells, whereas SKNO-1 cells showed an increased subG1- and G1-population and a decreased S- and G2/M- population. (E) Nicoletti assay showed that M23 induced significantly more apoptosis (% of subG1 cell population) in RUNX1/ETO-positive SKNO-1 cells than in RUNX1/ETO-negative THP-1 cells. QVD (Q-VD-OPh), a pan-caspase inhibitor, was used as a control to inhibit apoptosis induction. (F) Caspase 3/7 Glo assay after 24 h of treatment showed significantly more apoptosis induction in SKNO-1 cells in comparison to THP-1 cells. NC-NC cells served as B-cell control and no apoptosis induction was observed. (G) Differentiation analysis of SKNO-1, THP-1, and K562 cells after daily treatment with compound M23 for three days (c = 25 µM/50 µM). The percentage of CD11b-positive cells is depicted. Results are shown as means ± SD of 3 independent experiments. p values were determined by a two-tailed unpaired t-test (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001).
pKa value determination by NMR
The pKa value is a determinant of physicochemical and pharmacokinetic properties of small molecules. Here, we used NMR titration experiments in the pH range from 2 to 13 to determine the pKa values of M23 and M27. Both compounds contain exchangeable (labile) NH protons (the secondary amines of M23 and M27) that upon (de-)protonation induce different chemical environments for nearby reporter protons (protons 1’’’ and 2’’, Fig. 7, Figure S8). This results in distinct resonance frequencies of the reporter protons at different pH (Fig. 7A for M23, Figure S8A for M27); M10 does not contain a labile proton (Figure S9). Sodium 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) was used for chemical shift referencing and calibrated to a zero ppm value in each case. The pKa value was computed from the chemical shift perturbations at the respective pH by fitting to the Henderson-Hasselbalch Eq. (34) (Fig. 7B, Figure S8B). The pKa of M23 is 6.6–6.7, the one of M27 is ~ 7.8 (Fig. 7B, Figure S8B). These pKa values are ~ 4 and ~ 3 log units lower than that of dimethylamine (pKa ~10.7)34, which results from the electron-withdrawing properties of the nearby aromatic systems. At physiological pH of 7.5, these compounds are thus negligibly protonated (M23) or in the ratio ~ 1:1 (M27).
Determination of the pKa value of M23 by NMR. (A) Resolved, high-field portion of the 700 MHz 1H NMR spectrum of 300 µM M23 (chemical structure shown on top) measured in the pH range of 2 to 13 in 50 mM sodium phosphate, 100 mM sodium chloride (80% (v/v) H2O, 10% (v/v) D2O, 10% (v/v) DMSO-d6). Sodium 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) was used for chemical shift referencing and calibrated to a zero ppm value. (B) Chemical shift values of reporter protons (labeled as 1’’’ and 2’’, marked by arrows in the chemical structure) were plotted against pH. The pKa value was calculated by fitting to the Henderson-Hasselbalch equation. The pKa value of the secondary amine is 6.69 ± 0.01 and 6.63 ± 0.02 as indicated by protons 1’’’ and 2’’, respectively.
Membrane permeability prediction of selected compounds
A determining factor for the bioavailability of drugs is their capacity to passively cross cellular membranes35. Free energy profiles of the permeation process obtained by MD simulations can predict experimentally determined permeabilities from parallel artificial membrane permeability assays (PAMPA), as we previously showed for 7.4420,36. Here, we predicted the permeabilities of M23, M27 (both protonated and deprotonated), and M10 through a PAMPA membrane from MD simulations and configurational free energy computations (Fig. 8A), considering experimental data from nine reference compounds for calibration (Fig. 8B, Table S2), as done before (see Supporting Information). The computed permeabilities (Table 2) classify the species prevalent at physiological pH, non-protonated M23 and M10, as highly permeable (Peff > 4.7 × 10− 6 cm sec− 1). The same holds for non-protonated M27, which exists in a ~ 1:1 ratio with protonated M27; the latter is low to medium permeable (Peff = 7 × 10− 7 − 2.1 × 10− 6 cm sec− 1)36.
Prediction of membrane permeabilities. (A) Potential of mean force (PMF) of permeation of M23, M27, and M10. M23 and M27 are additionally considered in their protonated form (M23_H and M27_H, respectively). As a reaction coordinate, the distance to the center of the DOPC membrane bilayer was used, setting the profile in the water phase to zero. M10 and deprotonated M23 and M27 display favorable free energy in the membrane center, while protonated M23_H and M27_H display a high energy barrier in the membrane center. (B) Calibration curve obtained previously by us20 between the calculated permeabilities obtained from MD simulations and the experimental values obtained from PAMPA experiments from36. Using this calibration curve, M23, M27, and M10 are predicted to be highly permeable (note the overlap between their values), while M23_H and M27_H are predicted to be impermeable or low to medium permeable (Table 2). Peff is the effective permeability, and P0 is the unit factor corresponding to 1 cm sec−1. Shaded areas correspond to the standard deviation obtained by splitting each calculation into ten slices of 5 ns simulation time.
