Abstract
Maternal smoking during pregnancy is known to have an adverse impact on child development. This study aimed to elucidate whether maternal smoking during pregnancy has a direct adverse effect on the child development or whether it exerts an indirect effect through birth weight reduction or cadmium exposure. Mothers and infants enrolled in the Japan Environment and Children’s Study were evaluated. A total of 4794 mother–child pairs were included in the analysis. They were classified into four groups according to their smoking status during pregnancy (non-smokers [Q1, n = 2966], ex-smokers who quit before pregnancy [Q2, n = 575], ex-smokers who quit during pregnancy [Q3, n = 505] and smokers [Q4, n = 168)]). Children’s development was assessed at 2 and 4 years of age using a standardized tool, the Kyoto Scale of Psychological Development. Among male children, Q1 exhibited higher scores in all domains except posture-motor than Q4. Female children did not show lower scores in Q4 relative to the other groups. Path analysis revealed that maternal smoking increased cadmium exposure, a direct negative effect of maternal smoking was observed. The findings of this study confirm that maternal smoking during pregnancy has a direct negative impact on the development of children, particularly males.
Similar content being viewed by others
Introduction
High cognitive ability in children is associated with academic achievement and may influence their subsequent socioeconomic status1,2. Therefore, it is crucial to identify the factors that negatively affect cognitive ability in children. Child development is influenced by genetic, socioeconomic, and educational factors. Additionally, environmental factors can affect child development. One of the most common environmental factors that negatively affects child development is maternal smoking during pregnancy. It is well documented that children born to women who smoked during pregnancy have lower cognitive and language scores3, lower intelligence quotients (IQ)4, and male children among them are four times more likely to develop behavioral disorders by adolescence than children born to women who did not smoke during pregnancy5. Furthermore, children born to mothers who smoked during pregnancy are approximately three times more likely to develop attention deficit hyperactivity disorder (ADHD)6. Prenatal exposure to cigarette smoke is thought to contribute to cognitive deficits by exposing the developing fetal brain to chemicals such as nicotine7.
It is known that smoking during pregnancy cause a reduction in birth weight. Simpson was the first researcher to report that children born to pregnant women who smoked during pregnancy had lower birth weights than children born to non-smoking women, and to warn against smoking among pregnant women8. Since then, many studies have confirmed that smoking during pregnancy adversely affects fetal development9,10. Additionally, in Japan, maternal smoking during pregnancy has been found to be associated with lower birth weight11,12. It is a well-documented fact that a child’s future development is influenced by their birth weight13,14. Additionally, smoking during pregnancy is known to be one of the sources of exposure to cadmium (Cd)15. In fact, it has been demonstrated that blood/urine Cd exposure levels are significantly higher in smokers than in non-smokers16, and that indoor Cd concentrations are higher in rooms with smokers than in rooms without smokers17,18. Cd exposure has also been identified to have an impact on child development. For example, it has been reported that exposure to Cd in urine during the first trimester of pregnancy increases the risk of autism spectrum disorder19,20,21 and ADHD21, and that exposure to Cd in blood during the second/third trimester of pregnancy lowers scores on developmental tests22.
In this context, it is worth investigating whether smoking during pregnancy has direct or indirect negative effects on child development. Direct effect is when an independent variable influences a dependent variable directly. Indirect effect is when it influences the dependent variable through another independent variable. Some reports have indicated that smoking during pregnancy directly affects child development, and others have demonstrated that birth weight was not a mediating factor for the effects of maternal smoking on IQ23. Despite a substantial body of evidence derived from geographically diverse populations, a direct causal link between maternal smoking during pregnancy and fetal neurodevelopment has not yet been established. In this study, we used data from a birth cohort study conducted in Japan (Japan Environment and Children’s Study, [JECS]) to (1) compare the developmental indices at ages 2 and 4 years between children born to women who smoked during pregnancy and those who did not, and (2) determine whether smoking during pregnancy directly affects child development or indirectly affects it via lower birth weight or Cd exposure.