Pharmacokinetic and toxicological property predictions
Pharmacokinetic and toxicological properties were predicted for M23, M27, and M10 (Table 3). Using Qikprop from the Schrödinger software suite37, we predicted the central nervous system (CNS) activity, the brain-blood partition coefficient, and the IC50 value for blockage of HERG K+ channels (Table 3, No. 1). All three molecules can be CNS-active, but are predicted to not block HERG K+ channels. No violations of Lipinski’s rule of five38 or Jorgensen’s rule of three39 are found, judging bioavailability. Furthermore, none of the three compounds has properties falling outside the 95% range of similar values for known drugs. Toxicological properties were predicted using the softwares DEREK Nexus for a variety of endpoints and SARAH Nexus for mutagenicity predictions40,41 (Table 3, No. 2). No toxicophores were identified in any of the compounds, and the compounds are predicted to be inactive with respect to bacterial mutagenicity.
Discussion
We identified M23, M27, and M10 as non-charged analogs of 7.44 using a combination of ligand-based virtual screening, in vivo hit identification, biophysical and in vivo hit validation, as well as integrative modeling and ADMET predictions to characterize interactions with NHR2 or pharmacokinetic and toxicological properties. All three compounds interact with the NHR2 domain and show KD, app values of 39–114 µM in Microscale Thermophoresis (MST) experiments as well as IC50 values of 33–77 µM as to cell viability in RUNX1/ETO-positive KASUMI cells. All three compounds can interact with the previously identified binding epitope of 7.44, where M23 is predicted to form interactions with the hot spot residues W498 and W502. NMR-based pKa value determination reveals that M23 and M27 are negligibly protonated or in a ~ 1:1 ratio at physiological pH, while M10 has no (de-)protonatable group. The non-protonated species are predicted to be highly membrane-permeable, along with other favorable pharmacokinetic and toxicological properties.
The biophysical characterizations by STD-NMR, nanoDSF, MST, and pKa determination as well as the integrative modeling to determine the binding epitope and predictions of membrane permeability and toxicological properties were performed as recently established or applied by us to characterize 7.44 binding to NHR2 or 7.44 properties20. For the in vivo experiments, RUNX1/ETO-positive or –negative cell lines were used that had been applied before to characterize 7.4416,19.
Remarkably, M23, M27, and M10 show a ~ 5 to 10-fold increase in potency over 7.44 in a cell viability assay with RUNX1/ETO-positive KASUMI cells, although 7.44 is ~ 17 to 50-fold more affine to NHR2 according to the KD, app. M23 is also ~ 10-fold more potent than 7.44 in inhibiting cell proliferation of AML cells. This increased in vivo potency can likely be attributed to the prevailing neutral charge of the compounds compared to 7.44, resulting in improved membrane permeability. Furthermore, M23 and M27 are weak bases that might become enriched intracellularly due to a lower intracellular pH42, which would result in their protonated forms being less membrane permeable. Note that it is important to conserve the low pKa values of the secondary amines for this behavior in subsequent optimization studies. The selectivity factor of ~ 2 of the three compounds with respect to RUNX1/ETO-positive over -negative cells is similar to the selectivity found for 7.44 in in vitro16 and in vivo19 assays. Further biological characterization of M23 in relevant RUNX1/ETO-positive -and negative cell lines indicates that M23 promotes differentiation in RUNX1/ETO-positive AML cells.
A change in the screening strategy performed now compared to identifying 7.44 can explain the selection of the non-charged 7.44 analogs M23, M27, and M10. Previously, we performed virtual screening focusing on the side chains of hot spot residues in the NHR2 dimer-of-dimers interface as a template, which contained D533, E536, and W54016. Unsurprisingly, this resulted in hit suggestions that optimally mimic the hot spot functional groups and frequently contained carboxylic acid(s) or bioisosteres. In addition, we initially evaluated the hit suggestions in vitro measuring the inhibition of NHR2 tetramerization, which favored hits with good affinity to NHR2 but neglected the question of availability. By contrast, our virtual screening for analogs now evaluated hit suggestions according to pharmacophore and molecular shape similarity, which allowed for the identification of molecules that only sterically mimic the carboxyl group of 7.44 with an isopropyl (M23) or cyclopropyl (M27) moiety. Next, this time, we first evaluated hit suggestions now using a cell viability assay, which allowed us to assess properties such as cell permeability in addition to a putative action on the target. Only in the second step were the interactions with the target directly probed by STD-NMR. Although we performed only two such steps of complementary virtual screening/hit evaluation campaigns, the positive results suggest that such a strategy could be used to iteratively optimize affinity and pharmacokinetic, in particular, availability, properties.
It may be surprising at first glance why the carboxyl group of 7.44 can be replaced with a neutral carbonyl (M10) or even hydrophobic groups (M23, M27) while keeping the anti-leukemic effect in RUNX1/ETO-dependent cells. Yet, in the predicted binding mode of 7.44 at the NHR2 tetramer, the carboxyl group is oriented towards the solvent whereas the 1,3-benzodioxole moiety inserts in between the helices20. Thus, an effect-conserving replacement of the carboxyl group is plausible. By contrast, the most active compound M23 identified here now buries the structurally homologous isopropyl group between the NHR2 helices, allowing the remainder of the compound to interact with hot spots W498 and W502. Although the number of compounds investigated here is too small to derive a quantitative SAR, these findings and qualitative comparison of 7.44 versus M23, M27, and M10 suggest that the terminal 1,3-benzodioxole moiety (M10), bioisosteres thereof (1,4-benzodioxane (M27) or 1,5-benzodioxepine (M23)), or even sterically and functionally reduced moieties (methoxyphenyl (M10) or methoxypyridyl (M27)) together with a distance of three atoms between them are more relevant for interactions with the target. These insights can be exploited in future compound optimizations.
In summary, the identified M23, M27, and M10 together with 7.44 might serve as lead structures for further optimization of binding affinity, bioavailability, and anti-leukemic effects of compounds inhibiting RUNX1/ETO oncogenic function in t(8;21) acute myeloid leukemia. To improve the anti-leukemic effects, one might also consider combinations of (derivates of) the lead structures with traditional chemotherapeutic agents such as cytarabine or daunorubicin, or with inhibitors that target transcription factors, such as AP-1, or epigenetic regulators, such as DNA methyltransferase or HDAC inhibitors, which might provide synergistic effects for treating acute myeloid leukemia.
Materials and methods
NHR2 protein expression and purification
The expression and purification of the NHR2 domain (residues 485–552 of human RUNX1/ETO fusion protein) was performed as described earlier20. Briefly, the protein was expressed in Escherichia coli Rosetta (DE3)pLysS (Invitrogen) cells that had codon-optimized gene in the pETSUMO vector. The protein was purified by nickel affinity chromatography on a HisTrap column, and the 6His-SUMO tag was removed by incubation with the SUMO protease (1:100 ratio) 50–150 µg/ml followed by affinity chromatography. NHR2 was then purified by S-75 size exclusion chromatography (SEC buffer- 50 mM sodium phosphate, 50 mM sodium chloride, 1 mM DTT, pH 8) (GE Healthcare Life Sciences) and analyzed using SDS-PAGE. For NMR experiments, isotope labeling of NHR2 was achieved in ~ 98% D2O-containing M9 media supplemented with 13C-glucose and 15N-NH4Cl as sole carbon and nitrogen sources, respectively. The labeled protein was purified with the same protocol as the unlabeled protein.
Inhibitor compounds
All chemical compounds (except 7.44) were purchased from commercial suppliers (Table S1) via MolPort (www.molport.com) and used as received unless otherwise indicated. The compound 7.44 was kindly provided by the Developmental Therapeutics program, National Cancer Institute, USA (NCI code 162496). All compounds were dissolved in DMSO-d6 as a 50 or 100 mM stock solution and stored at − 20 °C for future use.
Cell culture
The cell lines K-562 (#ACC 10, BCR-ABL1+ CML), SKNO-1 (#ACC 690, RUNX1-RUNXT1 positive AML), KASUMI (#ACC 220, RUNX1-RUNXT1 positive AML), HL-60 (#ACC 3, AML), THP-1 (#ACC 16, KMT2A-MLLT3+ AML), and NC-NC (#ACC 120, Blymphoblastoid cells) were purchased from DSMZ (German Collection of Microorganisms and Cell Cultures) and validated by STR DNA profiling. If not stated otherwise, indicated cells were cultured in RPMI 1640 GlutaMax (Gibco, #61870-070) supplemented with 10% FCS (Sigma-Aldrich, #121031) and 1% Penicillin-Streptomycin (Gibco, #15140-122). SKNO-1 cells were additionally supplemented with 10 ng/ml GM-CSF (Peprotech, #300-03). Cells were kept in a 37 °C humidified incubator (BINDER, #BD056) with 5% CO2.
Cell viability screening
Micro-robotic drug library screening was performed as previously described31. In short, compounds or appropriate controls were printed (randomized) on white 384-well plates (Corning, #3570) spanning eight concentrations in triplicates [1–450 µM] in a targeted titration approach utilizing micro-robotics (D300e Digital Dispenser, Tecan). All wells were normalized to the highest vehicle (DMSO) concentration. 30 µL cells were seeded (Multidrop, Thermo Fisher Scientific) with a previously optimized seeding concentration of 3125 cells/ml (K562) or 7000 cells/ml (SKNO-1, KASUMI, HL-60, THP-1, NC-NC) in the preprinted plates. After 72–96 h of incubation (37 °C, 5% CO2), cell viability was assessed utilizing CellTiter-Glo® luminescence assay (Promega, #G755A) as per manufacturer’s instructions and measured with the SPARK 10 M reader (Tecan). The 50% inhibitory concentration (IC50) was calculated using a sigmoid dose-response curve and nonlinear regression of the raw data normalized to the corresponding DMSO controls (GraphPadPrism).
Colony-forming unit (CFU) assay
Assessment of colony-forming units was performed by treating 1 × 103 Kasumi cells with indicated compounds for 48 h in liquid RPMI1640. Subsequently, treated cells were harvested and plated into 24-well culture plates in triplicates in semisolid MethoCult™ (#04100, STEMCELL) media33. Colonies were counted after 14 days31,43.