Materials and methods
Study design and participants
This study included mothers and children who were registered in the JECS. The study design and protocol of the JECS have been described in detail elsewhere24,25. In brief, the JECS is a nationwide birth cohort study investigating the environmental factors that might affect children’s health and development. From January 2011 to March 2014, the Research Co-ordinaters explained the study to pregnant women at 15 Regional Centres across the country. If they consented, they were registered in the JECS. The JECS has registered 104,059 records and follows the growth and development of the enrolled children. The JECS conducts a questionnaire survey twice a year targeting all registered participants. Additionally, a Sub-Cohort Study was conducted on 5000 children, which represents 5% of the total mother–child pairs enrolled in the JECS. For the Sub-Cohort Study, children born after April 1, 2013 who met the eligibility criteria were randomly selected. The eligibility criteria included the following: (1) questionnaire and medical record data from the first trimester to 6 months of age, and (2) biospecimens collected from the child and their mothers from the first trimester to the second/third trimester and at birth. In the Sub-Cohort Study, in addition to questionnaire surveys, detailed assessments were conducted, including environmental measurements and face-to-face examinations (developmental tests and medical examinations). For a detailed description of the Sub-Cohort Study, refer to Sekiyama et al.26, which has already been published. The present study used the results of developmental tests conducted on children aged 2 and 4 years. The developmental tests performed are explained in the outcomes section. This study employed datasets released in September 2019 and April 2021 (jecs-ta-20190930 and jecs-qa-20210401) for the analysis.
The JECS protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the Ethics Committees of all the participating institutions (Ethical Number: No.100910001). Written informed consent was obtained from all the participants included in the study. All data collection procedures were performed in accordance with the Japanese ethics guidelines and regulations.
Exposure biomarker
The smoking status of the pregnant women was evaluated based on two types of questionnaires administered during pregnancy. The questionnaires were administered in the first and second/third trimesters of pregnancy. Participants were classified into three groups based on their smoking status during pregnancy: non-smokers (Q1), ex-smokers who quit before pregnancy (Q2), ex-smokers who quit smoking during pregnancy (Q3), and smokers who continued smoking during pregnancy (Q4).
The levels of Cd exposure during pregnancy were determined from whole blood samples collected during the second or third trimester. The procedures for collecting and analyzing the blood samples have been described in detail elsewhere27. In brief, blood was collected into 9 mL tubes containing a coagulant, transported to the laboratory within 48 h of collection, and stored at − 80 °C until analysis. Cd concentration was quantified using inductively coupled plasma mass spectrometry (Agilent 7700, Tokyo, Japan). The detection limit of Cd was calculated based on a previously described method28. All the measurements were above the detection limit.
Outcomes
The Kyoto Scale of Psychological Development (KSPD)29 was administered to 5000 mother–child pairs enrolled in the JECS sub-cohort study. The KSPD is a standardized developmental test that evaluates development from birth to adulthood. Testers assess the child’s behavior and reactions in a face-to-face setting. The test consists of 328 items, starting with the item corresponding to the child’s chronological age and evaluates whether the child passes or fails that item. If a child is able to pass an item, they move on to a more difficult item. Conversely, if a child fails an item, the item for the younger age group is implemented, and the score is calculated based on the number of items passed. The scores are divided into three domains: cognitive-adaptive (C-A), language-social (L-S), and posture-motor (P-M). The developmental quotient (DQ) for all domains is calculated from the scores of each domain. The C-A domain assesses nonverbal reasoning and visuospatial awareness. The L-S domain assesses interpersonal relationships, sociability, and language skills. The P-M domain assesses gross motor function. All scores are designed to have a mean of 100 and a standard deviation (SD) of 15. To ensure the reliability of administration, the testers were trained and certified by the Programme Office of the JECS. In clinical settings, the test is sometimes administered over two or three days, depending on the child’s situation at the time of the test. However, the JECS was conducted on a single day, as it was not conducted in a clinical setting. This approach correlates with the Bayley Scales of Infant Development, 2nd edition, which has been standardized in the United States30.