Proliferation assay
To measure the cell proliferation, cells were seeded at equal density (0.1·10^6 cells/mL), treated with M23 (DMSO, 25 µM or 50 µM), and the viable cell number was measured after 10 days with the cell counter (Beckman Coulter). The obtained amount was normalized to the cell number at day 0.
Cell cycle analysis (Nicoletti assay)
The cells (0.25·10^6 cells/mL) were treated with the compound M23 (50 µM and 100 µM; ± QVD) or DMSO ± QVD as control. After incubation for 24 h at 37 °C, 1 mL was taken, centrifuged, and resuspended in Nicoletti assay buffer (Sodium citrate (1%), Triton X-100 (0.1%), PI (50 µg/mL) in dH2O). Cells were subsequently measured with the CytoFLEX (Beckman Coulter).
Caspase-Glo® 3/7 assay
The Caspase 3/7 assay (#G8090, Promega) was performed according to manufacturer instructions. The cells (0.25·10^6 cells/mL) were treated with the compound M23 (50 µM and 100 µM) or DMSO as control. Cells were incubated for 24 h and diluted with Caspase-Glo® 3/7 Reagent 1:1 (Caspase-Glo® 3/7 Substrate + Caspase-Glo® buffer was previously mixed). After 30 min of incubation at RT, luminescence was measured using the SPARK 10 M reader (Tecan). The Signal was normalized to DMSO and the cell count after 24 h of incubation.
Differentiation (CD11b) assay
The cells (0.25·10^6 cells/mL) were treated daily with the compound M23 (25 µM or 50 µM) or DMSO as control for three days. After three days of incubation, cells were centrifuged, washed one time with PBS, and resuspended in antibody solution (CD11b APC (BD Biosciences #333143) diluted 1:100 in PBS). After incubation at 4 °C for 10 min, cells were measured with the CytoFLEX (Beckman Coulter). CD11b positive population was calculated in comparison to DMSO treated sample.
Nano differential scanning fluorimetry (nanoDSF) assay
To determine (changes in) the thermal unfolding transition (Tm) of NHR2 in the presence of the compounds, samples were prepared by mixing 20 µM of NHR2, and 20, 40, 80, and 200 µM of respective compounds (M10, M23, M27) to the final volume of 50 µl in a buffer consisting of 50 mM sodium phosphate, 50 mM sodium chloride, pH 8.0, 10% (v/v) DMSO. After incubation for 15 min at room temperature, approximately 10 µl of the sample was loaded into a capillary. The thermal denaturation curves were measured by using intrinsic tryptophan fluorescence of NHR2 (with and without compound) on nanoDSF (NanoTemper, Prometheus NT.40, Germany). The temperature was increased from 20 to 95 °C at a rate of 1 °C/min. The excitation wavelength was 280 nm, and the fluorescence intensity was recorded at both 330 and 350 nm wavelengths27. The fluorescence intensity ratio F350/F330 was used for the data evaluation26. For data analysis and fitting to obtain the Tm, the software provided by NanoTemper was used. Duplicates of the same dilution were measured.
NMR spectroscopy
An STD NMR experiment was performed as previously described20 at 35 °C on a Bruker Avance III HD spectrometer operating at 750 MHz, equipped with a 5 mm triple resonance TCI (1H, 13C, 15N) cryoprobe and a shielded z-gradient. For STD NMR experiments, 20–30 µM of 13C, 15N-NHR2 protein were prepared in buffer containing 20 mM sodium phosphate, 50 mM sodium chloride, 0.5 mM tris(2-carboxyethyl)phosphine, pH 6.5 (80% (v/v) H2O, 10% (v/v) DMSO-d6, 10% (v/v) D2O). The compound concentration was 1–3 mM in the complex. Selective saturation of protein resonances (on-resonance spectrum) was performed by irradiating at 0.267 ppm for a total saturation time of 2 s. For the reference spectrum (off-resonance), the samples were irradiated at − 30 ppm23. To determine the binding epitope mapping, the STD intensity of the largest STD effect was set to 100% as a reference, and the relative intensities were determined22,23. Data was acquired with 256 scans, then processed and analyzed by the TopSpin 3.6.1 (Bruker BioSpin) software. Sodium 2,2-dimethyl-2-silapentane-5-sulfonate (DSS) was used for chemical shift referencing.
For pKa value determination, 300 µM of M10, M23, or M27 were prepared in buffer containing 50 mM sodium phosphate, 50 mM sodium chloride (80% (v/v) H2O,10% (v/v) DMSO-d6, 10% (v/v) D2O) and a pH range of 2–13 (pH of 0.5 steps).1D 1H-NMR data was acquired on a Bruker Avance III HD spectrometer operating at 700 MHz spectrometer at 25 °C with 128 scans for each sample. 1H chemical shift values of reporter protons were extracted by the TopSpin 3.6.1 (Bruker BioSpin) software and analyzed by the Origin software (OriginLab Corporation, USA). The pKa value was calculated for M23 and M27 (M10 has no labile protons in the tested pH range) compounds by applying the Henderson-Hasselbalch equation as explained by Gift et al.44. DSS was used for chemical shift referencing.