Birth weight was also used as an outcome measure. This information was obtained from the medical record transcripts.
Covariates
The covariates included in the analysis of covariance (ANCOVA) model were as follows: pre-pregnancy body mass index (BMI), calculated as weight in kilograms divided by the height in square meters (kg/m2) (< 18.5, 18.5–24.9, and ≥ 25); maternal age at parturition (< 20, 20–24, 25–29, 30–34, 35–39, and ≥ 40 years); annual household income (< 4, 4–7.9, ≥ 8 million JPY); parity (1, 2 or more); and maternal blood Cd concentration. Covariates data were collected from the self-administered questionnaires and medical record transcripts.
Statistical analysis
Due to the observed child sex differences in the KSPD score and birthweight, the analysis was stratified by sex. Analysis of variance (ANOVA) and ANCOVA with covariate adjustment were employed to compare the KSPD scores among the three smoking behavior groups. The Bonferroni multiple comparison test was used to examine the differences among the three smoking behavior groups. All analyses with two-sided p-values were conducted using SPSS (version 29.0; SPSS Japan, Tokyo, Japan), with a significance level of 5% set for all tests. Path analyses were performed using R (Version 4.0.2). The hypothesized model was subjected to path analysis using the “lavaan” package31. In this multivariate theoretical assumptions, maternal smoking during pregnancy was used as predictors, maternal blood Cd levels and birth weight were considered as mediators, and the KSPD scores were used as the outcome variables. For direct and indirect effects, significance was indicated by p values under 0.05. The root mean square error of approximation (RMSEA), goodness of fit index (GFI), adjusted goodness-of-fit index (AGFI), and comparative fit index (CFI) were used in the present study to determine the model fit. The values of RMSEA should be lower than 0.0532, the values of AGFI, GFI, CFI should be higher than 0.9.
Results
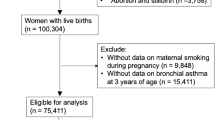
Supplemental Fig. 1 shows a flowchart of the JECS participants who were analyzed in this study. Table 1 presents the characteristics of the participants according to sex. Among all mothers, 3.4% of those with male children and 3.6% of those with female children smoked during pregnancy. Furthermore, women who smoked during pregnancy (Q4) were younger at parturition, and more likely to have a higher pre-pregnancy BMI, lower annual household income, and two or more births. Women who had smoked during pregnancy (Q4) also had higher blood Cd concentrations in the second or third trimester than those in the other groups. Additionally, the infants born to women classified as Q4 had a lower birth weight.
Table 2 present the KSPD scores by smoking group during pregnancy, along with the results of the ANOVA. Among male children, at 2 and 4 years of age, the KSPD score of Q4 was lower than that of Q1, except for the P-M domain. Among 2-year-old female children, L-S domain score of Q4 was lower than that of Q1, and P-M domain score of Q4 was higher than that of Q1. At 4 years of age, significant differences were observed in domains other than the P-M domain, with Q4 scores lower than those of Q1. Table 3 and Supplemental Fig. 2 presents the results of the ANCOVA. As with the results of the ANOVA, the KSPD scores of Q4 were lower than Q1, except for the P-M domain, for males at 4 years of age. For 2-year-old females, Q4 scores were significantly higher in the P-M domain. Moreover, no significant differences were observed between Q1 and Q4 at the age of 4 years.
A path analysis was conducted to examine whether maternal smoking during pregnancy had a direct impact on child development or exerted an indirect effect through its association with a lower birth weight and Cd exposure. The model fitting was performed using four distinct pathways: a direct path from maternal smoking during pregnancy to the KSPD; a path from maternal smoking during pregnancy via birth weight to the KSPD scores; a path from maternal smoking during pregnancy via maternal blood Cd concentration to the KSPD scores; and a path from maternal smoking during pregnancy via maternal blood Cd concentration via birth weight to the KSPD scores. The overall fit of the path model was acceptable. The standardized regression coefficients of the hypothesized model are depicted in Supplemental Figs. 3 and 4, and Table 4 lists the mediation results. Regardless of the child’s sex or age, the direct effect of maternal smoking on the KSPD scores was considerable compared to other path coefficients, demonstrating that maternal smoking during pregnancy has a substantial direct effect on the KSPD scores. In domains other than P-M, the results indicated the presence of numerous negative path coefficients, suggesting that maternal smoking during pregnancy has an adverse effect on child development.
Discussion
This study examined the direct and indirect effects of maternal smoking during pregnancy on child development. If smoking during pregnancy has an adverse effect on child development, it would be beneficial to ascertain whether this is a direct consequence of smoking or through indirect effects of smoking such as reduced birth weight or prenatal exposure to Cd. The findings of this study indicate that maternal smoking during pregnancy has a direct adverse impact on child development rather than indirectly through an effect on birth weight or Cd concentrations.
Several epidemiological, clinical, and animal studies have shown that children born to mothers who smoke during pregnancy are more likely to have behavioral problems and lower IQs5,6,33,34,35. The study by Blood-Siegfried and Rende35 provided a rationale for the direct impact of smoking on child development. Chronic exposure to nicotine during pregnancy in rats and mice has been demonstrated to be related to the behavioral outcomes in the offspring. One hypothesis is that nicotine reduces the blood flow to the placenta, limiting the oxygen and nutrients delivered to the developing fetus, which may result in damage to the fetal brain at a critical developmental stage. However, there are numerous aspects to consider with regards to smoking during pregnancy. For example, women who smoke during pregnancy have been found to have lower educational levels36,37, lower socioeconomic status38, a younger age at parturition, and higher parity39. Smokers also have lower IQs40,41. Therefore, it is important to elucidate the mechanisms by which smoking during pregnancy negatively affects child development.
Although the results of this study indicate that maternal smoking during pregnancy has an adverse impact on child development, no statistically significant differences in the P-M domain scores were observed between the smoking and non-smoking groups. Additionally, participants aged 2 years scored higher in this domain in Q4. One probable reason for this result is the characteristics of the KSPD measurement items utilized in this study. Maternal smoking during pregnancy is reportedly associated with an increased risk of ADHD and developmental coordination disorders (DCD)42,43. Children with ADHD and DCD show deficits in both gross and fine motor skills, particularly in activities such as throwing and kicking a ball, writing, and using scissors44. Conversely, one of the characteristics of children with ADHD is a proclivity for running, climbing, and jumping repetitively during early childhood45. In the P-M domain of the KSPD, which evaluates 2–4-years-olds, evaluates motor skills such as climbing stairs and jumping, but does not evaluate motor skills such as using a ball or scissors. In other words, the P-M domain does not evaluate the motor skills that children with ADHD or DCD are weak at, but rather evaluates the motor skills that they are good at. It is hypothesized that this is the reason why the P-M domain had a high score in Q4.
Exposure to maternal smoking during pregnancy been shown to have adverse effects on the development of male children. No statistically significant differences were observed in the maternal blood Cd concentrations (males, median 0.658 [95%CI 0.327–1.430] ng/g; females, 0.653 [0.322–1.460] ng/g, p = 0.529) or cord blood Cd concentrations (males, 0.041 [0.0.26–0.072] ng/g; females, 0.042 [0.026–0.073] ng/g, p = 0.153) between male and female children as measured by the JECS. Although there was no significant difference in Cd concentrations between males and females, the negative effects of smoking during pregnancy were observed only in males, indicating that males may be more susceptible to these effects. It has been reported that children born to women who smoked during pregnancy are significantly more likely to be males with DCD than children born to women who did not smoke46. Moreover, the birth rate of males is lower47, and the perinatal mortality rate of males is higher48. In other words, male children are more susceptible to the effects of maternal smoking than female children49,50. Therefore, it is necessary to consider the child sex when evaluating the reproductive toxicity of a substance.