Microscale thermophoresis
NHR2 labeling was performed with the reactive Alexa Fluor® 488 dye using N-hydroxysuccinimide (NHS)–ester chemistry (NHS Ester Protein Labeling kit, ThermoFisher Scientific, USA), which reacts efficiently with primary amines of proteins to form stable dye–protein conjugates. The labeling of NHR2 was performed as described earlier20. Briefly, 115 µM of NHR2 and 350 µM of Alexa Fluor® 488 dye (410 µl reaction volume) were incubated for one hour at room temperature, followed by 35 min at 30 °C in a buffer consisting of 50 mM sodium phosphate, 50 mM sodium chloride, pH 8.0 (MST buffer). The dye-labeled protein was purified from unreacted free dye using a PD-10 column containing Sephadex G-25 Medium (GE Healthcare Life Sciences) and then centrifuged at 50,000 rpm for 30 min. The concentrations and the efficiency of the labeling were determined as indicated in the manual.
To determine the constant of dissociation (KD) of the compounds from NHR2, 16 dilution samples were generated by serial dilution (1:1) mixing 200 nM of labeled NHR2 and 1 mM or 2 mM of the respective compound to the final volume of 20–25 µl in MST buffer containing 10% (v/v) DMSO. The samples were incubated overnight in the dark before the experiment. The samples were loaded into the Monolith™ NT.115 standard coated capillaries (NanoTemper Technologies), and thermophoresis was measured in the instrument (Monolith NT.115, NanoTemper Technologies) at an ambient temperature of 24 °C, 50% light-emitting diode, and 40% infrared laser (IR) power (MST power) with IR laser on/off times of 30/5 seconds. Triplicates of the same dilution were measured.
The data analysis and the fit (by either thermophoresis with T-jump or thermophoresis or T-jump methods, based on the response amplitude and/or standard error of the MST traces) to obtain the apparent KD (considering a 1:1 binding model) was done using the MO affinity analysis software (NanoTemper, Germany)29. The processed data were plotted with the Origin software (OriginLab Corporation, USA).
Pharmacokinetic and toxicological prediction
Pharmacokinetic parameters were predicted for the compounds M23, M27, and M10 using the QikProp-Schrödinger suite (Release 2023-1)37 (https://www.schrodinger.com/platform/products/qikprop/). Toxicological parameters were predicted using the toxicology modeling tools DEREK Nexus® (Derek Nexus: 6.0.1, Nexus: 2.2.2)41 and SARAH Nexus® (Sarah Model—2.0 Sarah Nexus: 3.0)40 from Lhasa Limited, Leeds, UK (https://www.lhasalimited.org/).
Virtual screening
For the virtual screening, the eMolecules (eMolecules Corporate Office, San Diego, CA, USA) and ZINC1545 databases were downloaded as SMILES. After removing redundant entries, reactive molecules, fragments, and otherwise unsuitable compounds were filtered out using the OpenEye filter tool with the drug filter (https://www.eyesopen.com/). The remaining compounds were protonated at physiological pH using fixpka, and conformers were generated using omega2 (up to 200 conformers per compound). The final dataset was indexed for FastROCS TK46,47 (https://www.eyesopen.com/). Using 7.44 as a template (Fig. 1A), the similarity search was performed on a subset of the eMolecules and ZINC15 databases containing 5.88 million compounds. The Tanimoto-Combo score of each molecule was calculated by considering the shape and pharmacophore according to the automatically assigned standard ROCS color features (donor, acceptor, anion, cation, hydrophobe, rings), and then molecules were ranked. The top 650 most similar analogs showing the best Tanimoto-Combo scores were further inspected visually considering the main pharmacophore points, i.e., if structural elements equivalent to the 1,3-benzodioxole moiety enter into a hydrophobic grove formed by W502, L505, L509, and L523 or equivalent to the carboxyl group interact with R527 and R528 of NHR216. Finally, we selected (molecular weight < 400 Da) and purchased 30 candidates from commercial suppliers via MolPort (www.molport.com) for the experimental testing (Table S1).
Molecular dynamics simulations
System preparation
To investigate how the ligands M10, M23, and M27 interact with NHR2 on a molecular level, we performed unbiased molecular dynamics (MD) simulations of the diffusion of the ligands around the NHR2 complex. The tetrameric structure of the NHR2 domain (PDB ID 1WQ610, hereafter residue numbering according to the RUNX1-ETO fusion protein48) was prepared using Maestro49; protonation states, Asn/Gln flips, and the tautomeric and flip state of histidines were determined using the built-in implementation of PROPKA. The ligand structures were geometry-optimized in Gaussian 09 (https://gaussian.com/) at the HF/6-31G* level of theory. For M27, the singly deprotonated species was used. Partial charges were assigned according to the restraint electrostatic potential (RESP) fitting procedure50. For the ligands, GAFF251,52 parameters were used, while ff14SB53 parameters were used for the protein. For each ligand, initial configurations were built with PACKMOL54 such that a single ligand molecule was placed at least 20 Å from the central axis of the NHR2 helices. Potassium ions were placed randomly to counter excessive negative charges. Finally, the system was solvated in a truncated octahedron of TIP3P55 water, leaving at least 11 Å between the edges and any solute molecule. For each system, twenty initial starting configurations were generated.