This study has several limitations. One limitation is that the data on maternal smoking status during pregnancy were collected via self-reported questionnaires completed by the pregnant women themselves. Previous studies have demonstrated that self-reported smoking status may result in an underestimation of actual prevalence51. In this study, we examined the urinary cotinine concentrations during the second or third trimester of pregnancy. Given that the cotinine concentrations in the smoking group were higher than those in the other groups, we believe that our results are reliable to a certain extent. Nevertheless, caution should be exercised when interpreting these findings. Data on cotinine levels and child development are not presented here and will be published separately. A second limitation of this study is that we were unable to include maternal IQ as a confounding factor. While the JECS currently collects data on maternal IQ, it was not possible to assess it at the ages 2 or 4 years; thus, it could not be included as a variable in this analysis. In this study, annual income, which has a certain positive correlation with IQ, was used as an alternative variable; however, further analysis is required to confirm these findings. The final limitation of this study is that sources of Cd exposure other than smoking were not included in the analysis. The primary source of Cd exposure in the Japanese population is dietary52, with rice consumption contributing the most significant amount53, followed by vegetables and seafood54. While the JECS administered a food frequency questionnaire to evaluate dietary habits during pregnancy, calculating the intake of rice alone is difficult. In future studies, it will be important to include analyses of Cd exposure sources other than smoking.
In conclusion, our findings indicate that maternal smoking during pregnancy is associated with reduced birth weight and Cd exposure. However, maternal smoking during pregnancy also has a direct adverse effect on child development rather than affecting child development through low birth weight or Cd exposure. Additionally, there was no effect on the developmental test scores of the children whose mothers quit smoking after finding out that they were pregnant. The findings of this study highlight the adverse effects of smoking during pregnancy. In Japan, the goal is to reduce the smoking rates during pregnancy to zero. The smoking rate, which was 5.0% in 2009, decreased gradually to 3.8% in 2013, 2.7% in 2017, 2.0% in 2020, and 1.9% in 202155. This achievement can be attributed to the efforts of medical professionals who work with pregnant women who smoke. Our intention is to continue raising awareness regarding the harmful effects of maternal smoking and promote the healthy development of children.
Data availability
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
References
Agrawal, A. et al. The effects of maternal smoking during pregnancy on offspring outcomes. Prev. Med. 50(1–2), 13–18. https://doi.org/10.1016/j.ypmed.2009.12.009 (2010).
Kristjansson, A. L. et al. Maternal smoking during pregnancy and academic achievement of offspring over time: A registry data-based cohort study. Prev. Med. 113, 74–79. https://doi.org/10.1016/j.ypmed.2018.05.017 (2018).
Fried, P. A., O’Connell, C. M. & Watkinson, B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: Cognitive and language assessment. J. Dev. Behav. Pediatr. 13(6), 383–391 (1992).
Frydman, M. The smoking addiction of pregnant women and the consequences on their offspring’s intellectual development. J. Environ. Pathol. Toxicol. Oncol. 15(2–4), 169–172 (1996).
Weissman, M. M., Warner, V., Wickramaratne, P. J. & Kandel, D. B. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J. Am. Acad. Child Adolesc. Psychiatry 38(7), 892–899. https://doi.org/10.1097/00004583-199907000-00020 (1999).
Milberger, S., Biederman, J., Faraone, S. V., Chen, L. & Jones, J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children?. Am. J. Psychiatry 153(9), 1138–1142. https://doi.org/10.1176/ajp.153.9.1138 (1996).
Secker-Walker, R. H., Vacek, P. M., Flynn, B. S. & Mead, P. B. Smoking in pregnancy, exhaled carbon monoxide, and birth weight. Obstet. Gynecol. 89(5 Pt 1), 648–653. https://doi.org/10.1016/s0029-7844(97)00103-8 (1997).
Simpson, W. J. A preliminary report on cigarette smoking and the incidence of prematurity. Am. J. Obstet. Gynecol. 73(4), 807–815 (1957).
Abraham, M. et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE 12(2), e0170946. https://doi.org/10.1371/journal.pone.0170946 (2017).
Hamadneh, S. & Hamadneh, J. Active and passive maternal smoking during pregnancy and birth outcomes: A study from a developing country. Ann. Glob. Health 87(1), 122. https://doi.org/10.5334/aogh.3384 (2021).
Suzuki, K., Shinohara, R., Sato, M., Otawa, S. & Yamagata, Z. Association between maternal smoking during pregnancy and birth weight: An appropriately adjusted model from the Japan Environment and Children’s Study. J. Epidemiol. 26(7), 371–377. https://doi.org/10.2188/jea.JE20150185 (2016).
Tatsuta, N. et al. Timing of maternal smoking cessation and newborn weight, height, and head circumference. Obstet. Gynecol. 141(1), 119–125. https://doi.org/10.1097/AOG.0000000000004991 (2023).
Jackson, D. B. & Beaver, K. M. Sibling differences in low birth weight, dopaminergic polymorphisms, and ADHD symptomatology: evidence of GxE. Psychiatry Res. 226, 467–473. https://doi.org/10.1016/j.psychres.2015.01.025 (2015).
Rahman, M. S. et al. Elevated risk of attention deficit hyperactivity disorder (ADHD) in Japanese children with higher genetic susceptibility to ADHD with a birth weight under 2000 g. BMC Med. 19(1), 229. https://doi.org/10.1186/s12916-021-02093-3 (2021).
Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 68, 167–182. https://doi.org/10.1093/bmb/ldg032 (2003).
Järup, L., Berglund, M., Elinder, C. G., Nordberg, G. & Vahter, M. Health effects of cadmium exposure: A review of the literature and a risk estimate. Scand. J. Work Environ. Health 24(Suppl 1), 1–51 (1998).
Drago, G. et al. Relationship between domestic smoking and metals and rare earth elements concentration in indoor PM2.5. Environ. Res. 165, 71–80. https://doi.org/10.1016/j.envres.2018.03.026 (2018).
Landsberger, S., Larson, S. & Wu, D. Determination of airborne cadmium in environmental tobacco smoke by instrumental neutron activation analysis with a compton suppression system. Anal. Chem. 65(11), 1506–1509. https://doi.org/10.1021/ac00059a004 (1993).
Dou, J. F. et al. Exposure to heavy metals in utero and autism spectrum disorder at age 3: A meta-analysis of two longitudinal cohorts of siblings of children with autism. Environ. Health 23(1), 62. https://doi.org/10.1186/s12940-024-01101-2 (2024).
Błażewicz, A. & Grabrucker, A. M. Metal profiles in autism spectrum disorders: A crosstalk between toxic and essential metals. Int. J. Mol. Sci. 24(1), 308. https://doi.org/10.3390/ijms24010308 (2022).
Błażewicz, A. et al. Research into the association of cadmium and manganese excretion with thyroid function and behavioral areas in adolescents with autism spectrum disorders. J. Clin. Med. 11(3), 579. https://doi.org/10.3390/jcm11030579 (2022).
Ma, C. et al. Association of prenatal exposure to cadmium with neurodevelopment in children at 2 years of age: The Japan Environment and Children’s Study. Environ. Int. 156, 106762. https://doi.org/10.1016/j.envint.2021.106762 (2021).
Corrêa, M. L. et al. Maternal smoking during pregnancy and intelligence quotient of offspring aged 18 and 30 years: Evidence from two birth cohorts in southern Brazil. Prev. Med. 156, 106983. https://doi.org/10.1016/j.ypmed.2022.106983 (2022).
Kawamoto, T. et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 14, 25. https://doi.org/10.1186/1471-2458-14-25 (2014).
Michikawa, T. et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 28(2), 99–104. https://doi.org/10.2188/jea.JE20170018 (2018).
Sekiyama, M. et al. Study design and participants’ profile in the sub-cohort study in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 32(5), 228–236. https://doi.org/10.2188/jea.JE20200448 (2022).