Thermalization and production runs
All MD simulations were performed using the graphics processing unit (GPU) version of pmemd implemented in Amber1852,56,57 (https://ambermd.org/). The Langevin thermostat58 was used to maintain the temperature at 300 K with a collision frequency of 2 ps− 1, while the Monte Carlo barostat was used to keep the pressure at 1 bar. The particle mesh Ewald method59 was used for handling long-range interactions with a cutoff of 8.0 Å. The SHAKE algorithm60 was used to constrain bonds to hydrogens. Hydrogen mass repartitioning was applied to increase the integration time step to 4 fs for the productive simulations61.
For thermalization, sequentially water molecules, solute molecules, and, finally, the whole system were minimized for 3000 steps of steepest descent followed by 2000 steps of conjugate gradient algorithm. Positional restraints of 5 kcal mol Å−2 were applied to keep other atoms fixed during the minimization. Then, the system was gradually heated to 300 K over 25 ps (NVT), employing a time step of 2 fs, followed by 975 ps (NPT) of density adaptation. The integration timestep was increased to 4 fs, and the system was simulated for further 6,000 ps (NPT), gradually reducing the positional restraints on solute atoms by 0.2 kcal mol Å−2 every 600 ns. Finally, the system was simulated for another 2,000 ps (NPT) without any restraints.
After thermalization, all systems (20 starting configurations * 7 ligands) were subjected to 1 µs of unbiased simulation, amounting to a total of 140 µs of MD simulations. Frames were written out at a frequency of 100 ps.
Analysis
To identify epitopes on the NHR2 tetramer where a ligand would bind, we calculated the grid densities of ligand heavy atoms mapped onto the tetramer structure using cpptraj62 from AmberTools of Amber2063. For this, we only considered frames where the ligand was in contact (heavy atom-heavy atom distance < 4 Å) with at least five different residues of NHR2 and fitted the NHR2 tetramer structure to a standardized conformer. To characterize the ligand binding, we applied an interaction fingerprint-based hierarchical clustering approach, as described before20. PyMOL was used for visualization of all molecular data64.
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
References
Peterson, L. F. & Zhang, D. E. The 8; 21 translocation in leukemogenesis. Oncogene 23, 4255–4262 (2004).
Okuda, T. et al. Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood 91, 3134–3143 (1998).
Reikvam, H., Hatfield, K. J., Kittang, A. O., Hovland, R. & Bruserud, O. Acute myeloid leukemia with the t(8;21) translocation: clinical consequences and biological implications. J. Biomed. Biotechnol. 2011, 104631 (2011).
van Dijk, A. D., de Bont, E. & Kornblau, S. M. Targeted therapy in acute myeloid leukemia: Current status and new insights from a proteomic perspective. Expert Rev. Proteom. 17, 1–10 (2020).
Dohner, H., Weisdorf, D. J. & Bloomfield, C. D. Acute myeloid leukemia. N Engl. J. Med. 373, 1136–1152 (2015).
Marcucci, G. et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): A cancer and leukemia group B study. J. Clin. Oncol. 23, 5705–5717 (2005).
Kim, J. & Hurria, A. Determining chemotherapy tolerance in older patients with cancer. J. Natl. Compr. Canc Netw. 11, 1494–1502 (2013).
Kayser, S. & Levis, M. J. Updates on targeted therapies for acute myeloid leukaemia. Br. J. Haematol. 196, 316–328 (2022).
Arora, R., Sawney, S. & Saluja, D. Potential therapeutic approaches for the treatment of acute myeloid leukemia with AML1-ETO translocation. Curr. Cancer Drug Targets 16, 215–225 (2016).
Liu, Y. et al. The tetramer structure of the nervy homology two domain, NHR2, is critical for AML1/ETO’s activity. Cancer Cell. 9, 249–260 (2006).
Sun, X. J. et al. A stable transcription factor complex nucleated by oligomeric AML1-ETO controls leukaemogenesis. Nature 500, 93–U120 (2013).
Zhang, J. S. et al. Oligomerization of ETO is obligatory for corepressor interaction. Mol. Cell. Biol. 21, 156–163 (2001).
Mannari, D., Gascoyne, D., Dunne, J., Chaplin, T. & Young, B. A novel exon in AML1-ETO negatively influences the clonogenic potential of the t(8;21) in acute myeloid leukemia. Leukemia 24, 891–894 (2010).
Rejeski, K., Duque-Afonso, J. & Lubbert, M. AML1/ETO and its function as a regulator of gene transcription via epigenetic mechanisms. Oncogene 40, 5665–5676 (2021).
Kwok, C., Zeisig, B. B., Qiu, J. H., Dong, S. O. & So, C. W. E. Transforming activity of AML1-ETO is independent of CBF beta and ETO interaction but requires formation of homo-oligomeric complexes. P Natl. Acad. Sci. USA. 106, 2853–2858 (2009).