Nakayama, S. F. et al. Blood mercury, lead, cadmium, manganese and selenium levels in pregnant women and their determinants: The Japan Environment and Children’s Study (JECS). J. Expo. Sci. Environ. Epidemiol. 29(5), 633–647. https://doi.org/10.1038/s41370-019-0139-0 (2019).
Currie, L. A. Detection and quantification limits: Origins and historical overview. Anal. Chim. Acta. 391, 127–134. https://doi.org/10.1016/S0003-2670(99)00105-1 (1999).
Ikuzawa, M., Matsushita, Y. & Nakase, A. Kyoto Scale of Psychological Development 2001 (Kyoto International Social Welfare Exchange Centre, 2002).
Tatsuta, N. et al. Comparison of Kyoto scale of psychological development and Bayley scales of infant development second edition among Japanese infants. J. Spec. Educ. Res. 2, 17–24 (2013).
Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 48(2), 1–36 (2012).
Hu, L. & Bentler, P. M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model 6, 1–55. https://doi.org/10.1080/10705519909540118 (1999).
Richardson, S. A. & Tizabi, Y. Hyperactivity in the offspring of nicotine-treated rats: Role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol. Biochem. Behav. 47(2), 331–337. https://doi.org/10.1016/0091-3057(94)90018-3 (1994).
Orlebeke, J. F., Knol, D. L. & Verhulst, F. C. Increase in child behavior problems resulting from maternal smoking during pregnancy. Arch. Environ. Health 52(4), 317–321. https://doi.org/10.1080/00039899709602205 (1997).
Blood-Siegfried, J. & Rende, E. K. The long-term effects of prenatal nicotine exposure on neurologic development. J. Midwifery Womens Health 55(2), 143–152. https://doi.org/10.1016/j.jmwh.2009.05.006 (2010).
Schaap, M. M. et al. Effect of nationwide tobacco control policies on smoking cessation in high and low educated groups in 18 European countries. Tob. Control 17(4), 248–255. https://doi.org/10.1136/tc.2007.024265 (2008).
Kandel, D. B., Griesler, P. C. & Schaffran, C. Educational attainment and smoking among women: Risk factors and consequences for offspring. Drug Alcohol. Depend. 104(Suppl 1), S24-33. https://doi.org/10.1016/j.drugalcdep.2008.12.005 (2009).
Galiatsatos, P. et al. Association between neighborhood socioeconomic status, tobacco store density and smoking status in pregnant women in an urban area. Prev. Med. 136, 106107. https://doi.org/10.1016/j.ypmed.2020.106107 (2020).
Lu, Y., Tong, S. & Oldenburg, B. Determinants of smoking and cessation during and after pregnancy. Health Promot. Int. 16(4), 355–436. https://doi.org/10.1093/heapro/16.4.355 (2001).
Weiser, M., Zarka, S., Werbeloff, N., Kravitz, E. & Lubin, G. Cognitive test scores in male adolescent cigarette smokers compared to non-smokers: A population-based study. Addiction 105(2), 358–363. https://doi.org/10.1111/j.1360-0443.2009.02740.x (2010).
Corley, J., Gow, A. J., Starr, J. M. & Deary, I. J. Smoking, childhood IQ, and cognitive function in old age. J. Psychosom. Res. 73(2), 132–138. https://doi.org/10.1016/j.jpsychores.2012.03.006 (2012).
He, Y., Chen, J., Zhu, L. H., Hua, L. L. & Ke, F. F. Maternal smoking during pregnancy and ADHD: Results from a systematic review and meta-analysis of prospective cohort studies. J. Atten. Disord. 24(12), 1637–1647. https://doi.org/10.1177/1087054717696766 (2020).
Chen, D. et al. The correlation between prenatal maternal active smoking and neurodevelopmental disorders in children: A systematic review and meta-analysis. BMC Public Health 23(1), 611. https://doi.org/10.1186/s12889-023-15496-z (2023).
Biotteau, M., Albaret, J. M. & Chaix, Y. Developmental coordination disorder. Handb. Clin. Neurol. 174, 3–20. https://doi.org/10.1016/B978-0-444-64148-9.00001-6 (2020).