Metz, A., Schanda, J., Grez, M., Wichmann, C. & Gohlke, H. From determinants of RUNX1/ETO tetramerization to small-molecule protein-protein interaction inhibitors targeting acute myeloid leukemia. J. Chem. Inf. Model. 53, 2197–2202 (2013).
Wichmann, C. et al. Dimer-tetramer transition controls RUNX1/ETO leukemogenic activity. Blood 116, 603–613 (2010).
Wang, L. et al. Therapeutic peptides: Current applications and future directions. Signal. Transduct. Target. Ther. 7, 48 (2022).
Schanda, J. et al. Suppression of RUNX1/ETO oncogenic activity by a small molecule inhibitor of tetramerization. Haematologica 102, e170–e174 (2017).
Gopalswamy, M. et al. Biophysical and Pharmacokinetic characterization of a small-molecule inhibitor of RUNX1/ETO tetramerization with anti-leukemic effects. Sci. Rep. 12, 14158 (2022).
Hawkins, P. C. D., Skillman, A. G. & Nicholls, A. Comparison of shape-matching and docking as virtual screening tools. J. Med. Chem. 50, 74–82 (2007).
Gossert, A. D. & Jahnke, W. NMR in drug discovery: A practical guide to identification and validation of ligands interacting with biological macromolecules. Prog Nucl. Mag Res. Sp 97, 82–125 (2016).
Mayer, M. & Meyer, B. Characterization of ligand binding by saturation transfer difference NMR spectroscopy. Angew Chem. Int. Ed. 38, 1784–1788 (1999).
Zhao, X., Ghaffari, S., Lodish, H., Malashkevich, V. N. & Kim, P. S. Structure of the Bcr-Abl oncoprotein oligomerization domain. Nat. Struct. Biol. 9, 117–120 (2002).
Chen-Wichmann, L. et al. Compatibility of RUNX1/ETO fusion protein modules driving CD34 + human progenitor cell expansion. Oncogene 38, 261–272 (2019).
Kotov, V. et al. In-depth interrogation of protein thermal unfolding data with moltenprot. Protein Sci. 30, 201–217 (2021).
Bruce, D., Cardew, E., Freitag-Pohl, S. & Pohl, E. How to stabilize protein: Stability screens for thermal shift assays and nano differential scanning fluorimetry in the virus-X project. Jove-J Vis. Exp. (2019).
Tjernberg, A., Markova, N., Griffiths, W. J. & Hallen, D. DMSO-related effects in protein characterization. J. Biomol. Screen. 11, 131–137 (2006).
Wienken, C. J., Baaske, P., Rothbauer, U., Braun, D. & Duhr, S. Protein-binding assays in biological liquids using microscale thermophoresis. Nat. Commun. 1 (2010).
Cala, O. & Krimm, I. Ligand-orientation based fragment selection in STD NMR screening. J. Med. Chem. 58, 8739–8742 (2015).
Bhatia, S. et al. Targeting HSP90 dimerization via the C terminus is effective in imatinib-resistant CML and lacks the heat shock response. Blood 132, 307–320 (2018).
Gohlke, H. et al. Binding region of alanopine dehydrogenase predicted by unbiased molecular dynamics simulations of ligand diffusion. J. Chem. Inf. Model. 53, 2493–2498 (2013).
Franken, N. A. P., Rodermond, H. M., Stap, J., Haveman, J. & van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 1, 2315–2319 (2006).
Perrin, D. D. Dissociation constants of organic bases in aqueous solution. IUPAC Chem. Data Ser., 1–383 (Butterworths, London, 1965).
Kansy, M., Avdeef, A. & Fischer, H. Advances in screening for membrane permeability: High-resolution PAMPA for medicinal chemists. Drug Discov Today Technol. 1, 349–355 (2004).
Bennion, B. J. et al. Predicting a drug’s membrane permeability: A computational model validated with in vitro permeability assay data. J. Phys. Chem. B 121, 5228–5237 (2017).
QikProp, S. R. (2023). http://gohom.win/ManualHom/Schrodinger/Schrodinger_2012_docs/qikprop/qikprop_user_manual.pdf (Schrödinger, LLC, 2023).
Benet, L. Z., Hosey, C. M., Ursu, O. & Oprea, T. I. BDDCS, the rule of 5 and drugability. Adv. Drug Deliver Rev. 101, 89–98 (2016).
Lionta, E., Spyrou, G., Vassilatis, D. K. & Cournia, Z. Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Curr. Top. Med. Chem. 14, 1923–1938 (2014).
Barber, C. et al. Evaluation of a statistics-based ames mutagenicity QSAR model and interpretation of the results obtained. Regul. Toxicol. Pharm. 76, 7–20 (2016).
Greene, N., Judson, P. N., Langowski, J. J., and Marchant, C. A. (1999) Knowledge-based expert systems for toxicity and metabolism prediction: DEREK, StAR and METEOR. SAR QSAR Environ. Res. 10, 299–314.
Madshus, I. H. Regulation of intracellular pH in eukaryotic cells. Biochem. J. 250, 1–8 (1988).
Bhatia, S. et al. Control of AC133/CD133 and impact on human hematopoietic progenitor cells through nucleolin. Leukemia 29, 2208–2220 (2015).
Gift, A. D., Stewart, S. M. & Bokashanga, P. K. Experimental determination of pK(a) values by use of NMR chemical shifts, revisited. J. Chem. Educ. 89, 1458–1460 (2012).
Irwin, J. J. & Shoichet, B. K. ZINC–a free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 45, 177–182 (2005).
Petrovic, D. et al. Virtual screening in the cloud identifies potent and selective ROS1 kinase inhibitors. J. Chem. Inf. Model. 62, 3832–3843 (2022).
Rush, T. S. 3rd, Grant, J. A., Mosyak, L. & Nicholls, A. A shape-based 3-D scaffold hopping method and its application to a bacterial protein-protein interaction. J. Med. Chem. 48, 1489–1495 (2005).
Miyoshi, H. et al. The t (8; 21) translocation in acute myeloid leukemia results in production of an AML1-MTG8 fusion transcript. EMBO J. 12, 2715–2721 (1993).
Schrödinger Release 2018-1: Maestro. (Schrödinger, LLC, New York, NY, 2018).
Bayly, C. I., Cieplak, P., Cornell, W. & Kollman, P. A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 97, 10269–10280 (1993).
Wang, J. M., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
Case, D. A. et al. Xiao, L., York, D. M., and P. A. AMBER 2018 (University of California, San Francisco, 2018).
Maier, J. A. et al. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 11, 3696–3713 (2015).
Martinez, L., Andrade, R., Birgin, E. G. & Martinez, J. M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Salomon-Ferrer, R., Götz, A. W., Poole, D., Le Grand, S. & Walker, R. C. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. J. Chem. Theory Comput. 9, 3878–3888 (2013).
Le Grand, S., Gotz, A. W. & Walker, R. C. SPFP: Speed without compromise—a mixed precision model for GPU accelerated molecular dynamics simulations. Comput. Phys. Commun. 184, 374–380 (2013).
Pastor, R. W., Brooks, B. R. & Szabo, A. An analysis of the accuracy of Langevin and molecular-dynamics algorithms. Mol. Phys. 65, 1409–1419 (1988).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Ryckaert, J. P., Ciccotti, G. & Berendsen, H. J. C. Numerical-integration of cartesian equations of motion of a system with constraints-molecular-dynamics of N-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Hopkins, C. W., Le Grand, S., Walker, R. C. & Roitberg, A. E. Long-time-step molecular dynamics through hydrogen mass repartitioning. J. Chem. Theory Comput. 11, 1864–1874 (2015).
Roe, D. R., Cheatham, T. E. PTRAJ and CPPTRAJ: software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 9, 3084–3095 (2013).
Case, D. A. et al. Software of Amber and AmberTools. AMBER 2020 (University of California, San Francisco, 2020).
PyMOL. The PyMOL Molecular Graphics System. 2.1.0 Ed., Schrödinger, LLC.
Acknowledgements
This work was supported by a grant from the state of North-Rhine Westphalia and the European Fonds for Regional Development EFRE.NRW 2014-2020 to HG. We are grateful for computational support and infrastructure provided by the “Zentrum für Informations- und Medientechnologie” (ZIM) at the Heinrich Heine University Düsseldorf and the computing time provided by the John von Neumann Institute for Computing (NIC) to HG on the supercomputer JUWELS at Jülich Supercomputing Centre (JSC) (user ID: HKF7, VSK33). Financial support by Deutsche Forschungsgemeinschaft (DFG) through funds (INST 208/704-1 FUGG to HG) to purchase the hybrid computer cluster used in this study, GRK 2158/2 (project number 270650915) to HG, and Heisenberg grant ET 103/5-1 to ME is gratefully acknowledged. The authors acknowledge access to the Jülich-Düsseldorf Biomolecular NMR Center. HG is grateful to OpenEye Scientific Software for granting a Free Public Domain Research License.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.G.: Methodology, investigation, formal analysis, writing—original draft; D.B.: Investigation, formal analysis, writing—review and editing; N.D.: Investigation, formal analysis; J.W.T.: Investigation, formal analysis; M.V.: Investigation, formal analysis; S.S.V.: Investigation, formal analysis, writing—review and editing; S.B.: Investigation, formal analysis, writing—review and editing; M.E.: Investigation, formal analysis, writing—review and editing; H.G.: Conceptualization, supervision, project administration, funding acquisition, writing—original draft.
Corresponding author
Ethics declarations
Competing interests
The author(s) declare no competing interests except that HG is a coauthor on the patent EP/30.04.13/EPA 13165993, 2014.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gopalswamy, M., Bickel, D., Dienstbier, N. et al. Identification of non-charged 7.44 analogs interacting with the NHR2 domain of RUNX1-ETO with improved antiproliferative effect in RUNX-ETO positive cells. Sci Rep 15, 17720 (2025). https://doi.org/10.1038/s41598-025-02422-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02422-z