DuPaul, G. J., Power, T. J., Anastopoulos, A. D. & Reid, R. ADHD Rating Scale–IV: Checklists, Norms, and Clinical Interpretation (Guilford Press, 1998).
Wakschlag, L. S. et al. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch. Gen. Psychiatry 54(7), 670–676. https://doi.org/10.1001/archpsyc.1997.01830190098010 (1997).
Fukuda, M., Fukuda, K., Shimizu, T., Andersen, C. Y. & Byskov, A. G. Parental periconceptional smoking and male: Female ratio of newborn infants. Lancet 359(9315), 1407–1408. https://doi.org/10.1016/S0140-6736(02)08362-9 (2002).
Xu, B., Järvelin, M. R. & Rantakallio, P. Maternal smoking in pregnancy and sex differences in perinatal death between boys and girls. Soc. Biol. 45(3–4), 273–277. https://doi.org/10.1080/19485565.1998.9988978 (1998).
Obi, E. et al. Elevated prenatal methylmercury exposure in Nigeria: evidence from maternal and cord blood. Chemosphere 119, 485–489. https://doi.org/10.1016/j.chemosphere.2014.07.038 (2015).
Taylor, C. M., Kordas, K., Golding, J. & Emond, A. M. Effects of low-level prenatal lead exposure on child IQ at 4 and 8 years in a UK birth cohort study. Neurotoxicology 62, 162–169. https://doi.org/10.1016/j.neuro.2017.07.003 (2017).
Shipton, D. et al. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross-sectional study. BMJ 339, b4347. https://doi.org/10.1136/bmj.b4347 (2009).
Ma, C. et al. Health risk assessment and source apportionment of mercury, lead, cadmium, selenium, and manganese in Japanese women: An adjunct study to the Japan Environment and Children’s Study. Int. J. Environ. Res. Public Health 17(7), 2231. https://doi.org/10.3390/ijerph17072231 (2020).
Horiguchi, H. Current status of cadmium exposure among Japanese, especially regarding the safety standard for cadmium concentration in rice and adverse effects on proximal renal tubular function observed in farmers exposed to cadmium through consumption of self-grown rice. Nihon Eiseigaku Zasshi 67(4), 447–454. https://doi.org/10.1265/jjh.67.447 (2012) (in Japanese).
Watanabe, T., Kataoka, Y., Hayashi, K., Matsuda, R. & Uneyama, C. Dietary exposure of the japanese general population to elements: Total diet study 2013–2018. Food Saf. (Tokyo) 10(3), 83–101. https://doi.org/10.14252/foodsafetyfscj.D-22-00003 (2022).
Ministry of Health, Labor, and Welfare. Healthy parents and children 21: (Second phase) After the mid-term evaluation. https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://sukoyaka21.cfa.go.jp/wp-content/uploads/2024/03/sukoyaka21tyukanshihyou.pdf&ved=2ahUKEwjX7OTKqOKHAxV1iq8BHUZILI0QFnoECB4QAQ&usg=AOvVaw3Heb53STuDFEJ9DXqj2YTD. (2022) (in Japanese).
Acknowledgements
We would like to thank the participants and the Co-operating health care providers for their cooperation in JECS. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government.
Funding
This study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the government.
Author information
Authors and Affiliations
Consortia
Contributions
NT: Conceptualization, Investigation, Writing—original draft. KN: Investigation, Formal analysis, Writing—review and editing. MS: Methodology, Writing—review and editing. YT: Data curation, Writing—review and editing. MI: Methodology, Writing—review and editing. MT: Methodology, Writing—review and editing. YK: Methodology, Writing—review and editing. TI: Methodology, Writing—review and editing. SN: Formal analysis, Supervision, Funding acquisition. SY: Project administration, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tatsuta, N., Nakai, K., Sekiyama, M. et al. Direct and indirect effects of smoking during pregnancy on child development: The Japan Environment and Children’s Study. Sci Rep 15, 18053 (2025). https://doi.org/10.1038/s41598-025-02684-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-02684-7